Abstract
The nature of neurochemical changes in autism spectrum disorder (ASD) remains controversial. We compared medial prefrontal cortex (mPFC) neurochemistry of twenty high-functioning children and adolescents with ASD without associated comorbidities and fourteen controls. We observed reduced total N-acetylaspartate (tNAA) and total creatine, increased Glx/tNAA but unchanged glutamate + glutamine (Glx) and unchanged absolute or relative gamma-aminobutyric acid (GABA+) in the ASD group. Importantly, both smaller absolute and relative GABA+ levels were associated with worse communication skills and developmental delay scores assessed by the autism diagnostic interview—revised (ADI-R). We conclude that tNAA is reduced in the mPFC in ASD and that glutamatergic metabolism may be altered due to unbalanced Glx/tNAA. Moreover, GABA+ is related to autistic symptoms assessed by the ADI-R.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Autism Spectrum Disorder (ASD) is an early-onset neurodevelopmental disorder clinically expressed by impairments in social communication and interaction, presence of stereotyped and atypical and restricted areas of unusual interest (American Psychiatric Association 2013). Prevalence estimates are quite heterogeneous and may vary between 1.4 and 116.1 in 10 000 (Oliveira et al. 2007; Zaroff and Uhm 2012), being higher in boys than in girls, with a ratio around 4:1 (Fombonne 2003). Associated cognitive phenotypes vary from intellectual disability to high functioning profiles with normal or above normal global intelligence (Mouga et al. 2016). However, dysfunctions in other clinical areas are frequent in ASD such as motor and sensory abnormalities (Geschwind 2009), which suggests the involvement of distinct brain circuits. Importantly, comorbid neuropsychiatric or neurological conditions are highly prevalent among ASD patients, namely attention-deficit hyperactivity disorder (ADHD), anxiety, and epilepsy (Lai et al. 2014; Lever and Geurts 2016), pointing towards shared pathophysiological mechanisms.
It has been hypothesized that an early cortical excitation/inhibition (E/I) imbalance with a bias for increased excitation involving circuits related to sensory processing, social and emotional cognition, and language may underlie the pathophysiology of ASD (Rubenstein and Merzenich 2003). The prefrontal cortex (PFC) and the anterior cingulate cortex (ACC), two regions of the frontal lobe, are of particular interest due to their roles in high order functions such as inferring others’ mental states, executive functions, social and emotional cognition and ability to adjust behaviour (Apps et al. 2016; Badre and D’Esposito 2007; Van Overwalle 2009). These cognitive functions are strikingly impaired in patients with ASD (Brunsdon and Happé 2014). Importantly, the above mentioned regions rely in key cortico-cortical, subcortico-cortical and fronto-cerebellar connections that drive these types of behaviour (Arnold Anteraper et al. 2014; Daly et al. 2014; Hodge et al. 2010; Morris et al. 2016). Widespread altered brain circuity involving these connections has been put forward as the basis for the neurobiological and behavioural features of ASD (Markram et al. 2007). Control of the levels of Glutamate, the main excitatory neurotransmitter, and Gamma-aminobutyric acid (GABA), the main inhibitory neurotransmitter, is pivotal in keeping the physiological E/I balance (Marín 2012; Takahashi et al. 2012). Excitatory glutamatergic neurons, which make up to 80% of the cortical neurons, are responsible for cortico-cortical and cortico-subcortical connections that form local/short- or distant/long-range circuits giving rise to large-scale information processing networks (Zikopoulos and Barbas 2013). Inhibitory GABAergic interneurons, which make up to 20% of the cortical neurons, are important in shaping the responses of local cortical columns, although long range interactions have also been reported, with an important role in learning and neuroplasticity (Letzkus et al. 2015). In line with the E/I imbalance hypothesis, several postmortem studies directly support altered GABAergic function in the frontal lobe of patients with ASD, namely reduced GABAA and GABAB receptors densities, as well as reduced protein expression and benzodiazepine binding sites (Fatemi et al. 2002, 2009, 2010; Oblak et al. 2010, 2009). In vivo studies also provide both indirect and direct evidence for glutamatergic and/or GABAergic dysfunction in ASD, such as a high rate of epileptiform discharges without seizures detected in ASD patients (Spence and Schneider 2009), which frequently originate in the frontal lobe (Hashimoto et al. 2001; Yasuhara 2010). Likewise, reduced benzodiazepine receptors in the medial frontal cortex of patients with ASD and normal intellectual level (Mori et al. 2012) and reduced GABAA binding potential in a Positron Emission Tomography study (Mendez et al. 2013) support an E/I imbalance.
Proton magnetic resonance spectroscopy (1H-MRS) is a promising tool allowing for the in vivo non-invasive quantification of biologically relevant compounds (i.e. neurotransmitters and metabolic intermediates), providing information regarding the tissue concentration of main neurotransmitters as well as associated neural metabolism (Rae 2014). Regarding the E/I balance in ASD, neurochemical studies have mainly used conventional spectroscopy sequences that only allow to investigate the glutamatergic pool, i.e. glutamate (Glu) + glutamine (Gln), reported as Glx. The literature reveals mixed findings of increased, decreased or unchanged Glx levels (see below). Nonetheless, when changes are present most studies suggest regional- and age-dependent metabolic changes, especially regarding the ACC where children and adolescent show increased Glx, while adult levels may be decreased or unchanged (Ford and Crewther 2016; Naaijen et al. 2015; Rojas et al. 2014a). However, in these studies, and particularly at 1.5T which has suboptimal spectral resolution and low signal to noise (SNR), the Glx signal measured between 2.0 the 2.4 parts per million (ppm) also contains contribution from the most prominent peak observable by 1H-MRS, the n-acetylaspartate (NAA) + n-acetylaspartylglutamate (NAAG), at 2 ppm and from GABA at 1.9 ppm (Ford and Crewther 2016; Rae 2014; Rojas et al. 2014a). The signal of NAA + NAAG (tNAA), in turn, has the contribution of NAA which is synthesized only in the neuronal mitochondria, and is usually referred to as a marker of neuronal mitochondrial metabolism (Rae 2014); and NAAG, which is synthesized from NAA and glutamate. The latter is an important regulator of glutamatergic neurotransmission due to its interactions with both metabotropic and ionotropic glutamate receptors (Benarroch 2008). A meta-analysis of NAA studies showed decreased levels in the ACC of children and adolescents with ASD while results in adults are less clear (Aoki et al. 2012b; Ford and Crewther 2016). In any case the biological significance of these findings remains under debate (Chugani 2012; Rae 2014). Consequently, in conventional spectroscopy studies, changes in the 2–2.4 ppm Glx signal are difficult to interpret in the light of the E/I imbalance hypothesis since this signal represents a mixture of metabolites and information regarding GABA is lacking. Currently, with field strengths of 3T or above, combined with recent spectral editing sequences, such as the MEGA-PRESS method, it is possible to quantify GABA by resolving its signal from the prominent creatine + phosphocreatime (tCr) signal at 3 ppm (Mescher et al. 1998). Usually macromolecules also contribute to this signal, with the global signal denoted as GABA+ (Shungu et al. 2016). MEGA-PRESS has been the main spectral-editing sequence used at 3T to quantify GABA+ either in the healthy brain (Cleve et al. 2015; Long et al. 2015; Maddock et al. 2016) or in pathological conditions where an imbalance in the E/I may be also present, such as schizophrenia (Chowdhury et al. 2015; Rowland et al. 2015), attention deficit hyperactivity disorder (ADHD) (Bollmann et al. 2015), neurofibromatosis type 1 (Ribeiro et al. 2015; Violante et al. 2013, 2016), and epilepsy (Chowdhury et al. 2015).
Regarding GABA+ quantification in ASD, decreased or unchanged GABA+ levels have been reported with MEGA-PRESS, with most studies assessing children and adolescents (Brix et al. 2015; Cochran et al. 2015; Drenthen et al. 2016; Gaetz et al. 2014; Goji et al. 2017; Harada et al. 2011; Ito et al. 2017; Puts et al. 2016; Rojas et al. 2014b), two studies including only adults (Ajram et al. 2017; Robertson et al. 2015) and one study assessing children and adults (Port et al. 2016). Decreased GABA+ measurements were reported in the ACC (Ito et al. 2017), left frontal (Harada et al. 2011), motor (Gaetz et al. 2014), auditory (Gaetz et al. 2014; Port et al. 2016; Rojas et al. 2014b), right sensorimotor (Puts et al. 2016), and left cerebellum (Ito et al. 2017) regions in children and adolescents with ASD. On the other hand, unchanged GABA+ levels in ASD patients were reported in the left (Brix et al. 2015) and middle prefrontal cortex and anterior cingulate (Ajram et al. 2017; Cochran et al. 2015; Goji et al. 2017); left lenticular nucleus (Harada et al. 2011); bilateral occipital cortex (Brix et al. 2015; Drenthen et al. 2016; Gaetz et al. 2014; Puts et al. 2016; Robertson et al. 2015), left motor cortex (Robertson et al. 2015) and left cerebellum (Goji et al. 2017), both in children and adults. Importantly, GABA+ levels either changed or unchanged likely play an important role in the pathophysiology of ASD since its levels have been linked to altered sensory processing (Port et al. 2016; Puts et al. 2016; Robertson et al. 2015) and to symptom’s severity (Brix et al. 2015; Gaetz et al. 2014).
