Abstract
The high prevalence of age-related diseases among the population explosion of senior citizens has gained significance in the pursuit of therapeutic agents capable of slowing the progression of degenerative disorders and preventing premature aging. Since our societies’ rising longevity is correlated with an increase in morbidity, preserving health in old age has become the main objective for biomedicine. Natural antioxidants are increasingly used as healthy ingredients in the nutrition, cosmetics, and pharmaceutical industries. Some synthetic drugs used as health supplements are enzyme inhibitors mediating several disease processes. However, due to concerns regarding their toxicity and adverse effects, several newly discovered heterogeneous phenolic and biphenolic structures have sparked a search for novel, safe, and effective agents, especially from natural sources. Natural antioxidants are one of the most well-studied natural product groups due to the wide range of biological effects they have. This review summarizes current knowledge on the various antioxidants and their action against age-related diseases like rheumatoid arthritis, skin aging, eye degeneration, metabolic syndrome, and neuroinflammation. According to our study, a wide variety of antioxidants have been examined, and although some potential therapeutic molecules have been identified based on their antioxidant action, further in vivo studies and evaluation are needed before a possible therapeutic implementation as drug candidates.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Humanity has wanted to attain immortality since the dawn of civilization, and the modern human need for medicines is so extensive that it is thought to be a deep evolutionary behavior (Hardy 2021). Satisfactory living conditions and the increase in medical technology have prolonged human life significantly in the last hundred years. Aging is a process that affects all parts of the body, such as cardiovascular diseases, neurological conditions, and cancer, resulting in irreversible physiological deterioration. Patients, their families, and society as a whole bear a heavy economic and psychological burden as a result of these age-related diseases (Hano and Tungmunnithum 2020). The average life expectancy has risen steadily in recent decades, reaching around 71.4 years in 2015. Based on global population demographics between 2000 and 2050, the population is projected to rise from 605 million to 2 billion people during the next 60 years. While increased life expectancy is an example of increased human progress, it also presents a new obstacle. In reality, neurological and biological degenerations, such as physical frailty, psychological deficiency, and cognitive loss, are all linked to being older (Elfawy and Das 2019). In the twenty-first century, age-related illnesses have emerged as the largest health concern. Aging is an innate, systemic, multifactorial, and progressive phenomenon marked by progressive loss of function and eventually increased mortality rate (Bosch-Morell et al. 2020).
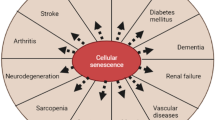
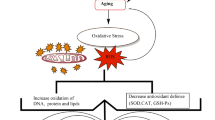
Age-related diseases are linked to structural changes in mitochondria, as well as differences in physicochemical characteristics of the membranes, such as changes in electron transport chain complex functions and reduced fluidity, which lead to energy imbalance and mitochondrial loss. These changes disrupt cellular homeostasis and mitochondrial activity, increasing oxidative stress vulnerability (Elfawy and Das 2019). Owing to a decrease in the efficacy of their endogenous antioxidant processes, aged people are more vulnerable to oxidative stress (Fig. 1). Organs like the brain and heart, which have large oxygen intake rates but low respiration speeds, are especially susceptible to this effect, which helps understand why cardiovascular diseases (CVDs) and neurological problems are so common in older people (Bosch-Morell et al. 2020).
Natural compounds may be able to alleviate oxidative stress and increase immune function, according to emerging research evidence (Liguori et al. 2018). Indeed, oxidative stress is highly dependent on hereditary or acquired defects in redox-mediated signaling enzymes. Thus, the function of molecules with antioxidant activity in promoting healthy aging and mitigating oxidative stress warrants further discussion (Elfawy and Das 2019). Polyphenols and flavonoids (active redox agents) are widely spread in nature and fully recognized as antioxidants or scavengers. The pharmacological potential of medicinal plant material is probably due to the capability of polyphenols and flavonoids to interact with important cellular processes in which key enzymes such as cyclooxygenase, lipoxygenase, phospholipase A2, NADH oxidase, or glutathione reductase are involved. Catechols in flavonoids and polyphenols are also able to form chelates with metals and are reactive in their oxidized form (like quinones) with nucleophiles present in lateral protein chains such as cysteine and lysine. To illustrate the potential effects of aging, we highlighted the list of antioxidants and their mechanism of action in the prevention of age-related diseases in this review.
Search Strategy
Search terms related to studies of most potent natural antioxidants for new age-related disorders were compiled in the following manner: oxidative stress/mitochondrial dysfunction/age-related disorders, polyphenols/EGCG/epigallocatechin gallate/withaferin A/salvianolic acid (A, B, C, D, E, F, and G)/extra virgin olive oil/olive oil derivatives/curcumin/metabolites of curcumin and phenol/phytochemicals, as well as health benefits/human diseases/oxidative stress/oxidative damage/neurodegenerative diseases/cardiovascular diseases/cancer/rheumatoid arthritis/eye diseases/skin ailments, and also baicalein/plumbagin/sulforaphane/vitamins/resveratrol/quercetin/omega-3 fatty acids/olive oil and phytochemicals/dietary supplements/bioavailability/mechanism of action/nano-formulations/in vitro/in vivo animal studies. The following electronic scientific databases were searched for data collection: Google Scholar, Science Direct, Springer, Elsevier, PubMed, Wiley Online Library and Taylor, and Francis Library. PubMed Advanced Search Builder was used to search research papers between 2012 and 2021.
Discussion
Aging Diseases
Oxidative stress plays a significant role in the disease process and its development in model animals, humans, and microorganisms. The oxidative stress theory of aging asserts that a progressive and incremental increase in oxidative exposure caused by reactive oxygen species (ROS) has a direct effect on core aspects of aging biology, leading to impaired physiological mechanisms, increased disease prevalence, and a reduced life span. Oxidative stress is defined as an interruption and deregulation of the signaling and redox system that can be caused by an imbalance in the production of oxidant and antioxidant species. To counterbalance the oxidant species, there is an antioxidant system formed by enzymes and nonenzymatic molecules. Though, during pathological events, there may be an increase in the production of oxidant species not neutralized by the antioxidant system, resulting in oxidative stress that promotes cellular damage through protein denaturation, changes in the functions of nucleic acids, lipid peroxidation, and cell death.
It is a well-known fact that mitochondria are the energy house of the cell and help in the production of energy through oxidative phosphorylation and are also involved in the metabolism of lipids, fats, proteins, and carbohydrates. But during all these processes, it releases free radicals. These free radicals are implicated in various processes such as aging, mutagenesis, and pathological events. Free radical production and their targeted action on biomolecules can also increase oxidative stress in the brain, ultimately leading to neurodegenerative disorders. Oxidative damage includes mitochondrial dysfunction, α-synuclein aggregation, glial cell activation, dopamine auto-oxidation, alterations in calcium signaling, and excess free iron. Moreover, neurons encounter more oxidative stress as a counteracting mechanism with advancing age that does not function properly (Elfawy and Das 2019). Age-related eye disorder is the progressive loss of vision or other vision-related problems owing to increased production and imbalance of free radical scavenging (Bosch-Morell et al. 2020).