Some of these studies also reported Glu or Glx and tNAA levels, with mostly no changes detected in these metabolites (Ajram et al. 2017; Brix et al. 2015; Cochran et al. 2015; Goji et al. 2017; Harada et al. 2011; Ito et al. 2017; Robertson et al. 2015), and reduced tNAA (Gaetz et al. 2014; Goji et al. 2017) or increased Glu/tCr (Drenthen et al. 2016; Ito et al. 2017) in others. Additionally, one study performed at 1.5T reported decreased levels of GABA+, Glx and tNAA in the left and right frontal lobe of children with ASD (Kubas et al. 2012). However the authors did not use specialized GABA quantifying methods which is challenging at 1.5T, making the interpretation of results unclear and possibly attributable to a Glx decrease (Ford and Crewther 2016). The current knowledge on the functional and clinical impact of GABA levels in ASD is still scarce due to the reduced number of available studies. Importantly, research including core clinical measures may help to shed light on the impact of GABA+ both in the behavioural and clinical outcomes of ASD (Brix et al. 2015; Ford and Crewther 2016; Gaetz et al. 2014). Nonetheless, a recent meta-analysis of GABA+ studies in ASD suggested that overall levels are reduced in children and adolescents with ASD (Schür et al. 2016), though less than half (5 out of 12) of the published studies were included in the meta-analysis.
The current study aimed at assessing the E/I balance in a sample of high functioning children and adolescents with ASD without any other neuropsychiatric or neurodevelopmental comorbidity. We quantified GABA+, Glx and tNAA in a voxel comprising the mPFC and ACC. Importantly, given the previous association between GABA+ measures in the frontal lobe and ASD clinical severity (Brix et al. 2015), we further explored the relation between GABA+, Glx and tNAA with the gold standard ASD diagnostic tools, as this has not been previously done with standardized tools. We additionally investigated whether intellectual level could be related to medial frontal cortex GABA+, Glx or tNAA measures either in healthy controls or in the ASD participants. Measures of GABA+ and Glx normalized to the two most common internal references, tNAA and tCr, were also investigated.
Methods
Participants
Fifty participants were enrolled in the study: 27 patients (age range: 9–18 years old) and 23 control participants (age range 10–18 years old).
Patients were recruited from a Paediatric Hospital which is a leading national centre for the diagnosis of Autism Spectrum Disorder and other neurodevelopmental disorders. All patients were previously diagnosed and assessed in the clinical setting at least twice a year by a team of neurodevelopmental paediatricians and psychologists with extensive experience in ASD assessment and diagnosis. Thus, a comprehensive clinical characterization was used to ensure that the patients met the inclusion and exclusion criteria by the time of the MRS session. Inclusion criteria for patients were age below 18 years old, positive results on the direct parental or caregiver interview Autism Diagnostic Interview—Revised (ADI-R) (Rutter et al. 2003) and proband assessment with Autism Diagnostic Observation Schedule, ADOS (Lord et al. 1999). ADI-R is a structured interview used for diagnosing autism, planning treatment, and distinguishing autism from other neurodevelopmental disorders. It can be used for diagnostic purposes for anyone with a mental age of at least 18 months and provides quantitative measures of behaviour in the areas of (i) reciprocal social interactions, (ii) language and communication, and (iii) repetitive behaviours/interests. ADOS is used for assessing and diagnosing autism and pervasive developmental disorder across ages, developmental levels, and language skills. It consists of a series of structured and semi-structured tasks that involve social interaction between the examiner and the subject. The examiner observes and identifies segments of the subject’s behaviour and assigns these to predetermined observational categories, which are combined to a score. The score is assessed through cut-offs in (i) reciprocal social interaction and (ii) communication and language that identify the potential diagnosis of ASD. Additionally, the diagnostic criteria for autism obtained with the DSM-IV were revised according to the Diagnostic and Statistical Manual of Mental Disorders 5, DSM-5 (American Psychiatric Association 2013). Exclusion criteria were full-scale intelligence quotient (FSIQ) below 70 as measured by the Wechsler Intelligence Scales, so to exclude subjects with intellectual disability using the World Health Organization International Classification of Diseases-10 criteria (World Health Organization 1992), genetic syndrome (i.e. Tuberous Sclerosis, Fragile X Syndrome), neuropsychiatric, neurodevelopmental and neurologic comorbidities (such as anxiety, severe mood or sleep disturbances, attention-deficit and hyperactivity disorder or epilepsy). Six patients were under medication for ASD-related symptomatology, in particular irritability and agitation, at the time of the MRS exam (risperidone n = 5 and methylphenidate n = 1).
Subjects included in the control group were recruited from local schools. Inclusion criteria were age under 18 years old. Exclusion criteria were medication, FSIQ below 70, history of psychiatric or neurological conditions, neuropsychiatric, neurodevelopmental or neurologic conditions (an anamnesis questionnaire was completed by the parents), and altered MRI examinations. Parents of control participants completed the Social Communication Questionnaire (SCQ) (Rutter et al. 2003) and Social Responsiveness Scale (SRS) (Constantino and Gruber 2005) to exclude autism. Both groups were assessed with the Portuguese version of the Wechsler Intelligence Scale for children (WISC-III) (Wechsler 2003) or for adults (WAIS-III) (Wechsler 2008), when applicable.
Informed consent was obtained from the parents/guardians of the participants. Children and adolescents also gave oral informed consent.
The study was approved by the ethics committee of the Faculty of Medicine of the University of Coimbra and was conducted in accordance with the declaration of Helsinki.
Magnetic Resonance Spectroscopy
Acquisition
MRS data were acquired in a 3T Siemens TimTrio scanner (Erlangen, Germany), using a 12-channel birdcage head coil. No sedation was used.
For each participant a T1-weighted Magnetization Prepared Rapid Acquisition Gradient-Echo (MPRAGE) sequence was acquired with 1 × 1 × 1 mm3 voxel size, repetition time (TR) = 2.3 s, echo time (TE) = 2.98 ms, flip angle (FA) = 9°, field of view (FOV) = 256 × 256, 160 slices, and used for spectroscopy voxel positioning.
A single-voxel with 3 × 3 × 3 cm was acquired with the MEGA-PRESS method (Edden and Barker 2007; Mescher et al. 1998). Acquisition parameters were: repetition time 1.5 s, echo time 68 ms, 392 averages, 1024 data points, bandwidth 2000 Hz with automatic shimming and chemical shift selective (CHESS) weak water suppression with a bandwidth of 35 Hz, resulting in an acquisition time of approximately 10 min. No saturation bands were used. During the odd number acquisitions, a frequency-selective inversion pulse was applied to the GABA-C3 resonance at 1.9 ppm (edited spectrum). During even number acquisitions, the pulse was applied at 7.5 ppm, which does not affect the regularly observed peaks using conventional spectroscopy sequences, i.e. Glx, tCr, tNAA, Cho and mIns (unedited spectrum). The Gaussian editing pulse bandwidth was 44 Hz. Subtracting the unedited from the edited spectrum reveals the signal of the metabolites affected by the 1.9 ppm selective pulse, thus GABA, Glx, tNAA and macromolecules are the only peaks observed. The difference spectrum was exported from the scanner for further analysis with dedicated software. Macromolecules signal at 0.9 ppm also contribute to the GABA signal at 3 ppm (Shungu et al. 2016), thus our measures represent GABA+ macromolecules levels, referred to as GABA+.
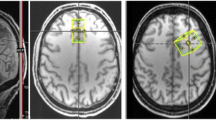
The voxel was placed above the orbitofrontal cortex in the bilateral medial prefrontal cortex, centered in the midline of the coronal and axial planes and rotated in the sagittal view to avoid bone. In the sagittal view the lower edge was aligned with the rostrum of the corpus callosum. Thus, the voxel targets prefrontal cortex and anterior cingulate (Fig. 1a–c), hereafter referred as mPFC.
Example of the position of the MEGA-PRESS voxel (3 × 3 × 3 cm) in the medial prefrontal cortex and anterior cingulate of one participant; a sagittal view; b coronal view; c axial view; and d difference spectrum from a representative participant (black line) and LCModel fit (red line). A anterior, P posterior, R right, L left, Glx glutamate + glutamine, GABA+ gamma-aminobutyric acid + macromolecules, tNAA n-acetylaspartate + n-acetylaspartilglutamate, MM macromolecules, ppm parts per million. (Color figure online)
All participants watched cartoons during the scanning protocol. This helped participants to stay still during scanning. SensoMotoric Instruments (SMI) (Teltow, Germany) eye-tracker was used to monitor subjects’ position during the session. If strong movement was detected the acquisition was stopped and restarted. One participant of the ASD group was unable to finish the MRS acquisition, thus only 26 datasets were further included.
Data Processing and Quality Check
We quantified GABA+, Glx and tNAA with LCModel version 6.3–1D (Stephen Provencher Inc., Oakville, Canada) which allows for the quantification of MEGA-PRESS data from the difference spectrum. LCModel analyzes in vivo spectra as linear combination model of in vitro basis set spectra from each metabolite using the complete spectra model (Provencher 1993, 2001). The software analyzes data in the frequency-domain and has been shown to provide very good reproducibility (O’Gorman et al. 2011). Absolute values of metabolites in the difference spectrum were quantified using the standard external calibration procedure of LCModel which scales the in vivo spectra to a basis set containing GABA, Glu, Gln, NAA, NAAG and glutathione (further details in the LCModel manual for version 6.3–1D, http://s-provencher.com/lcmodel.shtml). Moreover, no T1 and T2 relaxation time corrections or water-scaling were performed, therefore metabolites values are in institutional units. Additionally, we also report metabolite ratios using the internal reference tNAA. Since it is common to report GABA+ and Glx as ratios to total creatine (tCr) we conducted an additional analysis where we calculated GABA+/tCr and Glx/tCr by dividing GABA+ or Glx by the tCr obtained from the LCModel analysis of the unedited off spectrum. Since this spectrum is equivalent to a standard PRESS it was scaled to the appropriate basis set, i.e. PRESS (TE = 68 ms).
Difference spectra were visually inspected for fitting quality and standard deviation of the Cramer-Rao lower bound (%CRLB) for GABA+ measurement was set at a threshold of 10% (one case from the control group with 11% for GABA was also accepted). Importantly, SNR and linewidth also influence the viability of metabolite quantification (Bartha 2007), and only data with a SNR equal or above 18 (maximum was 35) and a linewidth (expressed as full-width-at-half-maximum) between 0.032 and 0.079 parts per million (ppm) (or between 4 and 10 Hz) showed good fitting quality. After thorough data quality assessment, 15 fifteen datasets were excluded from the final analysis (6 datasets in the ASD group and 9 datasets from the control group). The included data had standard deviation of %CRLB estimates ranging between 4 and 11 for GABA+, between 3 and 6 for Glx, and between 1 and 2 for NAA + NAAG. Thus, only data with good metabolite level estimation were further analyzed. Figure 1 d shows an example of a good quality difference spectrum and the respective LCModel fit from one patient (see supplementary Fig. 1S for MEGA-PRESS spectra fitting for each group). The corresponding unedited spectra all showed good fitting quality with %CRLB ranging between 1 and 3 for tCr, SNR ranging between 17 and 43 and FWHM linewidth ranging between 0.032 and 0.071 (or between 4 and 10 Hz).
In-house developed software running in Matlab8 (R2013a, The Mathworks Inc., Natik, Massachussets, USA) using the VBM8 toolbox (http://www.neuro.uni-jena.de/vbm/) from SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/, University College London, London, UK) was used to perform MRS voxel segmentation of gray matter (GM), white matter (WM) and cerebrospinal fluid (CSF) to assess differences in tissue composition and to correct absolute metabolite concentrations for the CSF content of the voxel, given that CSF has negligible metabolite content. This correction was achieved with the following calculation: \([{Metabolite}_{LCModel}/(1 - {proportion}_{CSF}\left)\right]\), with the proportion of CSF falling in a 0 to 1 interval (Drenthen et al. 2016; Horder et al. 2013), and can be interpreted as a normalization of the metabolites signal to the overall proportion of tissue inside the voxel.
Statistical Analysis
Demographic, neuropsychological, voxel tissue composition and spectroscopy data were analyzed with IBM SPSS statistics version 23 (IBM Corporation, IL, USA).
All data were checked for normal distribution with the Shapiro–Wilk’s test and for homogeneity of variance with Levene’s test. Between group differences were then investigated with two-tailed independent samples t-test if data assumption of normality was confirmed or with Mann–Whitney U test if data did not show normal distribution. All data showed homogeneity of variance. Independent samples t-test, effect size and power were calculated for the statistically significant group differences regarding neurochemical differences with G*Power 3.1.9 software (Faul et al. 2007, 2009). Correction for multiple comparisons using the Benjamini-Hochberg false discovery rate (FDR) method (Benjamini and Hochberg 1995) (α = 0.05) was performed as adequate for the number of between group tests regarding metabolites comparisons and are indicated in the respective results.
Correlation analyses between metabolites and clinical diagnostic or intellectual level scores were assessed with partial correlation analysis controlling for age given the reported metabolite age-related changes in the ASD literature (see “Introduction” and “Discussion”). Statistical significance threshold was defined with α = 0.05. Correlation analyses were not corrected for multiple comparisons and are, therefore, exploratory.
Results
Demographic Data, Voxel Tissue Composition and Spectroscopy Data Quality Comparison Between Groups
In the current work we report data from 20 patients (mean age 13 years ± 2, range 11–18 years) and 14 control participants (mean age 13 years ± 2, range 10–18 years), after thorough quality control (see “Methods” for details). Patients and controls were matched in age (U = 137, p = 0.931) and performance IQ (PIQ) (U = 177.5, p = 0.191), while full scale (FSIQ) and verbal IQ (VIQ) were different between groups (U = 240.5, p < 0.001 for both). Table 1 summarizes demographic and neuropsychological data. Voxel tissue composition did not differ between groups (Table 2), thus any differences in our results were not likely driven by differences in tissue composition. Measures of data quality, i.e., SNR ratio and FWHM linewidth (Bartha 2007) were not different between groups either for the difference or for the unedited off spectra (all p > 0.274). Also, estimates of spectroscopy model fit quality, i.e. %CRLB bounds (Alger 2011), were not different between groups (p > 0.274), except for Glx that showed a marginal statistical difference (p = 0.047) (see supplementary Table S1 for details).
Metabolite Absolute Values Between-Group Comparisons
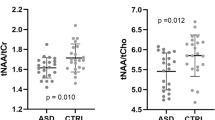
The tNAA levels were reduced in the ASD group [t(32) = − 2.810, p = 0.008, Cohen’s d = 0.95, power = 0.75; 9% reduction] (Fig. 2a) as well as tCr (t(32) = − 2.419 p = 0.021, Cohen’s d = 0.85, power = 0.65; 9% reduction) (Fig. 2b), with both results surviving FDR correction for four comparisons. GABA+ and Glx were not different between ASD and control participants [t(32) = − 0.897, p = 0.377 and t(32) = − 1.198, p = 0.240, respectively] (Fig. 2c and d) (see supplementary table S2 for a summary). An additional correction that has been recently proposed assuming that GABA in white matter is half of that found in grey matter (Harris et al. 2015) did not change this result. Excluding medicated patients (n = 6) did not change results since both tNAA and tCr were still significantly reduced in the ASD group [tNAA: t(26) = − 2.915 p = 0.007, Cohen’s d = 1.12, power = 0.81, 10% tNAA reduction; tCr: t(26) = − 2.907 p = 0.046, Cohen’s d = 0.79, power = 0.60, 9% reduction] with the remaining metabolites not differing between groups (p > 0.196). The tNAA reduction survived FDR correction for 4 multiple comparisons also when excluding the medicated patients.
Metabolites quantified the in the medial prefrontal cortex and anterior cingulate of patients with ASD (circles, n = 20) and controls (squares, n = 14): a tNAA, b tCr, c GABA+, and d Glx. Graphs depict individual values, mean and standard deviation. tNAA, n-acetylaspartate + n-acetylaspartilglutamate; tCr creatine + phosphocreatine, GABA+ gamma-aminobutyric acid + macromolecules, Glx glutamate + glutamine, p statistical value for between group comparisons. Results remained significant after Benjamin-Hochberg false-discovery rate correction for multiple four comparisons (α < 0.05)
Metabolite Ratios to tNAA or tCr Between-Group Comparisons
The Glx/tNAA was significantly increased in the ASD group [t(32) = 2.095, p = 0.044, Cohen’s d = 0.97, power = 0.77; 3% increased] (Fig. 3a) while Glx/tCr was marginally increased in the ASD group (p = 0.092) (Fig. 3b). Regarding GABA+/tNAA and GABA+/tCr, none of the ratios showed differences between groups (both p > 0.452) (Fig. 3c and d) (see supplementary table S3 for a summary). The Glx/tNAA result did not survive FDR correction for multiple comparisons. When removing medicated patients (n = 6) the Glx/tNAA ratio showed a trend for being increased in the ASD group (p = 0.076), while all the remaining comparisons showed no differences (all p > 0.242). We also compared the inhibitory index calculated as \([\text{GABA}/(\text{GABA}+\text{Glx}\left)\right]\) (Ajram et al. 2017), which was not different between groups (p > 0.882), not even when removing medicated patients (p = 0.652) (data not shown).
Relative measures to tNAA and tCr of the main excitatory and inhibitory neurotransmitters quantified in the medial prefrontal and anterior cingulate cortex of patients with ASD (circles, n = 20) and controls (squares, n = 14): a Glx/tNAA, b Glx/tCr, c GABA+/tNAA, and d GABA+/tCr. Graphs depict individual values, mean and standard deviation. Glx glutamate + glutamine, GABA+ gamma-aminobutyric acid + macromolecules, tCr creatine + phosphocreatine, tNAA n-acetylaspartate + n-acetylaspartilglutamate, p statistical value for between group comparisons
Metabolite Absolute Values Correlations with Behaviour and Cognition
GABA+ was negatively correlated with two of the four components of the ADI-R questionnaire, the communication score (r = − 0.567, p = 0.011) (Fig. 4a) and the developmental delay score (r = − 0.538, p = 0.018) (Fig. 4b), with lower GABA+ values corresponding to worse communication and greater severity in early development delay scores. No other correlations were found between the remaining ADI-R scores or any of the ADOS scores and the quantified metabolites. Regarding correlation with intellectual scores, a dimensional analysis across groups (N = 34) showed that tNAA was positively correlated with FSIQ (r = 0.492, p = 0.004) and VIQ (r = 0.513, p = 0.002); that tCr was positively, although with less strength, correlated with FSIQ (r = 0.375, p = 0.032) and VIQ (r = 0.356, p = 0.042); and that Glx was mildly correlated with VIQ (r = 0.358, p = 0.041), while GABA+ showed no correlation with intelligence scores (all p > 0.236). When patients and control groups were analyzed separately, however, the pattern of the correlations found was distinct between groups. In the ASD group, only tNAA and PIQ were positively correlated (r = 0.520, p = 0.022) (Fig. 4c), meaning that higher tNAA were associated with higher PIQ scores. On the other hand, the control group only showed trend level correlations between tNAA and VIQ (r = 0.534, p = 0.060) as well as between Glx and VIQ (r = 0.514, p = 0.072). Again, GABA+ did not show any significant correlation with intelligence scores either in the ASD (all p > 0.278) or in the controls group (all p > 0.096).