A multifaceted condition including insulin dysfunction which leads to abnormal homeostasis of glucose as well as the metabolism of lipids is called diabetes (Adeshara et al. 2016). During the pathology, macrophages and endothelial cells are supposed to release reactive oxygen species and cytotoxic nitric oxide. Specifically, in type 2 diabetes, vascular complications increased due to the release of free radicals (Son 2012). Free radical increase in diabetes may be contributed to a decrease in the natural antioxidant system present in the body (Korac et al. 2021). Moreover, vascular diseases caused by free radicals are the main culprit of death in diabetes instead of hyperglycemia (Korac et al. 2021). Free radicals affect several biochemical and molecular processes inside the cell, resulting in DNA damage that can cause cancer in the pancreas, mouth, stomach, and esophagus, inter alia. When tissue undergoes damage, either mechanical, chemical, or physical, it is trailed by an inflammatory response. Reactive oxygen species and reactive nitrogen species work to convey the message in inflammatory response by increasing free radical activity at the site of damage, which activates neutrophils to increase inflammation at the injury site (Srinivas et al. 2019) (Fig. 1).
Antioxidants are battalions against detrimental free radicals, which can securely react with free radicals and can dismiss the chain reaction before biomolecules are decayed. The harmful action of free radicals can be thwarted, to some extent, by the natural antioxidant enzymes (superoxide dismutase, catalases, glutathione peroxidase, glutathione S-transferase, glutathione reductase) of the body. These are involved in the prevention of cellular damage, by scavenging free radicals which cause premature aging and age-related disorders. A wide range of plant-based antioxidants are employed in the treatment of diseases caused by oxidative stress (Zhang et al. 2015). Plant-derived antioxidants contain various constituents such as organo-sulfuric compounds, phenolics, and flavonoids. The antioxidant properties of these phytochemicals are well established (Zhang et al. 2015). Natural antioxidants are well known to exhibit a wide range of biological effects including anti-inflammatory, antithrombotic, anticancer, antidiabetic, and vasodilatory activities. Antioxidant supply is not limited to the biological systems because one antioxidant molecule can react only with a single free radical (Zhang et al. 2015). Thus, there is a continuous need to replenish antioxidant resources, whether endogenously or through supplementation. When free radicals are produced in living organisms, many antioxidants come into action like catalase, glutathione peroxidase, superoxide dismutase, tocopherols, ascorbic acid, and flavonoids (Singh et al. 2019). The significance and effectiveness of these antioxidants depend on which reactive oxygen species is involved, and where it is generated. Thus, an antioxidant may be active in one system yet may fail in another. Sometimes a protective antioxidant may become aggressive. Also, the antioxidants of food articles may not necessarily be protective in the body. Antioxidants are powerful electron donors. In this process, the antioxidant is oxidized and must be regenerated or replaced. Thus, a produced antioxidant radical is relatively unreactive and unlikely to attack further molecules (Zhang et al. 2015).
Several chemicals of plant origin have been studied to benefit age-related pathology. Early and regular consumption of major phytochemicals can delay the onset of age-related diseases like cancer, arthritis, and psoriasis, as well as neurodegenerative and cardiovascular disorders. The role of the most potent and important chemicals of plant origin will be discussed in detail in the present review.
Neurodegenerative Diseases
Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, and amyotrophic lateral sclerosis are neurodegenerative diseases in which brain and spinal cord nerve cells degenerate, which can lead to sensory as well as functional loss. This can also lead to abnormalities in mitochondria and finally become the reason for aging and neurodegenerative diseases. Several factors, such as environmental factors, can be the cause of neurodegenerative diseases like heredity, but the excessive formation of free radicals due to faulty redox metal system in the body can be the leading cause of nerve cell death (Elfawy and Das 2019). Antioxidative enzymes present in our body help in regulating the free radical formation in the body, and also if there is no balance between free radical formation and its scavenging, then it can lead to neurodegeneration (Singh et al. 2019). Reactive oxygen species (nitric oxide, hydrogen peroxide, monoxide radicals, and superoxide anions) are considered as the real problem of neuronal degeneration (Elfawy and Das 2019). They are the cause of mitochondrial degeneration, and degenerated mitochondria act as the storehouse of the reactive oxygen species (Elfawy and Das 2019). Originally, it was assumed that reactive oxygen species were generated due to the imbalance between its production and elimination. But now, it is a well-known fact that controlled generation of reactive oxygen species can regulate biological processes at the cellular level (Gupta et al. 2020). However, free radicals have been identified as the primary cause of neuronal death, which leads to neurodegenerative diseases (Singh et al. 2019). The composition of the human brain is more prone to oxidative damage. The brain contains a high concentration of unsaturated lipids, which are highly labile to lipid peroxidation by free radicals, resulting in mitochondrial dysfunction and apoptotic cell death, which cause neurodegeneration (Singh et al. 2019).
Therapy for Neurodenegeration
Current medicines available in the market to cure neuroinflammation approved by USFDA are rivastigmine, galantamine, donepezil, and the N-methyl-d-aspartate receptor antagonist memantine. These drugs support cholinergic neurotransmission or block excitotoxic neuronal injury and death. However, these drugs provide only modest, temporary, and palliative benefits (Tan et al. 2014). The natural defense (antioxidant) system present in the body can help scavenge the free radicals, but when an imbalance occurs between the production of ROS and the natural defense (antioxidant) system in our body, consequently, cell death occurs. Excessive formation of free radicals causes mitochondrial dysfunction which is mainly due to an imbalance between the formation of free radicals and the antioxidant system as we age. This imbalance in oxidative stress leads to defective proteasome which is responsible for the degradation of abnormal protein aggregates like beta-amyloid, tau protein, and alpha-synuclein. Therefore, aggregation leads to neurodegeneration. The onset of the pathology can be delayed by antioxidant therapy which enhances natural antioxidant enzymes, scavenges free radicals, and establishes a healthy state with the normal functioning of the proteasome system for the degradation of aberrant proteins and the balance between the antioxidant enzymes superoxide dismutase (SOD), catalase (CAT), glutathione S-transferase (GSH), and free radical production. By supplementing our body with exogenous antioxidant phytochemicals, free radicals–mediated cell death can be managed to some extent as shown in Fig. 2. Numerous natural compounds are commercially viable, and studies are ongoing on herbal preparations that can effectively boost the endogenous antioxidant system’s ability to neutralize free radicals and reduce inflammation in the brain such as EGCG (1), the most abundant catechin in tea (Singh et al. 2016); withaferin A (2, Withania somnifera (L.) Dunal, Solanaceae) (Zahiruddin et al. 2020); polyunsaturated fatty acids (α-linolenic acid (3) (Eriksdotter et al. 2015, Madore et al. 2020); resveratrol (4, grapes and berries); sulforaphane (5, cruciferous vegetables such as broccoli, Brussels sprouts, and cabbages); salvianolic acid A (6; Salvia miltiorrhiza Bunge, Lamiaceae); curcumin (7; Curcuma longa L., Zingiberaceae); and blueberry polyphenols. Caloric restriction, as well as physical exercise, can help slow down neurodegeneration (Poulose et al. 2017).