Association between GABA+ and a the autism diagnostic interview—revised (ADI-R) communication (r = − 0.567, p = 0.011) and b delayed development (r = − 0.538, p = 0.018) scores, and c correlation between tNAA and PIQ in the autism group (r = 0.520, p = 0.022). GABA+ gamma-aminobutyric acid + macromolecules, tNAA n-acetylaspartate + n-acetylaspartilglutamate, PIQ Performance intelligence quotient, r partial correlation coefficient (correcting for age) p statistical value
Metabolite Ratios to tNAA or tCr Correlations with Behaviour and Cognition
Replicating the GABA+ absolute values analysis, GABA+/tNAA was negatively correlated with the ADI-R communication score (r = − 0.527, p = 0.020) and the developmental delay score (r = − 0.496, p = 0.031) as well as the GABA+/tCr which was correlated with the ADI-R communication score (r = − 0.464, p = 0.045) and the developmental delay score (r = − 0.488, p = 0.034), further showing the same link between lower GABA+ measures and symptom severity (correlation plots were very similar to the absolute values plots and are, thus, not shown). Again, we also investigated a dimensional correlation between ratios and intellectual level across groups which did not show any significant correlations (all p > 0.121). Similarly, no significant correlations were found between ratios measures and intellectual scores either in the ASD (all p > 0.088) or in the control group (all p > 0.209).
Discussion
In this study, we investigated the hypothesis of prefrontal lobe E/I imbalance in children and adolescents with ASD by quantifying three relevant 1H-MRS metabolites: GABA+, Glx (glutamate + glutamine) and tNAA (NAA + NAAG). We also aimed to investigate whether these metabolites were associated with the clinical and intellectual profile in the ASD group using standardized observational tools and interviews. Importantly, our high functioning ASD group presented no other neuropsychiatric, neurodevelopmental or neurological comorbidity which is a major distinction from previous studies assessing E/I balance in ASD, as participants with anxiety (Cochran et al. 2015; Robertson et al. 2015), ADHD (Cochran et al. 2015; Puts et al. 2016) or epilepsy (Brix et al. 2015) have been previously included in patient or even the control groups (Cochran et al. 2015). Disrupted E/I has also been identified in these conditions (Bozzi et al. 2017; Li et al. 2017; Maltezos et al. 2014; Solleveld et al. 2017), thus by excluding them our aim was to investigate the neurochemistry related only to ASD clinical symptomatology and to overcome possible confounding factors in previous works leading to sample heterogeneity. We focused in a voxel comprising the mPFC due to its important role in executive functions (i.e. behavioural inhibition, cognitive flexibility and working memory), as well as social learning and cognition, which are strikingly impaired in ASD patients (Apps et al. 2016; Badre and D’Esposito 2007; Brunsdon and Happé 2014; Van Overwalle 2009). We report absolute metabolite concentrations (in institutional units) as well as ratio values obtained from the edited MEGA-PRESS difference spectrum which allows for a complete signal separation of GABA+ at 3 ppm from Glx at 3.75 ppm and from tNAA at 2 ppm (Cleve et al. 2015). Previous studies quantifying GABA+ with MEGA-PRESS reported Glx or tNAA levels either from conventional spectroscopy sequences (Brix et al. 2015; Cochran et al. 2015; Harada et al. 2011; Ito et al. 2017) or from the unedited MEGA-PRESS spectrum (Gaetz et al. 2014; Robertson et al. 2015; Rojas et al. 2014b), which is the equivalent to a conventional PRESS sequence where peaks overlap. We additionally report GABA+/tCr and Glx/tCr for comparison with previous reports.
In this work, we found reduced tNAA and tCr, an increased Glx/tNAA, possibly driven by the reduction in tNAA, and unchanged GABA+ and Glx levels in the mPFC of children and adolescents with ASD. These results were found in the absence of voxel tissue composition differences. Importantly, excluding medicated patients did not change the overall results, except for the Glx/tNAA ratio which only showed a non-significant trend for an increase. Interestingly, in the ASD group tNAA was positively correlated with PIQ scores, while lower GABA+, GABA+/tNAA and GABA+/tCr values were all associated with worse communication/development scores obtained with the ADI-R questionnaire.
Our findings of decreased tNAA are consistent with 1H-MRS observations in the ASD literature. In fact, reduced frontal lobe levels of tNAA in children and adolescents with ASD, especially in the ACC, have been reported in different studies (see Aoki et al. 2012 for a meta-analysis and; Ford and Crewther 2016 for a comprehensive review; Goji et al. 2017). tNAA is thought to reflect neuroenergetics or neuronal metabolic function (Moffett et al. 2013), nevertheless, the biological meaning of this reduction in ASD is still a matter of debate (Chugani 2012; Rae 2014). The acetyl-aspartate signal measured by 1H-MRS at 2 ppm mainly accounts for NAA, with NAAG also contributing to about 10–25% of the total signal, depending on the brain region (Pouwels and Frahm 1997). Both molecules play distinct roles: NAA is considered a proxy measure for neural density and/or metabolism, while NAAG (synthesized from NAA and glutamate) is a neurotransmitter. NAAG interacts mainly with glutamatergic receptors (Benarroch 2008) and is thought to play a role in neuroprotection and synaptic plasticity due to modulatory properties of neurotransmission involving glutamate, GABA and other monoamines (Neale et al. 2000). In sum, NAAG plays an important role in the E/I balance. We found a 10% decrease in the tNAA signal in the ASD group, which could be due to decreased NAA, decreased NAAG or both. This finding provides further support for a role of mPFC tNAA levels in the neurobiology of ASD. Interestingly, frontal cortex tNAA measures when different appear to show age-related changes with decreased levels reported in children (Fujii et al. 2010; Goji et al. 2017; Mori et al. 2013) and the opposite being found in adults (Aoki et al. 2012a; Murphy 2002), but not always (Horder et al. 2013; Tebartz Van Elst et al. 2014). tNAA levels have been moderately linked with IQ measures in neurotypical individuals (Patel et al. 2014). In our ASD sample, tNAA was positively correlated with PIQ, while in the control group this correlation was absent. Instead, the control group showed trend level correlation between tNAA and VIQ. This suggests that tNAA may be related with different aspects of cognition in both groups. Currently, in vivo spectral editing techniques allow for the differentiation of NAA from NAAG (Edden et al. 2007; Landim et al. 2015) and future studies separating the contribution of each molecule to the overall signal should shed light into the nature and role of altered tNAA levels in ASD.
Regarding GABA, reports in the literature are less abundant and recent studies assessing GABA+ in ASD have shown reduced levels in auditory regions (Gaetz et al. 2014; Port et al. 2016; Rojas et al. 2014b), decreased (Gaetz et al. 2014; Puts et al. 2016) or unchanged (Robertson et al. 2015) levels in motor/sensorimotor regions, decreased (Harada et al. 2011; Ito et al. 2017) or unchanged (Ajram et al. 2017; Brix et al. 2015; Cochran et al. 2015; Goji et al. 2017) levels in frontal regions, decreased (Ito et al. 2017) or unchanged (Goji et al. 2017) levels in the left cerebellum, and unchanged levels in the occipital lobe (Drenthen et al. 2016; Gaetz et al. 2014; Puts et al. 2016; Robertson et al. 2015). Our results showed no changes either in the absolute or relative GABA+ levels in the medial prefrontal cortex of children and adolescents with ASD. This remained also true for the absolute GABA+ value corrected to take into account the assumption that GABA in white matter is half of that found in grey matter (Harris et al. 2015), which was expected given the lack of differences between voxel tissue composition. Our results are in line with the work from Cochran et al. (Cochran et al. 2015) and Goji et al. (Goji et al. 2017), which voxel position overlaps with ours, but also with Brix et al. (Brix et al. 2015) which voxel position partially overlaps with ours. In a recent study Ajram et al. (Ajram et al. 2017) used 1H-MRS to investigate the E/I responsivity to an antiglutamatergic and pro-GABA drug in adults with ASD. Our results are in line with the observed unchanged levels of glutamate and GABA+, and the inhibitory index, i.e. \([\text{GABA}/(\text{GABA}+\text{Glx}\left)\right],\) in the dorsomedial prefrontal cortex of adults with ASD (Ajram et al. 2017), despite the age differences between studies. Interestingly, in that study, after a single dose of Riluzole, the inhibitory index was increased in patients while the opposite was observed for controls, an effect thought to be driven by GABA in patients and by Glx in controls (Ajram et al. 2017), supporting altered E/I regulation even when basal levels are not different. Moreover, the relationship between medial prefrontal GABA+/tCr levels and behaviour appears to be reversed (as compared to controls) in neurofibromatosis type 1 (NF1). NF1 is a single-gene neurodevelopmental disorder with high prevalence of autism comorbidity and for which cortical GABA+ levels were found to be reduced (Violante et al. 2013, 2016). However, lower levels of GABA+/tCr in the medial prefrontal cortex were associated with a more cautious response style in patients while in controls the opposite was observed (Ribeiro et al. 2015). In summary, in the presence of a neurodevelopmental condition, the relationship between GABA and brain function may be different from what is observed in the neurotypical brain, which is relevant for interventional approaches. In our ASD group, both absolute GABA+ levels and ratios either to tNAA or tCr, while not differing between groups, were negatively correlated with ADI-R communication and developmental delay scores, with less GABA+ corresponding to worse behavioural scores. This is in line with the Brix et al. study where lower left anterior cingulate GABA+/tCr levels were associated with higher symptom severity as measured by the Autism Spectrum Screening Questionnaire (Brix et al. 2015), despite not being different from the control group. On the other hand, GABA+ measures, either absolute or relative, did not show correlations with intellectual level scores either across or within each group. Thus, our findings together with others suggest a relevant link of GABA and, specifically, important features of the clinical phenotype. Remarkably, GABA levels have been linked to behavioural traits in the neurotypical population, such as anxiety and impulsivity (Ende 2015), but also to adaptive behaviour (Scholl et al. 2017), which are typically altered in ASD (Brunsdon and Happé 2014; Lever and Geurts 2016), and it would be relevant to investigate the impact of GABA in these behavioral patterns in ASD. On the other hand, our results do not replicate the findings of reduced left frontal GABA+ or GABA+/tNAA in young children (2–11 years old) found in the study from Harada et al. (Harada et al. 2011) or the reduced GABA+/tCr in the ACC and left cerebellum of children (4–14 years old) with ASD and low IQ (Ito et al. 2017). This could be either due to the age range of the participants in those studies being different from our work (10–18 years old), or to the voxels being positioned in more posterior frontal region than ours, closer to the motor region were reduced GABA+/tCr levels have also been reported by others (Gaetz et al. 2014), suggesting that possible GABA reductions may be region specific. Also, all children were sedated during the MRS scan in those two studies (Harada et al. 2011; Ito et al. 2017), which is a possible confounding factor. Altogether, 1H-MRS GABA findings in typical and altered neurodevelopmental profiles appear to reflect the neurobiology underlying particular behaviours, warranting further investigation in ASD in order to characterize the role of GABA in particular clinical profiles which, in turn, could aid therapeutic interventions.
Concerning Glx, our results are consistent with unchanged Glx or Glu reported by others in the same region as ours (Cochran et al. 2015; Goji et al. 2017), while not replicating the increased absolute Glx levels reported in the frontal lobe in children and adolescents (Bejjani et al. 2012; Hassan et al. 2013; Joshi et al. 2013), or the decreased levels reported in one study (DeVito et al. 2007). The inconsistencies found may reflect the fact that Glx signal is a mixture of compounds (i.e. glutamate, glutamine, GABA and tNAA) depending on the type of sequence and field strength used, especially when assessed with conventional spectroscopy sequences (Cleve et al. 2015) and magnetic fields below 3T(Cecil 2013). Here we report Glx levels from the MEGA-PRESS difference spectrum were signals are better resolved, and could also account for the discrepant results. Also, not all studies position the voxel in the same frontal region and a previous multivoxel study suggested a right-lateralization of increased ACC Glx in children with ASD (Bejjani et al. 2012), which we could not address in our study. Importantly, studies quantifying only Glx or GABA+ give an incomplete view of the E/I imbalance as the question of whether GABA+ levels compensate the Glx decreases or increases cannot be investigated, and our results are in line with two recent studies reporting no changes either in Glx or Glu and GABA+ in a voxel which position overlapped with ours (Cochran et al. 2015; Goji et al. 2017). Interestingly, our data suggest that increased Glx/tNAA and trend for increased Glx/tCr were likely driven by the reduced absolute levels of tNAA and tCr. However, GABA+ relative measures to the same internal references yielded no significant results. This is an important aspect since it is common to use tNAA and tCr as internal references in spectroscopy studies and our work together with others (Goji et al. 2017; Horder et al. 2013) shows that in ASD, at least in the frontal lobe, these measures can be altered or may be highly variable (Ipser et al. 2012) which introduce biases in ratio analyses. Finally, given that NAA and glutamate are the precursors of NAAG, which in turn is a negative regulator of glutamatergic neurotransmission (Benarroch 2008), our results suggest that the glutamatergic (excitatory) metabolism or neurotransmission may be still be dysregulated in this region, or that neuronal energy metabolism may be impaired as suggested by the reduced tNAA levels (Moffett et al. 2013).
Limitations
Some methodological limitations of this study should be taken into account. GABA, glutamate and tNAA are involved both in neurotransmission and cellular metabolism where they are interchanged between neurons and glial cells (Moffett et al. 2013; Rae 2014), possibly representing different pathophysiological mechanisms and treatment targets (Varghese et al. 2017). While MRS is not able to differentiate between cellular pools, the notion that the neurochemistry of the medial prefrontal frontal region and diagnostic scores are related, even in the absence of group differences such as we report for GABA+ measures, suggests that MRS can be valuable tool not only for research on the underlying neurobiology of ASD, but also in the future from a clinical perspective. However, our correlation analyses between medial prefrontal cortex and behaviour and cognition were not corrected for multiple comparisons, and should thus, be interpreted with caution and replicated in larger cohorts. As an alternative to metabolite ratios, metabolite levels can be scaled to the water content of the spectroscopy voxel and corrected for T1 and/or T2 relaxation constants of the water and/or metabolites in the different tissue types (Gasparovic et al. 2006; Mlynrik et al. 2001). This was not the case in our study, which makes our metabolite absolute values less comparable with other studies. Nevertheless, there is no standard approach regarding different metabolite quantification correction and T1 and T2 relaxation constants are usually obtained from the literature were values vary, thus caution should be taken when comparing absolute metabolite values across studies (Harris et al. 2015).
Conclusion
We conclude that tNAA levels are reduced in the mPFC in children and adolescents with ASD and that GABA+ is associated with core symptoms of the clinical ASD phenotype, in particular, communication skills and developmental delay scores. Importantly, our results support the notion that, even when not different between groups, spectroscopy E/I measurements can show a link between the clinical profile and the neurobiology of ASD. Remarkably, not only the GABAergic system, but also the aspartate system may provide a substrate for future pharmacological interventions studies in ASD, as well as for stratifying the autism spectrum into more homogeneous neurophenotypes in terms of clinically interpretable behavioural phenotypes and associated neurochemistry prior to clinical trial development. Finally, while E/I-related neurotransmitter levels were not changed, it is not possible to exclude altered glutamatergic function in this region given that the ratio of Glx/tNAA was increased suggesting altered metabolism and/or overall neuronal function.
References
Ajram, L. A., Horder, J., Mendez, M. A., Galanopoulos, A., Brennan, L. P., Wichers, R. H., et al. (2017). Shifting brain inhibitory balance and connectivity of the prefrontal cortex of adults with autism spectrum disorder. Translational Psychiatry, 7(5), e1137. https://doi.org/10.1038/tp.2017.104.
Alger, J. R. (2011). Quantitative proton magnetic resonance spectroscopy and spectroscopic imaging of the brain a didactic review. Topics in Magnetic Resonance Imaging: TMRI, 21(2), 115–128.
American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th edn.). Arlington: American Psychiatric Publishing.
Arnold Anteraper, S., Triantafyllou, C., Sawyer, A. T., Hofmann, S. G., Gabrieli, J. D. & Whitfield-Gabrieli, S. (2014). Hyper-connectivity of subcortical resting-state networks in social anxiety disorder. Brain Connectivity, 4(2), 81–90. https://doi.org/10.1089/brain.2013.0180.
Aoki, Y., Abe, O., Yahata, N., Kuwabara, H., Natsubori, T., Iwashiro, N., et al. (2012). Absence of age-related prefrontal NAA change in adults with autism spectrum disorders. Translational Psychiatry, 2(10), e178. https://doi.org/10.1038/tp.2012.108.
Aoki, Y., Kasai, K., & Yamasue, H. (2012). Age-related change in brain metabolite abnormalities in autism: A meta-analysis of proton magnetic resonance spectroscopy studies. Translational Psychiatry, 2(1), e69. https://doi.org/10.1038/tp.2011.65.
Apps, M. A. J., Rushworth, M. F. S., & Chang, S. W. C. (2016). The anterior cingulate gyrus and social cognition: Tracking the motivation of others. Neuron, 90(4), 692–707. https://doi.org/10.1016/j.neuron.2016.04.018.
Badre, D., & D’Esposito, M. (2007). Functional magnetic resonance imaging evidence for a hierarchical organization of the prefrontal cortex. Journal of Cognitive Neuroscience, 19, 2082–2099. https://doi.org/10.1162/jocn.2007.19.12.2082.
Bartha, R. (2007). Effect of signal-to-noise ratio and spectral linewidth on metabolite quantification at 4 T. NMR in Biomedicine, 20(5), 512–521. https://doi.org/10.1002/nbm.1122.
Bejjani, A., O’Neill, J., Kim, J. A., Frew, A. J., Yee, V. W., Ly, R., et al. (2012). Elevated glutamatergic compounds in pregenual anterior cingulate in pediatric autism spectrum disorder demonstrated by1H MRS and 1H MRSI. PLoS ONE, 7(7), e38786. https://doi.org/10.1371/journal.pone.0038786.
Benarroch, E. E. (2008). N-Acetylaspartate and N-acetylaspartylglutamate: Neurobiology and clinical significance. Neurology, 70(16), 1353–1357. https://doi.org/10.1212/01.wnl.0000311267.63292.6c.
Benjamini, Y., & Hochberg, Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, 57(1), 289–300. https://doi.org/10.2307/2346101.
Bollmann, S., Ghisleni, C., Poil, S.-S., Martin, E., Ball, J., Eich-Höchli, D., et al. (2015). Developmental changes in gamma-aminobutyric acid levels in attention-deficit/hyperactivity disorder. Translational Psychiatry, 5, e589. https://doi.org/10.1038/tp.2015.79.
Bozzi, Y., Provenzano, G., & Casarosa, S. (2017). Neurobiological bases of autism-epilepsy comorbidity: A focus on excitation/inhibition imbalance. European Journal of Neuroscience. https://doi.org/10.1111/ejn.13595.
Brix, M. K., Ersland, L., Hugdahl, K., Grüner, R., Posserud, M.-B., Hammar, Å, et al. (2015). Brain MR spectroscopy in autism spectrum disorder—the GABA excitatory/inhibitory imbalance theory revisited. Frontiers in Human Neuroscience, 9(June), 1–12. https://doi.org/10.3389/fnhum.2015.00365.
Brunsdon, V. Ea, & Happé, F. (2014). Exploring the “fractionation” of autism at the cognitive level. Autism: The International Journal of Research and Practice, 18(1), 17–30. https://doi.org/10.1177/1362361313499456.