Proposed mechanism of antioxidant therapy for neurodegenerative diseases. Excessive formation of free radicals causes mitochondrial dysfunction which is mainly due to an imbalance between the formation of free radicals and the antioxidant system as we age. This imbalance in oxidative stress leads to defective proteasome which is responsible for the degradation of abnormal protein aggregates like beta-amyloid, tau protein, and alpha-synuclein. Therefore, aggregation leads to neurodegeneration. The onset of the pathology can be delayed by antioxidant therapy which enhances natural antioxidant enzymes, scavenges free radicals, and establishes a healthy state with the normal functioning of the proteasome system for the degradation of aberrant proteins and the balance between the antioxidant enzymes superoxide dismutase (SOD), catalase (CAT), glutathione S-transferase (GSH), and free radical production

Antioxidants found in fruits and vegetables help prevent the formation of free radicals and cytokines in activated neuronal cells (Elfawy and Das 2019). Serotonin and melatonin are two of these natural antioxidant molecules from plants capable to attenuate, or even prevent, stress oxidative-related disorders, which are widely found in Mediterranean foodstuffs, fruits, vegetables, and medicinal herb (Gonçalves et al. 2021). Additionally, polyphenols derived from plants have been shown to protect neuronal cells from inflammation by downregulating NF-κB and inhibiting transcription factors involved in pro-inflammatory cell signaling pathways. Chemicals present in olive oil (Olea europaea L., Oleaceae) like tyrosol (8) and 3-hydroxytyrosol (9), as well as the bitter glycosylated secoiridoids, oleuropein (10), and ligstroside (11) and their aglycon, and the phenylethanoid oleocanthal (12), are found to show anti-inflammatory activities (Rodríguez-Morató et al. 2015, Brunetti et al. 2020).



Resveratrol (4) is currently being studied to target SIRT1 because it helps activate secretase, which helps cleave amyloid precursor protein, protecting the brain from unwanted accumulation of the fibrillar protein amyloids, which are the main cause of neuroinflammation-mediated neurodegeneration (Sun et al. 2021), and several preclinical shreds of evidence show resveratrol (4) help treat hepatitis C virus (HCV). Another chemical that helps slow down neurodegeneration is curcumin (7) as it helps neutralize reactive oxygen species directly by enhancing the function of superoxide dismutase (Samarghandian et al. 2017). Curcumin (7) helps modulate different biochemical pathways, e.g., phosphatidylinositol 3-kinase/protein kinase B pathway, AMP-activated protein kinase pathway, and mitogen-activated protein kinase, and modulation of these pathways ultimately helps prevent oxidative burden (Thota et al. 2020). Other curcumin (7) derivatives, such as tetrahydrocurcumin (13), were able to increase dopamine levels in mouse models and inhibit the activity of monoamine oxidase, which degrades neurotransmitters (Chainoglou and Hadjipavlou-Litina 2020). Demethoxycurcumin (14) protects neurons by increasing glutathione activity (Chainoglou and Hadjipavlou-Litina 2020), and octahydrocurcumin (15) helps activate Nrf2 (nuclear erythroid-2-p45–related factor 2) pathway (Chainoglou and Hadjipavlou-Litina 2020). Curcumin (7) derivatives with phenyl hydroxy and phenyl methoxy groups have been shown to prevent the formation of amyloid plaques in a recent study. The keto-enol tautomerism of curcumin derivatives seems to be a novel modification for the design of amyloid-binding agents (Chainoglou and Hadjipavlou-Litina 2020).

In a recent report, several chemicals have been isolated from marine algae, like polyunsaturated fatty acids, polysaccharides, sterols, carotenoids, tocopherols, terpenes, phycobilins, phycocolloids, and phycocyanin. These bioactive compounds have been reported to show neuroprotective effect by neutralizing free radicals and slowing down neuroinflammation (Barbalace et al. 2019). The latest research on green, red, and brown algae showed that they can help alleviate the symptoms of Alzheimer’s, Parkinson’s, multiple sclerosis, and other chronic diseases (Pereira and Valado 2021). Astaxanthin (16), a blood red pigment produced by the freshwater microalga Haematococcus pluvialis and the yeast fungus Xanthophyllomyces dendrorhous, has been shown to relieve oxidative stress (Wu et al. 2020).

Ginseng and its main active triterpene saponins, ginsenosides (17), have also been reported to prevent the onset of neurodegenerative diseases (Huang et al. 2019). Coffee, tea, and dark chocolate (cacao) can promote brain health and may reduce the risk of age-related neurodegenerative disorders when consumed regularly (Camandola et al. 2019). In a recent study, the function of tannins in the treatment of neurodegenerative diseases was investigated (Camandola et al. 2019).

The combined treatment of epigallocatechin gallate (1), sulforaphane (5), and plumbagin (18), from species of the plant genus Plumbago, was found to be more effective than single treatment in relieving oxidative burden because it can alleviate endogenous antioxidant system, suppress nicotinamide adenine dinucleotide phosphate oxidases 1 and 2, and increase cell viability (Marrazzo et al. 2019). Monoterpenes, sterols, polyphenols, polyunsaturated fatty acids, polysaccharides, and carotenoids help stabilize the homeostasis of calcium in the brain to relieve inflammation (Welcome 2020). Studies conducted in vitro and in vivo proved that bioactive compounds like resveratrol (4) (Sun et al. 2021), curcumin (7) (Thota et al. 2020), and quercetin (19) have the potential to slow down brain damage by disassembly of abnormally folded proteins and abnormal mitochondria (Ay et al. 2017). The onset of neurodegenerative diseases can be delayed by continuous consumption of some vital nutritional ingredients like polyphenols and endogenous substances like coenzyme Q10 (20), acetyl-l-carnitine (21), and polyunsaturated fatty acids (Elfawy and Das 2019).

Nanoformulations of natural antioxidants like biopolymeric nanoparticles can be used to deliver bioactive compounds and enhance the effectiveness at the specific targeted site in the treatment of several diseases. Poly(lactic-co-glycolic acid) (PLGA) is one of the most successfully developed biodegradable polymers that has attracted considerable attention due to its desirable properties like high bioavailability (Liu et al. 2019). Rhinacanthus nasutus (L.) Kurz., Acanthaceae, is a herbal plant, native of Thailand, that has been reported to help with neurodegenerative diseases, but no clinical data is available (Brimson et al. 2020). Bioactive flavonoids from the dried fruits of Cullen corylifolium (L.) Medik. (syn. Psoralea corylifolia L.), Fabaceae, namely bavachin (22), bavachinin (7-O-methylbavachin) (23), bavachalcone (24), and isobavachalcone (25), have been established to relieve Alzheimer’s symptoms in vitro as well as in vivo by relieving oxidative stress, inhibiting neuroinflammation, and regulating acetylcholinesterase and glycogen synthase kinase (Zhou et al. 2020). Dried roots of Glycyrrhiza uralensis Fish. consist of chalcones, flavanones, coumarins, and triterpenoid saponins. Out of these phytochemicals, the most potent phytochemicals used to delay the progression of neurodegenerative disease were found to be the flavanone liquiritigenin (26) and its precursor chalcone isoliquiritigenin (27) (Ramalingam et al. 2018). Soya flavonoids found in soybean have been proven to show neuroprotective effects (Welcome 2020).