Cecil, K. M. (2013). Proton magnetic resonance spectroscopy: technique for the neuroradiologist. Neuroimaging Clinics of North America, 23(3), 381–392. https://doi.org/10.1016/j.nic.2012.10.003.
Chowdhury, F. A., O’Gorman, R. L., Nashef, L., Elwes, R. D., Edden, R. A., Murdoch, J. B., et al. (2015). Investigation of glutamine and GABA levels in patients with idiopathic generalized epilepsy using MEGAPRESS. Journal of Magnetic Resonance Imaging, 41(3), 694–699. https://doi.org/10.1002/jmri.24611.
Chugani, D. C. (2012). Neuroimaging and neurochemistry of autism. Pediatric Clinics of North America, 59(1), 63–73. https://doi.org/10.1016/j.pcl.2011.10.002.
Cleve, M., Gussew, A., & Reichenbach, J. R. (2015). In vivo detection of acute pain-induced changes of GABA + and Glx in the human brain by using functional 1H MEGA-PRESS MR spectroscopy. NeuroImage, 105, 67–75. https://doi.org/10.1016/j.neuroimage.2014.10.042.
Cochran, D. M., Sikoglu, E. M., Hodge, S. M., Edden, R. a. E., Foley, A., Kennedy, D. N., et al. (2015). Relationship among glutamine, γ-aminobutyric acid, and social cognition in autism spectrum disorders. Journal of Child and Adolescent Psychopharmacology, 25(4), 150428120459000. https://doi.org/10.1089/cap.2014.0112.
Constantino, J. N., & Gruber, C. P. (2005). Social responsiveness scale (SRS). Los Angeles, CA: Western Psychological Services.
Daly, E., Ecker, C., Hallahan, B., Deeley, Q., Craig, M., Murphy, C., et al. (2014). Response inhibition and serotonin in autism: A functional MRI study using acute tryptophan depletion. Brain, 137(9), 2600–2610. https://doi.org/10.1093/brain/awu178.
DeVito, T. J., Drost, D. J., Neufeld, R. W. J., Rajakumar, N., Pavlosky, W., Williamson, P., & Nicolson, R. (2007). Evidence for cortical dysfunction in autism: A proton magnetic resonance spectroscopic imaging study. Biological Psychiatry, 61(4), 465–473. https://doi.org/10.1016/j.biopsych.2006.07.022.
Drenthen, G. S., Barendse, E. M., Aldenkamp, A. P., van Veenendaal, T. M., Puts, N. A. J., Edden, R. A. E., et al. (2016). Altered neurotransmitter metabolism in adolescents with high-functioning autism. Psychiatry Research Neuroimaging, 256, 44–49. https://doi.org/10.1016/j.pscychresns.2016.09.007.
Edden, R. A. E., & Barker, P. B. (2007). Spatial effects in the detection of γ-aminobutyric acid: Improved sensitivity at high fields using inner volume saturation. Magnetic Resonance in Medicine, 58(6), 1276–1282. https://doi.org/10.1002/mrm.21383.
Edden, R. A. E., Pomper, M. G., & Barker, P. B. (2007). In vivo differentiation of N-acetyl aspartyl glutamate from N-acetyl aspartate at 3 T. Magnetic Resonance in Medicine, 57(6), 977–982. https://doi.org/10.1002/mrm.21234.
Ende, G. (2015). Proton magnetic resonance spectroscopy: Relevance of glutamate and GABA to neuropsychology. Neuropsychology Review, 25(3), 315–325. https://doi.org/10.1007/s11065-015-9295-8.
Fatemi, S. H., Halt, A. R., Stary, J. M., Kanodia, R., Schulz, S. C., & Realmuto, G. R. (2002). Glutamic acid decarboxylase 65 and 67 kDa proteins are reduced in autistic parietal and cerebellar cortices. Biological Psychiatry, 52(8), 805–810. https://doi.org/10.1016/S0006-3223(02)01430-0.
Fatemi, S. H., Reutiman, T. J., Folsom, T. D., Rooney, R. J., Patel, D. H., & Thuras, P. D. (2010). mRNA and protein levels for GABAAalpha4, alpha5, beta1 and GABABR1 receptors are altered in brains from subjects with autism. Journal of Autism and Developmental Disorders, 40(6), 743–750. https://doi.org/10.1007/s10803-009-0924-z.
Fatemi, S. H., Reutiman, T. J., Folsom, T. D., & Thuras, P. D. (2009). GABA(A) receptor downregulation in brains of subjects with autism. Journal of Autism and Developmental Disorders, 39(2), 223–230. https://doi.org/10.1007/s10803-008-0646-7.
Faul, F., Erdfelder, E., Buchner, A., & Lang, A.-G. (2009). Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behavior Research Methods, 41(4), 1149–1160. https://doi.org/10.3758/BRM.41.4.1149.
Faul, F., Erdfelder, E., Lang, A.-G., & Buchner, A. (2007). G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39(2), 175–191. https://doi.org/10.3758/BF03193146.
Fombonne, E. (2003). Epidemiological surveys of autism and other pervasive developmental disorders: an update. Journal of autism and developmental disorders, 33(4), 365–382. http://www.ncbi.nlm.nih.gov/pubmed/12959416.
Ford, T. C., & Crewther, D. P. (2016). A Comprehensive review of the 1H-MRS metabolite spectrum in autism spectrum disorder. Frontiers in Molecular Neuroscience. https://doi.org/10.3389/fnmol.2016.00014.
Fujii, E., Mori, K., Miyazaki, M., Hashimoto, T., Harada, M., & Kagami, S. (2010). Function of the frontal lobe in autistic individuals: a proton magnetic resonance spectroscopic study. The journal of medical investigation: JMI, 57(1–2), 35–44. http://www.ncbi.nlm.nih.gov/pubmed/20299741.
Gaetz, W., Bloy, L., Wang, D. J., Port, R. G., Blaskey, L., Levy, S. E., & Roberts, T. P. L. (2014). GABA estimation in the brains of children on the autism spectrum: Measurement precision and regional cortical variation. NeuroImage, 86, 1–9. https://doi.org/10.1016/j.neuroimage.2013.05.068.
Gasparovic, C., Song, T., Devier, D., Bockholt, H. J., Caprihan, A., Mullins, P. G., et al. (2006). Use of tissue water as a concentration reference for proton spectroscopic imaging. Magnetic Resonance in Medicine, 55(6), 1219–1226. https://doi.org/10.1002/mrm.20901.
Geschwind, D. H. (2009). Advances in autism. Annual Review of Medicine, 60, 367–380. https://doi.org/10.1146/annurev.med.60.053107.121225.
Goji, A., Ito, H., Mori, K., Harada, M., Hisaoka, S., Toda, Y., et al. (2017). Assessment of anterior cingulate cortex (ACC) and left cerebellar metabolism in asperger’s syndrome with proton magnetic resonance spectroscopy (MRS). Plos ONE, 12(1), e0169288. https://doi.org/10.1371/journal.pone.0169288.
Harada, M., Taki, M. M., Nose, A., Kubo, H., Mori, K., Nishitani, H., & Matsuda, T. (2011). Non-invasive evaluation of the GABAergic/glutamatergic system in autistic patients observed by MEGA-editing proton MR spectroscopy using a clinical 3 T instrument. Journal of Autism and Developmental Disorders, 41(4), 447–454. https://doi.org/10.1007/s10803-010-1065-0.
Harris, A. D., Puts, N. A. J., & Edden, R. A. E. (2015). Tissue correction for GABA-edited MRS: Considerations of voxel composition, tissue segmentation, and tissue relaxations. Journal of Magnetic Resonance Imaging, 42(5), 1431–1440. https://doi.org/10.1002/jmri.24903.
Hashimoto, T., Sasaki, M., Sugai, K., Hanaoka, S., Fukumizu, M., Kato, T., et al. (2001). Paroxysmal discharges on EEG in young autistic patients are frequent in frontal regions. Journal of Medical Investigation, 48(3–4), 175–180. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L33545846%5Cnhttp://sfx.library.uu.nl/utrecht?sid=EMBASE&issn=13431420&id=doi:&atitle=Paroxysmal+discharges+on+EEG+in+young+autistic+patients+are+frequent+in+frontal+regions.&stitle=.
Hassan, T. H., Abdelrahman, H. M., Abdel Fattah, N. R., El-Masry, N. M., Hashim, H. M., El-Gerby, K. M., & Abdel Fattah, N. R. (2013). Blood and brain glutamate levels in children with autistic disorder. Research in Autism Spectrum Disorders, 7(4), 541–548. https://doi.org/10.1016/j.rasd.2012.12.005.
Hodge, S. M., Makris, N., Kennedy, D. N., Caviness, V. S., Howard, J., McGrath, L., et al. (2010). Cerebellum, language, and cognition in autism and specific language impairment. Journal of Autism and Developmental Disorders, 40(3), 300–316. https://doi.org/10.1007/s10803-009-0872-7.
Horder, J., Lavender, T., Mendez, M., O’Gorman, R., Daly, E., Craig, M. C., et al. (2013). Reduced subcortical glutamate/glutamine in adults with autism spectrum disorders: a [1H]MRS study. Translational Psychiatry, 3(April), e279. https://doi.org/10.1038/tp.2013.53.
Ipser, J. C., Syal, S., Bentley, J., Adnams, C. M., Steyn, B., & Stein, D. J. (2012). 1H-MRS in autism spectrum disorders: A systematic meta-analysis. Metabolic Brain Disease, 27(3), 275–287. https://doi.org/10.1007/s11011-012-9293-y.
Ito, H., Mori, K., Harada, M., Hisaoka, S., Toda, Y., Mori, T., et al. (2017). A proton magnetic resonance spectroscopic study in autism spectrum disorder using a 3-tesla clinical magnetic resonance imaging (MRI) System: The anterior cingulate cortex and the left cerebellum. Journal of Child Neurology, 32(8), 88307381770298. https://doi.org/10.1177/0883073817702981.