Cardiovascular Diseases
CVD, including stroke, heart failure, heart attack, arrhythmia, and other inherited heart ailments, remains the principal cause of disease and death globally. In cardiovascular disease, patients’ formation of plaque from lipid peroxidation has become the main cause of death because of the rupturing of atherosclerotic plaque in arteries which is known as myocardial infarction (Chen et al. 2021). The hardening of arteries due to lipid peroxidation is known as atherosclerosis, and it is caused by oxidative changes in low-density lipoproteins (Chen et al. 2021). The antioxidant system in the heart aids in the prevention of low-density lipoprotein oxidation during blood vessel formation, although there are a variety of other mechanisms at work in the development of disease in which low-density lipoproteins become oxidized and cause disease (Chen et al. 2021). Oxysterols are oxidized derivatives of cholesterol or its precursors, and they are implicated as mediators of the metastatic effects of a high-fat diet and the etiology of neurodegeneration (Griffiths and Wang 2019). Arachidonic, docosahexaenoic, and eicosatetraenoic acids oxidize to form isoprostanes. These prostaglandin F2–like compounds are formed in vivo by nonenzymatic free radical–catalyzed peroxidation of arachidonic acid. Because these compounds are a series of isomers that contain the prostane ring of prostaglandins, they were termed F2-isoprostanes (Montuschi et al. 2004). These end products of peroxidation are used as biomarkers to determine the risk of cardiovascular disease (Chen et al. 2021). Free radicals are produced by a variety of factors, including genetic risk factors, aging, lifestyle choices, and environmental factors (Ghezzi 2020). Oxidative stress, caused by the presence of nitrogen oxides, causes blood vessels to be damaged more severely. Excessive formation of free radicals can damage blood vessel endothelial cells, resulting in blood vessel hardening (Chen et al. 2021). Supplementing with plant-based chemicals or antioxidants can aid in the scavenging of free radicals, thereby reducing cardiovascular damage.
Cardiovascular Therapy
CVDs are complex diseases with diverse pathophysiologic mechanisms, and increased oxidative stress has been viewed as one of the potential common etiologies (Chen et al. 2021). The oxidative burden due to excessive formation of free radicals causes damage to endothelial cells which leads to the expression of vascular adhesion molecules (VCAMs) that ultimately recruit monocytes into a sub-endothelial space, and hampers the protective role of nitric oxide (NO), and peroxidation of low-density lipoproteins (LDLs) cause inflammation altogether leading to accumulation of lipids in the arteries and ultimately to the development of cardiovascular disease. A variety of plant chemicals are available or are undergoing clinical trials to determine their effectiveness against this disease. A common mechanism of action of dietary phytochemicals is to relieve the oxidative burden, avoid oxidation of low-density lipoproteins, and enhance the protective effect of nitric oxide as shown in Fig. 3. Resveratrol (4) is a naturally isolated stilbene with diverse health applications. Among phenolic compounds, resveratrol is credited with playing a key role in red wine’s health-promoting effects. In vitro and in vivo studies have shown the promising performance of resveratrol on cardiac problems as shown in the 3xTg mouse model. Resveratrol has been shown to improve cardiac function in conjugation with exercise (Esfandiarei et al. 2019) as mentioned in Table S1, but the beneficial effects of resveratrol have not been deep rooted and are unsatisfactory (Chudzińska et al. 2020). Curcumin (7), another essential chemical present in turmeric, is a potent diarylheptanoid. Administration of curcumin to healthy subjects has been shown to decrease homocysteine level and increase high-density lipoproteins, but no significant change was observed in central blood pressure, endothelial function, and oxidative stress biomarkers (Campbell et al. 2019).
Proposed antioxidant therapy mechanism for protection on endothelial cells, low-density lipoprotein, inflammation, and thrombosis (top panel). The oxidative burden due to excessive formation of free radicals causes damage to endothelial cells and leads to the expression of vascular adhesion molecule (VCAM) which ultimately recruits monocytes and hinders the protective role of nitric oxide (NO) and the peroxidation of low-density lipoproteins (LDLs), causing inflammation and altogether leading to accumulation of lipids in the arteries and, ultimately, to the development of cardiovascular disease (bottom panel)
Nevertheless, curcumin has recently been classified as both a pan-assay interference compound (PAIN) and an invalid metabolic panacea (IMP) candidate. The likely false activity of curcumin in vitro and in vivo has resulted in > 120 clinical trials of curcuminoids against several diseases. No double-blinded, placebo-controlled clinical trial of curcumin has been successful. Curcumin (7) is an unstable, reactive, nonbioavailable compound and, therefore, a highly improbable lead (Nelson et al. 2017). Derivatives such as tetrahydrocurcumin (13) and bisdemethoxycurcumin (28) have been shown to protect cardiomyocytes and help reduce oxidative stress (Sunagawa et al. 2018). Sulforaphane (5) is an isothiocyanate, commonly found in broccoli and was found to prevent age-associated mitochondrial dysfunction and oxidative damage in cardiac and muscular disorders through Nrf2 signaling in C57BL/6 mice (Bose et al. 2020). A central regulator of cellular responses to electrophilic stress is the transcription factor Nrf2 shown to be essential for detoxification gene activity, including in mammalian cardiac cells and other components of the cardiovascular system (Bose et al. 2020).

Salvianolic acids, the most effective and abundant polyphenols extracted from Salvia miltiorrhiza Bunge, are well known for their good antioxidative activity. Danshen (S. miltiorrhiza) has been extensively used as a traditional medicine to treat cardiovascular-related diseases in China and other Asian countries for hundreds of years. In vivo and in vitro experiments have demonstrated that salvianolic acids can modulate signal transduction within fibroblasts and cancer cells. Salvianolic acids promote the apoptosis of cancer cells, due to the inhibition of cancer-associated epithelial-mesenchymal transition processes (Ma et al. 2019; Du et al. 2020). Salvianolic acid A (6) has been shown to suppress the NF-κB pathway, hence helps protect the cardiac tissue in cardiac fibroblasts (Oh et al. 2011). Salvianolic acid B (29) has also been shown to exert beneficial effects on the cardiovascular system by restoring endothelial function in angiotensin II–induced mice. Salvianolic acids C (30) and D (31) have been proven beneficial to relieve inflammation by inhibiting NF-κB activation. Salvianolic acid D (31) has also been proven beneficial clinically for an acute myocardial infarction (Du et al. 2020). Salvianolic acids E (32), F (33), and G (34) have also been isolated, characterized, and found to scavenge oxygen free radicals and thus can provide a way to prevent and treat free radical–mediated damage to vital organs (Du et al. 2020).

Omega-3 fatty acid (3) carried on male Wistar rats has been shown to increase the viability of cardiomyocytes, improve membrane potential, and reduce the release of lactate dehydrogenase (Varghese et al. 2017). Ferulic acid (35), a polyphenol found in vegetables and cereals, has been shown to improve heart health by lowering oxidative stress, improving lipid profiles, and decreasing inflammation in hyperlipidemic patients (Bumrungpert et al. 2018). 3-Hydroxytyrosol (9), another polyphenolic drug, has been shown to inhibit inflammatory pathways and reduce oxidative stress (Rodríguez-López et al. 2020). Oleuropein aglycone (36) restored autophagic defects in neonatal rat ventricular myocytes (Rodríguez-López et al. 2020).


Oleocanthal (12) has been shown in human studies to reduce inflammatory markers of CVD (Rodríguez-López et al. 2020). Plumbagin (18), an active constituent derived from the roots of the traditional medicinal plant Plumbago zeylanica L., has been shown to reduce ischemia–reperfusion (IR) injury in IR rats (Zaki et al. 2018). Quercetin (19), a flavonoid found in grapes, has been clinically studied. It has been shown in clinical trials to reduce plasma lipid profile and help reduce CVD pathology in human subjects (Muñoz-Bernal et al. 2021). Coenzyme Q10 (20) has also been studied clinically in human subjects, and it was discovered that when treated with coenzyme Q10 (20), the lipid content in blood plasma decreased (Jorat et al. 2018). Another highly effective treatment for cardiovascular disease that has been tested on rats is l-carnitine (37), a quaternary ammonium compound involved in the metabolism of fatty acids in most mammals and derived from the methylation of the amino acid lysine, which was found to improve caspase-3 expression in albino rats by its combining capacity with vitamin E (38) (Aboubakr et al. 2020). Postmenopausal women have an increased risk of CVD due to estrogen deficiency, which can be corrected by supplementing with isoflavones (39) or other phytoestrogens (Stojanov and Kreft 2020), according to a recent report on postmenopausal women (Stojanov and Kreft 2020). Similarly, a clinical trial on vitamin C (40) co-supplementation with other vitamins (A (41), B (42), D (43), and E (38)) and minerals was not consistent in patients with CVD (Ingles et al. 2020). Baicalin (44), a plant flavonoid found in several species in the genus Scutellaria (Lamiaceae), has recently been shown to facilitate the release of nitric oxide in CMEC cells (Bai et al. 2019).

Cancer
Cancer is a multistage process involving transformation and uncontrolled cell proliferation. Cancer is also one of a variety of serious chronic diseases caused by free radicals and eventually causes fatal illnesses, including cancer by unbalanced free radicals. The cause of these free radicals may be attributed to an unhealthy lifestyle, eating too much-processed food, environmental conditions, and heredity factors (Klaunig 2018). Free radicals operate in dual directions: on the one hand, causing the death of healthy cells within an organism, and on the other hand, acting as pro-oxidants for cancer cells or causing apoptosis in cancer cells. High-level generation of free radicals can lead to an oxidative burden on cells, and these free radicals can cause alterations at the cellular and DNA level which can cause mutations (Ligouri et al. 2018). Free radicals also cause damage to macromolecules like lipids, proteins, carbohydrates, and other important molecules of life (Ligouri et al. 2018). The relation between free radicals and abnormal cell proliferation has been well documented (Klaunig 2018). A balanced diet and nutrients can help prevent the onset of these diseases. There is a long list of antioxidants that can help ameliorate the consequences of free radicals, and evidence of the same is found in the literature. Several studies have demonstrated that some herbal nutrients help cope with adverse reactions inside living organisms (Hano and Tungmunnithum 2020).
Cancer Therapy
Under oxidative stress conditions, Keap1 (Kelch-like ECH–associated protein) releases Nrf2 from the complex and ultimately Nrf2 (nuclear factor-erythroid 2–related factor 2) is translocated into the cell for transcription; elevated Nrf2 causes abnormal cell division. But when the cell is supplemented with antioxidants, this action helps alleviate the expression of Keap1 and inhibit the expression of Nrf2, thus normalizing the function of Keap1 and inhibiting further transcription of Nrf2. Keap1 tightly regulates the expression of Nrf2 protein levels. Dimers of Keap1, E3 ubiquitin ligase, cullin 3 (Cul3), and ring box protein 1 (RBX1) make a complex through the N-terminal BTB domain to promote Nrf2 ubiquitination and degradation. β-TrCP-SKP1 (β-transducin repeat–containing protein)-cullin 1 (Cul1)-RBX1 and HRD1 (3-hydroxy-3-methylglutaryl reductase degradation) and E3 ubiquitin ligase complex is also elevated by antioxidants to regulate Nrf2 as shown in Fig. 4 (Rada et al. 2012).
Proposed mechanism of antioxidant role in cancer. Under oxidative stress conditions, Kelch-like ECH–associated protein 1 (Keap1) release nuclear factor-erythroid 2–related factor 2 (Nrf2) from complex and, ultimately, Nrf2 translocated into the cell for transcription; elevated Nrf2 causes abnormal cell division. The cell supplemented with antioxidants helps alleviate the expression of Keap1 and inhibit Nrf2 expression, thus normalizing the function of Keap1 and inhibiting further transcription of Nrf2. Keap1 tightly regulates the expression of Nrf2 protein level. Dimers of Keap1, E3 ubiquitin ligase, cullin3 (Cul3), and ring box protein 1 (RBX1) make a complex through the N-terminal BTB domain to promote Nrf2 ubiquitination and degradation. β-Transducin repeat-containing protein (β-TrCP-SKP1)-cullin1 (Cul1)-RBX1 and HRD1 and E3 ubiquitin ligase complex is also elevated by antioxidants to regulate Nrf2