Joshi, G., Biederman, J., Wozniak, J., Goldin, R. L., Crowley, D., Furtak, S., et al. (2013). Magnetic resonance spectroscopy study of the glutamatergic system in adolescent males with high-functioning autistic disorder: A pilot study at 4T. European Archives of Psychiatry and Clinical Neuroscience, 263(5), 379–384. https://doi.org/10.1007/s00406-012-0369-9.
Kubas, B., Kułak, W., Sobaniec, W., Tarasow, E., Lebkowska, U., & Walecki, J. (2012). Metabolite alterations in autistic children: a 1H MR spectroscopy study. Advances in Medical Sciences, 57(1), 152–156. https://doi.org/10.2478/v10039-012-0014-x.
Lai, M.-C., Lombardo, M. V., & Baron-Cohen, S. (2014). Autism. The Lancet, 383(9920), 896–910. https://doi.org/10.1016/S0140-6736(13)61539-1.
Landim, R. C. G., Edden, R. A. E., Foerster, B., Li, L. M., Covolan, R. J. M., & Castellano, G. (2015). Investigation of NAA and NAAG dynamics underlying visual stimulation using MEGA-PRESS in a functional MRS experiment. Magnetic Resonance Imaging, 34(3), 239–245. https://doi.org/10.1016/j.mri.2015.10.038.
Letzkus, J. J., Wolff, S. B. E., & Luthi, A. (2015). Disinhibition, a circuit mechanism for associative learning and memory. Neuron, 88(2), 264–276. https://doi.org/10.1016/j.neuron.2015.09.024.
Lever, A. G., & Geurts, H. M. (2016). Psychiatric Co-occurring symptoms and disorders in young, middle-aged, and older adults with autism spectrum disorder. Journal of Autism and Developmental Disorders. https://doi.org/10.1007/s10803-016-2722-8.
Li, C. T., Lu, C. F., Lin, H. C., Huang, Y. Z., Juan, C. H., Su, T. P., et al. (2017). Cortical inhibitory and excitatory function in drug-naive generalized anxiety disorder. Brain Stimulation, 10(3), 604–608. https://doi.org/10.1016/j.brs.2016.12.007.
Long, Z., Dyke, J. P., Ma, R., Huang, C. C., Louis, E. D., & Dydak, U. (2015). Reproducibility and effect of tissue composition on cerebellar γ-aminobutyric acid (GABA) MRS in an elderly population. NMR in Biomedicine, 28(10), 1315–1323. https://doi.org/10.1002/nbm.3381.
Lord, C., Rutter, M., DiLavore, P., & Risis, S. (1999). Autism diagnostic observation schedule (ADOS). Los Angeles: Western Psychological Services.
Maddock, R. J., Casazza, G. A., Fernandez, D. H., & Maddock, M. I. (2016). Acute modulation of cortical glutamate and GABA content by physical activity. Journal of Neuroscience, 36(8), 2449–2457. https://doi.org/10.1523/JNEUROSCI.3455-15.2016.
Maltezos, S., Horder, J., Coghlan, S., Skirrow, C., O’Gorman, R., Lavender, T. J., et al. (2014). Glutamate/glutamine and neuronal integrity in adults with ADHD: a proton MRS study. Translational Psychiatry, 4(3), e373. https://doi.org/10.1038/tp.2014.11.
Marín, O. (2012). Interneuron dysfunction in psychiatric disorders. Nature Reviews Neuroscience, 13(2), 107–120. https://doi.org/10.1038/nrn3155.
Markram, H., Rinaldi, T., & Markram, K. (2007). The intense world syndrome–an alternative hypothesis for autism. Frontiers in Neuroscience, 1(1), 77–96. https://doi.org/10.3389/neuro.01.1.1.006.2007.
Mendez, M. A., Horder, J., Myers, J., Coghlan, S., Stokes, P., Erritzoe, D., et al. (2013). The brain GABA-benzodiazepine receptor alpha-5 subtype in autism spectrum disorder: A pilot [11C]Ro15–4513 positron emission tomography study. Neuropharmacology, 68, 195–201. https://doi.org/10.1016/j.neuropharm.2012.04.008.
Mescher, M., Merkle, H., Kirsch, J., Garwood, M., & Gruetter, R. (1998). Simultaneous in vivo spectral editing and water suppression. NMR in Biomedicine, 11(6), 266–272. https://doi.org/10.1002/(SICI)1099-1492(199810)11:6<266::AID-NBM530>3.0.CO;2-J
Mlynrik, V., Gruber, S., & Moser, E. (2001). Proton T1 and T2 relaxation times of human brain metabolites at 3 T. NMR in Biomedicine, 14(5), 325–331. https://doi.org/10.1002/nbm.713.
Moffett, J. R., Arun, P., Ariyannur, P. S., & Namboodiri, A. M. A. (2013). N-Acetylaspartate reductions in brain injury: Impact on post-injury neuroenergetics, lipid synthesis, and protein acetylation. Frontiers in Neuroenergetics, 5(DEC), 1–19. https://doi.org/10.3389/fnene.2013.00011.
Mori, K., Toda, Y., Ito, H., Mori, T., Goji, A., Fujii, E., et al. (2013). A proton magnetic resonance spectroscopic study in autism spectrum disorders: Amygdala and orbito-frontal cortex. Brain and Development, 35(2), 139–145. https://doi.org/10.1016/j.braindev.2012.09.016.
Mori, T., Mori, K., Fujii, E., Toda, Y., Miyazaki, M., Harada, M., et al. (2012). Evaluation of the GABAergic nervous system in autistic brain: 123I-iomazenil SPECT study. Brain and Development, 34(8), 648–654. https://doi.org/10.1016/j.braindev.2011.10.007.
Morris, L. S., Baek, K., & Voon, V. (2016). Distinct cortico-striatal connections with subthalamic nucleus underlie facets of compulsivity. Cortex, 88, 143–150. https://doi.org/10.1016/j.cortex.2016.12.018.
Mouga, S., Café, C., Almeida, J., Marques, C., Duque, F., & Oliveira, G. (2016). Intellectual profiles in the autism spectrum and other neurodevelopmental disorders. Journal of Autism and Developmental Disorders, 46(9), 2940–2955. https://doi.org/10.1007/s10803-016-2838-x.
Murphy, D. (2002). Asperger syndrome: a proton magnetic resonance spectroscopy study of brain. Archives of General Psychiatry, 59(NOVEMBER), 885–891. http://archpsyc.jamanetwork.com/article.aspx?articleid=206824.
Naaijen, J., Lythgoe, D. J., Amiri, H., Buitelaar, J. K., & Glennon, J. C. (2015). Fronto-striatal glutamatergic compounds in compulsive and impulsive syndromes: A review of magnetic resonance spectroscopy studies. Neuroscience and Biobehavioral Reviews, 52, 74–88. https://doi.org/10.1016/j.neubiorev.2015.02.009.
Neale, J. H., Bzdega, T., & Wroblewska, B. (2000). N-acetylaspartylglutamate: The most abundant peptide neurotransmitter in the mammalian central nervous system. Journal of Neurochemistry, 75(2), 443–452. https://doi.org/10.1046/j.1471-4159.2000.0750443.x.
O’Gorman, R. L., Michels, L., Edden, R. A., Murdoch, J. B., & Martin, E. (2011). In vivo detection of GABA and glutamate with MEGA-PRESS: Reproducibility and gender effects. Journal of Magnetic Resonance Imaging, 33(5), 1262–1267. https://doi.org/10.1002/jmri.22520.
Oblak, a, Gibbs, T. T., & Blatt, G. J. (2009). Decreased GABAA receptors and benzodiazepine binding sites in the anterior cingulate cortex in autism. Autism Research : Official Journal of the International Society for Autism Research, 2(4), 205–219. https://doi.org/10.1002/aur.88.
Oblak, A. L., Gibbs, T. T., & Blatt, G. J. (2010). Decreased GABAB receptors in the cingulate cortex and fusiform gyrus in Autism. Journal of Neurochemistry, 114(5), 1414–1423. https://doi.org/10.1111/j.1471-4159.2010.06858.x.
Oliveira, G., Ataide, A., Marques, C., Miguel, T. S., Coutinho, A. M., Mota-Vieira, L., et al. (2007). Epidemiology of autism spectrum disorder in Portugal: prevalence, clinical characterization, and medical conditions. Developmental Medicine & Child Neurology, 49(10), 726–733. https://doi.org/10.1111/j.1469-8749.2007.00726.x.
Patel, T., Blyth, J. C., Griffiths, G., Kelly, D., & Talcott, J. B. (2014). Moderate relationships between NAA and cognitive ability in healthy adults: implications for cognitive spectroscopy. Frontiers in Human Neuroscience, 8(February), 39. https://doi.org/10.3389/fnhum.2014.00039.
Port, R. G., Gaetz, W., Bloy, L., Wang, D. J., Blaskey, L., Kuschner, E. S., et al. (2016). Exploring the relationship between cortical GABA concentrations, auditory gamma-band responses and development in ASD: Evidence for an altered maturational trajectory in ASD. Autism Research. https://doi.org/10.1002/aur.1686.
Pouwels, P. J. W., & Frahm, J. (1997). Differential distribution of NAA and NAAG in human brain as determined by quantitative localized proton MRS. NMR in Biomedicine, 10(2), 73–78. https://doi.org/10.1002/(SICI)1099-1492(199704)10:2<73::AID-NBM448>3.0.CO;2-4
Provencher, S. (1993). Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magnetic Resonance in Medicine, 30(6), 672–679
Provencher, S. W. (2001). Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR in Biomedicine, 14(4), 260–264. https://doi.org/10.1002/nbm.698.