Evidence has been found for several plant chemicals: resveratrol (4) helps induce apoptosis and arrest the cell cycle in prostate cancer cell lines (Ammar et al. 2020). Sulforaphane (5) controls the malignant proliferation of CSCs in TNBC via a Cripto-mediated pathway in BALB/c nude mice (Castro et al. 2019). Curcumin (7) also helps induce apoptosis in breast cancer cell lines like T47D, MCF7, and MDA-MB-231 (Tomeh et al. 2019). Salvianolic acid B (29) helps reduce oxidative stress and inflammation and shows a cytotoxic effect on human breast cancer adenocarcinoma (MCF-7) cells (Du et al. 2020). By lowering cancer inflammatory markers in female cancer patients (mean age: 58 years), omega-3 fatty acid (3) significantly reduces breast cancer (Dydjow-Bendek and Zagoźdźon 2020). Ferulic acid (35), in combination with trans-resveratrol (4) nanoparticles, helps increase cytotoxicity in HT-29 cell lines (Kumar et al. 2020). Hydroxytyrosol (9) helps reduce pain and inflammation when given in combination with curcumin (7) and omega-3 fatty acids (3) in early-stage breast cancer patients (Martínez et al. 2019). Oleuropein aglycon (36) shows antiproliferative activities in MDA-MB-231 and MCF-7 (MCF-7/TR) cell lines (Musial et al. 2021) Novel oleocanthal (12) formulation with xylitol solid dispersion shows cytotoxic effect in breast cancer cells (Qusa et al. 2019). Tetrahydrocurcumin (13) shows an antitumoral effect on the tumor-bearing mouse model (Tomeh et al. 2019). Demethoxycurcumin (14) can induce a caspase-dependent pathway to stop the growth of cancer cells in the human skin squamous cell carcinoma cell line A431 and the human keratinocyte cell line HaCaT (Tomeh et al. 2019). Another derivative of curcumin (bisdemethoxycurcumin 28) has also shown the cytotoxic effect on cancer cells (Tomeh et al. 2019). Bisdemethoxycurcumin (28) helps sensitize NSCLC cells to icotinib via apoptosis (Tomeh et al. 2019). Epigallocatechin gallate (1) helps regulate TLR4 signaling in male BALB/c mice (Musial et al. 2021). Plumbagin (18) helps block the STAT3 in cancer cells and suppress jaw fibrosarcoma in breast and gastric cancer cells, large cell lung cancer cells, and hepatic cancer cells (Yu et al. 2018). Quercetin (19) assists to initiate apoptosis in cancer cells in MCF-7 breast cancer cells and CT-26, LNCaP, MOLT-4, and Raji cell lines and has shown antitumoral effect on mice bearing tumors (Niazvand et al. 2019). Coenzyme Q10 (20) helps decrease the viability and migration of myeloma cells and, when supplemented with l-carnitine (18), improves the quality of life by reducing pain in the tumor-bearing mouse model (Awa et al. 2017). Isoflavones (39) show cytotoxic effect against breast cancer cell lines (Hatono et al. 2021). Vitamin C (40) was also found to show cytotoxic effect on KRAS-mutated cancer cells (Di Tano et al. 2020). In a human trial, vitamin E (38) has failed to show any consistent effect on cancer patients (Ammar et al. 2020). Attenuation of inflammatory markers was found when myeloma cancer cell lines were treated with withaferin A (2). The growth of HCT116 colon cancer cells was suppressed when treated with the cytotoxic baicalin (44) (Ammar et al. 2020).
Rheumatoid Arthritis
Rheumatoid arthritis is a chronic inflammatory bone joint disease that is manifested by joint pain, inflammation, slow joint movement, redness, and swelling (Ferreira et al. 2021). In the development of inflammatory arthritis, reactive oxygen species and reactive nitrogen species play a significant role as the damaging essential components of the bone structure (Ferreira et al. 2021). An imbalance between the antioxidant and oxidant systems plays a crucial role in pathogenesis (Ferreira et al. 2021). Hydrogen peroxide interferes with ATP synthesis and inhibits the enzyme G-3-P dehydrogenase in bone cells which is important for bone health, ultimately leading to free radical–mediated cartilage degradation. This will activate the phagocytes, leading to an inflammatory response by activating different inflammatory cytokines. Activated phagocytes produce reactive oxygen species and reactive oxygen species which further change the immunoglobulin G behavior and lead to the development of the disease (Ferreira et al. 2021).
Arthritis Therapy
As it is well known that free radicals play a critical role in rheumatoid arthritis pathogenesis, the phytochemicals which can scavenge the free radicals can delay the progression of the disease (Kour et al. 2021). Xanthine oxidase helps in purine metabolism as it converts purines to uric acid. Uric acid is required at the optimum level in the body for proper functioning. Elevated expression of enzyme xanthine oxidase in old age is the main reason for rheumatoid arthritis pathology as it hampers immunity (Kour et al. 2021). Adenosine and guanosine nucleosides convert into xanthine following the above-mentioned pathway, which is depicted in Fig. 5, where the enzyme xanthine oxidase converts xanthine to uric acid. Supplementing cells with antioxidants can block the active site of the enzyme by competing with hypoxanthine and xanthine for the active site (Fig. 5). Resveratrol (4) was found to inhibit specifically TNF-alpha and IL-1beta–induced NF-kappa B activation to stop the progression of rheumatoid arthritis in the rabbit model (Kour et al. 2021). In a recent study, resveratrol (4) has been shown to suppress p62 expression and reduce the serum levels of C-reactive protein and inflammatory cytokines in antigen-induced rheumatoid arthritis animals (Kour et al. 2021). Inhibition of the NF-κB signaling pathway and the promotion of macrophage apoptosis were found in collagen-induced arthritis (CIA) rats when treated with curcumin (7) (Makuch et al. 2021). Curcumin (7) directly controls microRNA which promotes cancer in T24 and SV-HUC-1 cell lines (Makuch et al. 2021). A randomized, double-blind, clinical study was directed to analyze the proportional effectiveness of two different dosages of curcumin (250 mg or 500 mg) twice daily for 90 days. Curcumin (7) has been found to significantly change the C-reactive protein level, erythrocyte sedimentation rate, disease activity score, and rheumatoid factor value (Jacob et al. 2018). Sulforaphane (5) was found to inhibit the interleukin-1β (IL-1β)–induced rheumatoid arthritis and activate Nrf2 (Moon et al. 2021). Similarly, omega-3 fatty acids (3) help reduce the pain of inflammation in rheumatoid arthritis (da Fonseca et al. 2019). Decreased TNF-α, decreased JAK2 levels, reduced inflammation, and inhibition of JAK/STAT pathway were found when complete Freund’s adjuvant (CFA) rats were treated with ferulic acid (35) (Zhu et al. 2020). Hydroxytyrosol (9) was found to suppress oxidative stress and reduce inflammation in chondrocytes from female Sprague Dawley rats (Castejón et al. 2020). Oleuropein aglycon (36) is also a chemical that helps reduce pain in CIA rats (Castejón et al. 2020). Oleocanthal (12) was found to inhibit IL-1β, TNF-α, and GM-CSF protein synthesis from LPS-stimulated macrophages in ATDC5 murine chondrogenic cells (Castejón et al. 2020). Tetrahydrocurcumin (13) was found to improve pain and reduce inflammation in human patients (Jacob et al. 2018). Demethoxycurcumin (14) helps attenuate all the inflammatory markers of RA in CIA rats (Jacob et al. 2018). Quercetin (19) was found to attenuate inflammatory markers of rheumatoid arthritis in fibroblast-like synoviocytes (da Fonseca et al. 2019). Coenzyme Q10 (20) in combination with probiotics and zinc helps reduce the level of inflammatory cytokines in CIA rats (da Fonseca et al. 2019). Supplementation of vitamin C (40) can improve the symptoms of rheumatoid patients (da Fonseca et al. 2019). Combination therapy of vitamin E (38) with vitamins A (41) and C (40) and methotrexate can attenuate the inflammatory markers in the blood in a clinical trial (da Fonseca et al. 2019). Baicalin (44) attenuates all the inflammatory cytokines in the rheumatoid mouse model (da Fonseca et al. 2019).