Puts, N. A. J., Wodka, E. L., Harris, A. D., Crocetti, D., Tommerdahl, M., Mostofsky, S. H., & Edden, R. A. E. (2016). Reduced GABA and altered somatosensory function in children with autism spectrum disorder. Autism Research, 10, 608–619. https://doi.org/10.1002/aur.1691.
Rae, C. D. (2014). A guide to the metabolic pathways and function of metabolites observed in human brain 1H magnetic resonance spectra. Neurochemical Research, 39(1), 1–36. https://doi.org/10.1007/s11064-013-1199-5.
Ribeiro, M. J., Violante, I. R., Bernardino, I., Edden, R. A. E., & Castelo-Branco, M. (2015). Abnormal relationship between GABA, neurophysiology and impulsive behavior in neurofibromatosis type 1. Cortex, 64, 194–208. https://doi.org/10.1016/j.cortex.2014.10.019.
Robertson, C. E., Ratai, E.-M., & Kanwisher, N. (2015). Reduced GABAergic action in the autistic brain. Current Biology, 26(1), 80–85. https://doi.org/10.1016/j.cub.2015.11.019.
Rojas, D. C., Becker, K. M., & Wilson, L. B. (2014a). Magnetic resonance spectroscopy studies of glutamate and GABA in autism: Implications for excitation-inhibition imbalance theory. Current Developmental Disorders Reports, 2, 46–57. https://doi.org/10.1007/s40474-014-0032-4.
Rojas, D. C., Singel, D., Steinmetz, S., Hepburn, S., & Brown, M. S. (2014b). Decreased left perisylvian GABA concentration in children with autism and unaffected siblings. NeuroImage, 86, 28–34. https://doi.org/10.1016/j.neuroimage.2013.01.045.
Rowland, L. M., Krause, B. W., Wijtenburg, S., McMahon, R. P., Chiappelli, J., Nugent, K. L., et al. (2015). Medial frontal GABA is lower in older schizophrenia: a MEGA-PRESS with macromolecule suppression study. Molecular Psychiatry. https://doi.org/10.1038/mp.2015.34.
Rubenstein, J. L. R., & Merzenich, M. M. (2003). Model of autism : increased ratio of excitation / inhibition in key neural systems. Genes, Brain and Behavior, 2(5), 255–267. https://doi.org/10.1046/j.1601-183X.2003.00037.x.
Rutter, M., Bailey, A., & Lord, C. (2003). Social communication questionnaire. Los Angeles: Western Psychological Services.
Rutter, M., Le Couteur, A., & Lord, C. (2003). The autism diagnostic interview-revised (ADI-R). Los Angeles, CA: Western Psychological Services.
Scholl, J., Kolling, N., Nelissen, N., Stagg, C. J., Harmer, C. J., & Rushworth, M. F. (2017). Excitation and inhibition in anterior cingulate predict use of past experiences. eLife, 6, 1–15. https://doi.org/10.7554/eLife.20365.
Schür, R. R., Draisma, L. W. R., Wijnen, J. P., Boks, M. P., Koevoets, M. G. J. C., Joëls, M., et al. (2016). Brain GABA levels across psychiatric disorders: A systematic literature review and meta-analysis of 1 H-MRS studies. Human Brain Mapping, 37(April), 3337–3352. https://doi.org/10.1002/hbm.23244.
Shungu, D. C., Mao, X., Gonzales, R., Soones, T. N., Dyke, J. P., van der Veen, J. W., & Kegeles, L. S. (2016). Brain gamma-aminobutyric acid (GABA) detection in vivo with the J-editing 1H MRS technique: a comprehensive methodological evaluation of sensitivity enhancement, macromolecule contamination and test-retest reliability. NMR in Biomedicine, 29(7), 932–942. https://doi.org/10.1002/nbm.3539.
Solleveld, M., Schrantee, A., Puts, N., Reneman, L., & Lucassen, P. (2017). Age-dependent, lasting effects of methylphenidate on the GABAergic system of ADHD patients. NeuroImage: Clinical. https://doi.org/10.1016/j.nicl.2017.06.003.
Spence, S. J., & Schneider, M. T. (2009). The role of epilepsy and epileptiform EEGs in autism spectrum disorders. Pediatric Research, 65(5), 5A–5A. https://doi.org/10.1203/01.pdr.0000352115.41382.65.
Takahashi, H., Katayama, K., Sohya, K., Miyamoto, H., Prasad, T., Matsumoto, Y., et al. (2012). Selective control of inhibitory synapse development by Slitrk3-PTPδ trans-synaptic interaction. Nature Neuroscience, 15(3), 389–398. https://doi.org/10.1038/nn.3040.
Tebartz Van Elst, L., Maier, S., Fangmeier, T., Endres, D., Mueller, G., Nickel, K., et al. (2014). Disturbed cingulate glutamate metabolism in adults with high-functioning autism spectrum disorder: evidence in support of the excitatory/inhibitory imbalance hypothesis. Molecular Psychiatry, 19(12), 1314–132562. https://doi.org/10.1038/mp.2014.62.
Van Overwalle, F. (2009). Social cognition and the brain: A meta-analysis. Human Brain Mapping, 30(3), 829–858. https://doi.org/10.1002/hbm.20547.
Varghese, M., Keshav, N., Jacot-Descombes, S., Warda, T., Wicinski, B., Dickstein, D. L., et al. (2017). Autism spectrum disorder: neuropathology and animal models. Acta Neuropathologica, 134(4), 537–566. https://doi.org/10.1007/s00401-017-1736-4.
Violante, I. R., Patricio, M., Bernardino, I., Rebola, J., Abrunhosa, A. J., Ferreira, N., & Castelo-Branco, M. (2016). GABA deficiency in NF1. Neurology, 87(9), 897–904. https://doi.org/10.1212/WNL.0000000000003044.
Violante, I. R., Ribeiro, M. J., Edden, R. A. E., Guimares, P., Bernardino, I., Rebola, J., et al. (2013). GABA deficit in the visual cortex of patients with neurofibromatosis type 1: Genotype-phenotype correlations and functional impact. Brain, 136(3), 918–925. https://doi.org/10.1093/brain/aws368.
Wechsler, D. (2008). Manual for intelligence scale for adults. Portuguese version (M.R. Simões, A. M. Rocha, and C. Ferreira. Lisboa: Cegoc-Tea.
Wechsler, D., Simoes, M. R., Rocha, A. M., & Ferreira, C. (2003). Wechsler Intelligence Scale for Children—Portuguese version. Lisboa: Cegoc-Tea.
World Health Organization. (1992). ICD-10 classifications of mental and behavioural disorder clinical descriptions and diagnostic guidelines. Geneva: World Health Organization.
Yasuhara, A. (2010). Correlation between EEG abnormalities and symptoms of autism spectrum disorder (ASD). Brain and Development, 32(10), 791–798. https://doi.org/10.1016/j.braindev.2010.08.010.
Zaroff, C. M., & Uhm, S. Y. (2012). Prevalence of autism spectrum disorders and influence of country of measurement and ethnicity. Social Psychiatry and Psychiatric Epidemiology, 47(3), 395–398. https://doi.org/10.1007/s00127-011-0350-3.
Zikopoulos, B., & Barbas, H. (2013). Altered neural connectivity in excitatory and inhibitory cortical circuits in autism. Frontiers in Human Neuroscience, 7(September), 609. https://doi.org/10.3389/fnhum.2013.00609.
Acknowledgments
The authors thank all participants and their families for their willingness to participate in this study. The authors thank Dr. Inês Bernardino for support in the neuropsychological evaluation of control participants and Dr. João Castelhano for creating Matlab scripts for MEGA-PRESS combined spectra visualization. The authors are also grateful to the staff of the Institute of Nuclear Sciences Applied to Health (ICNAS, http://www.uc.pt/en/icnas) for their technical assistance with the magnetic resonance scanning, particularly to Mr. Carlos Ferreira, Mr. João Marques and Ms. Sónia Afonso. This research was supported by the Portuguese Foundation for Science and Technology grants: BIGDATIMAGE, CENTRO-01-0145-FEDER-000016 financed by Centro 2020 FEDER, COMPETE, FLAD Life Sciences Ed 2 2016, COMPETE, POCI-01-0145-FEDER-007440, FCT. UID/NEU/04539/2013–2020, PAC – MEDPERSYST POCI-01-0145-FEDER-016428; IRV is funded by is funded by the Wellcome Trust (103045/Z/13/Z), SM is funded by Portuguese Foundation for Science and Technology (individual scholarship: SFRH/BD/102779/2014). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Conceived and designed the study: ACP IRV MC-B and GO. Acquired data: ACP and SM. Analysed the data: ACP and IRV. Interpreted the data ACP and MC-B. Wrote original draft: ACP and MC-B. All authors reviewed and edited the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no competing interests exist.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10803_2017_3406_MOESM1_ESM.docx
Figure 1S. MEGA-PRESS mean fit (red line) and standard deviation (shaded red) of the difference spectra for each group. Abbreviations: Glx, glutamate+glutamine; GABA+, gamma-aminobutyric acid + lipids and macromolecules; tNAA, N-acetylaspartate + N-acetylaspartylglutamate; MM. macromolecules; ppm, parts per million. (DOCX 184 KB)
Rights and permissions
About this article
Cite this article
Carvalho Pereira, A., Violante, I.R., Mouga, S. et al. Medial Frontal Lobe Neurochemistry in Autism Spectrum Disorder is Marked by Reduced N-Acetylaspartate and Unchanged Gamma-Aminobutyric Acid and Glutamate + Glutamine Levels. J Autism Dev Disord 48, 1467–1482 (2018). https://doi.org/10.1007/s10803-017-3406-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10803-017-3406-8