Proposed mechanism of antioxidants in the prevention of rheumatoid arthritis. Elevated expression of the enzyme xanthine oxidase in old age is the main culprit of rheumatoid arthritis pathology. Adenosine and guanosine nucleosides converted into xanthine by enzymes adenosine deaminase and guanosine deaminase, respectively, and the enzyme xanthine oxidase converts xanthine to uric acid. Supplementing cells with antioxidants can block the active site of the enzyme by competing with hypoxanthine and xanthine for the active site
Skin Aging
Oxidative stress in the skin plays a major role in the aging process. This is true for intrinsic or chronological aging and even more for extrinsic aging or photoaging. Intrinsic and extrinsic aging are terms used to describe cutaneous aging of the skin and other parts of the integumentary system, primarily involving the dermis. Intrinsic aging is influenced by internal physiological factors alone and extrinsic aging by many external factors, which include ultraviolet radiation, cigarette smoking, and air pollution, among others. Of all extrinsic causes, radiation from sunlight has the most widespread documentation of its negative effects on the skin. Although the results are quite different in the dermis and epidermis, extrinsic aging is driven, to a large extent, by oxidative stress caused by ultraviolet (UV) irradiation. They include a disruption of the epidermal calcium gradient in old skin with an accompanying change in the composition of the cornified envelope. This modified cornified envelope also leads to an altered antioxidative capacity and a reduced barrier function of the epidermis (Rinnerthaler et al. 2015). Oxidative stress induced by the accumulation of ROS can lead to lipid, protein, nucleic acid, and organelle damage, thus leading to the occurrence of cellular senescence, which is one of the core mechanisms mediating skin aging (Gu et al. 2020). As we age, there is an imbalance in the formation of free radicals and natural antioxidant systems, which eventually activates mitogen-activated protein kinase (MAPK), which induces transcription factors such as activator protein 1 (AP-1) and nuclear factor-B (NF-B), which leads to the activation of metalloproteinases such as collagenase, elastase, and hyaluronidase, which degrade collagen and elastin. This also inhibits the transforming growth factor-β which helps produce collagen. Several antiaging strategies can help scavenge free radicals, including topicals, energy-based procedures, and dermal fillers, and restore the molecular features of dermal aging with clinical efficacy (Gu et al. 2020).
Skin Anti-aging Therapy
As aging progresses, skin becomes hypersensitive to external stimuli like UV rays of the sun, pollution, and environmental factors, leading to the generation of excessive free radicals causing mitochondrial dysfunction. Further free radicals damage DNA and protein and increase the production of collagenase, elastase, and hyaluronidase which degrade collagen in the skin and damage it. But when the cell is under antioxidant protection, the free radical formation can be managed due to the scavenging potential of antioxidants. Furthermore, antioxidants block the DNA damage pathway; inhibit protein oxidation; inhibit the action of collagenase, elastase, and hyaluronidase; and protect premature aging as shown in Fig. 6. Resveratrol (4) was used to enhance the antioxidant system, ultimately helping to reduce skin aging as the formation of free radicals slows down and the activation of inflammatory markers is also suppressed (Michalak et al. 2021). Sulforaphane (5) has also been shown to increase the production of collagen in the keratinocyte/fibroblast co-culture system (Ko et al. 2020). Another chemical that is very potent to slow down skin aging by inhibiting metalloproteases is curcumin (7), which was also proven in a clinical trial on patients with severe photoaging (Michalak et al. 2021). Demethoxycurcumin (14) has been shown to protect human keratinocyte cell lines from free radical–induced death (Liu et al. 2016). Tetrahydrocurcumin was reported to improve skin aging when used in combination with extract of Centella asiatica (L.) Urb., Apiaceae (Astuti et al. 2021). l-Carnitine (18) helps attenuate UV-induced DNA damage markers in the skin in a rat model (Salama et al. 2018). Quercetin (19) also suppressed PKCδ and JAK2 in the skin JB6 P + and JB6 P + PKCδ DN cells (Michalak et al. 2021). Isoflavones (39) are also one of the natural chemicals that have been reported to reduce the signs of skin aging when administrated on hairless mice (Michalak et al. 2021). Similarly, baicalin (44) helps reduce skin inflammation (Kostyuk et al. 2018). Salvianolic acid B (29) has also been shown to reduce inflammatory cytokines in skin fibroblast proliferation by TGF-β/SMAD and MAPK/ERK pathway suppression in the bleomycin-induced SSc mouse model (Du et al. 2020). In a double-blind clinical trial, healthy young females were selected to see the effect of omega-3 fatty acids (3) and Aloe vera in combination. At the end of the study, improved skin hardness and skin elasticity were found in females treated with a combination of omega-3 fatty acids (3) and Aloe vera (L.) Burm.f., Xanthorrhoeaceae (Michalak et al. 2021). In another study, omega-3 fatty acids (3) have been shown to protect the skin from ultraviolet rays and free radicals and reduce skin inflammation (Michalak et al. 2021). Treatment of JB6 epidermal skin cells with omega-3 fatty acids (3) has been shown to stabilize the Nrf2 protein as it is the master regulator of antioxidant and anti-inflammatory gene expression (Michalak et al. 2021). Ferulic acid (35) in combination with vitamins C (40) and E (38) helped reduce signs of aging in human subjects (Kim et al. 2020). Hydroxytyrosol (9) has been shown to attenuate inflammatory markers of photoaging in keratinocytes and blastocytes (Castejón et al. 2020). Oleocanthal (12) has been reported to decrease the photoaging-induced inflammation in human skin fibroblasts (Castejón et al. 2020). Nanoparticles loaded with coenzyme Q10 (20) microemulsion regenerated skin in keratinocytes and fibroblasts (Michalak et al. 2021). In a clinical trial, vitamin C (40) has been shown to delay skin aging in human subjects (Michalak et al. 2021). A clinical trial on 45-year-old men and women found that foods high in antioxidants, such as vitamin E (38), can delay skin aging (Michalak et al. 2021).
Proposed mechanism of antioxidants in the prevention of age-related skin disease. Aging makes the skin extra-sensitive to external stimuli like UV rays of the sun, pollution, and environmental factors that lead to the generation of excessive free radicals causing mitochondrial dysfunction. Further free radicals damage DNA and protein and increase the production of collagenase, elastase, and hyaluronidase which degrade collagen in the skin and damage it. But when the cell is under antioxidant protection, the free radical formation can be managed due to the scavenging potential of antioxidants. Further antioxidants block the DNA damage pathway; inhibit protein oxidation; inhibit the action of collagenase, elastase, and hyaluronidase; and protect premature aging
Eye Diseases
Oxidative stress–induced free radicals affect the cellularity of the human trabecular meshwork (HTM) which increases the pressure inside the ocular region which leads to eye damage and abnormality that is glaucoma. Therefore, glaucoma is the result of the excessive generation of free radicals (Nita and Grzybowski 2016). Most of the anterior segment of the eye, i.e., cornea and lens, are exposed to UV radiation, leading to histopathological changes, which is the main cause of cornea damage also known as photokeratitis (Nita and Grzybowski 2016). According to the World Health Organization, corneal blindness is the fourth largest cause of blindness in the world. Excessive exposure to UV radiation can cause acute to severe damage to the anterior portion of the eye. But, normal cornea contains nonenzymatic antioxidants like vitamins C (40) and E (38), glutathione, and enzymatic antioxidant systems like catalase, superoxide dismutase, and others. But as we age and have age-related disorders, all these enzymatic as well as nonenzymatic systems fail, which produce free radicals and lead to corneal damage (Nita and Grzybowski 2016). Reactive oxygen species leads to activation of the NF-κB pathway which ultimately leads to the inflammation of the trabecular meshwork in the eye. Inflammation of the trabecular meshwork resists the flow of aqueous humor and increases the pressure inside the eye, which is the leading cause of glaucoma (Nita and Grzybowski 2016). Dry eye, an age-related condition, is a multifactorial disease of the tears and ocular surface that results in symptoms of discomfort, visual disturbance, and tear film instability. Environmental factors are also often implicated in dry eye, including exposure to pollutants, UV radiation, and ozone as well as the chronic use of preserved eye drops such as in the treatment of glaucoma. These factors increase oxidative stress and ocular surface inflammation. A small number of interventional studies suggest that oxidative stress may be directly targeted in topical therapy of dry eye treatment. For example, in vitro studies suggest that l-carnitine (18) and pterostilbene (trans-3,5-dimethoxy-4-hydroxystilbene), a blueberry component chemically related to resveratrol (4), 3,5,4′-trihydroxytrans-stilbene (3), may reduce oxidative stress (Seen and Tong 2018), and in in vivo studies, selenoprotein P and alpha-lipoic acid have been proven to relieve the symptoms of dry eyes (Seen and Tong 2018).
Eye Therapy
Increased production of reactive oxygen species during old age causes an imbalance between ROS and antioxidant system, leading to lipid peroxidation, protein damage, DNA damage, cell dysfunction, inflammation, apoptosis, and necrosis which ultimately cause age-related macular degeneration and diabetic retinopathy (Fig. 7). Conflicting results were found when vitamin E (38) was given to treat eye disorders in human subjects (Schwartz et al. 2020). In another study, patients with retinal vascular disease were found to have a good field of the visual index when treated with coenzyme Q10 (20) (Fernández-Vega et al. 2020). Omega-3 fatty acids (3) were also found to provide moisture to the dry eyes in human subjects (Giannaccare et al. 2019). Resveratrol (4) has been shown to improve streptozocin-induced retinal damage in rats (Chen et al. 2019). Sulforaphane (5) was found to help reduce diabetic-induced retinopathy in male Sprague Dawley rats (Li et al. 2019). In a clinical trial, 100 patients suffering from eye disorders were treated with curcumin (7) and found to recover retinal damage (Mazzolani and Togni 2013). Salvianolic acid B (29) protected retinal pigment epithelial (RPE) cells by Grx1 induction through activating the Nrf2 pathway in human fetal RPE cells (Liu et al. 2016). Examples of treatments used in clinical trials include vitamin B12 eye drops and iodide iontophoresis (Seen and Tong 2018). Therefore, the ultimate conclusion is that natural antioxidants, active redox agents, are helpful to scavenge free radicals or to fix the production of excessive free radicals.
Proposed mechanism of antioxidants in the prevention of age-related eye disease. Increased production of reactive oxygen species during old age causes an imbalance between ROS and antioxidant system, leading to lipid peroxidation, protein damage, DNA damage, cell dysfunction, inflammation, apoptosis, and necrosis which ultimately cause age-related macular degeneration and diabetic retinopathy
Perspectives and Future Directions
Plants are commonly available, inexpensive, and high in polyphenols, which is why they have been used in conventional and herbal medicine, as well as in studies on health-promoting compounds. However, keep in mind that this is already a long way off. Complete knowledge of bioactive extract’s chemical profiling and biological activity is important but not sufficient. Identification and authentication of plants, processing, and postharvest care are also essential considerations. Genetics and/or environmental factors can have a substantial effect on the phytochemical profile of an extract, impacting both its biological activity and consumer safety. The rational identification of bioactive components in the raw extract, as well as their molecular targets, is an equally critical process. An increasing amount of focus is being paid to environmentally sound and consumer-friendly extraction processes based on green chemistry concepts, in addition to renewable sources. As we already see so frequently in the eyes of the general public or the mass media, “plant extract” or “natural product” does not necessarily mean “safer” than industrial materials in fact, so contamination and/or possible side effects must be studied. Targeted compounds can be used in biomedical and medicinal science, ranging from in vitro to in vivo and clinical trials, to determine the candidate compounds’ stability, effectiveness, and side effects in the short and long terms.
Polyphenols, especially some stilbenes (resveratrol), are reported to increase the shelf life of diverse model organisms such as Drosophila melanogaster (~ 10%), Caenorhabditis elegans (~ 10%), and Saccharomyces cerevisiae (~ 70%) by activating SIRT1 and SIRT2 genes which are responsible for aging processes. Various clinical and human trials suggested that potential drug candidates for various polyphenolic compounds as they are effective against various noncommunicable diseases like type 2 diabetes mellitus, cancer, and cardiovascular and neurodegenerative disorders. Resveratrol was previously overlooked until it was linked to the French paradox. The phrase “French paradox” was introduced in 1992 about epidemiological data from French individuals who had a low incidence of cardiovascular disease despite high saturated fat consumption. This conundrum was attributable to their moderate wine intake (20–30 g/day), which was anticipated to inhibit platelet aggregation. Since 1992, the French paradox has changed significantly, and its explanation has been ascribed to the positive benefits of resveratrol. Polyphenols are, and will undoubtedly continue to be, serious potential candidates in the medicinal and medical fields to improve human wellbeing, avoid, and treat multiple diseases, through all of these challenges until the identification of an active molecule that will become a lead compound.
Conclusions
Aging is a common biochemical phenomenon that can lead to a variety of age-related complications, including cancers, type 2 diabetes, metabolic syndrome, and neurodegenerative problems. Mitigating the aging process will help people live longer and live healthier lives. Traditional dietary therapies have been designed to combat age-related illnesses and can also help people live longer lives. The effects of plant chemicals on longevity and healthspan by specifically targeting the aging process have only recently been investigated. As a result, the majority of phytochemical research to date has been concentrated on the impact of plant secondary metabolites on the biochemical and physiological features of age-related diseases. Numerous endogenous and external activities generate reactive oxygen species. Oxidative stress is caused by an imbalance in the generation of ROS and antioxidant defenses and is mainly associated with the “aging theory.” Additionally, oxidative stress is associated with several chronic illnesses and, in conjunction with chronic inflammation, with sarcopenia and frailty in the elderly. Oxidative stress biomarkers may be useful as a diagnostic tool or therapeutic target. Antioxidant therapy, such as resveratrol and other dietary supplements, in combination with moderate aerobic exercise, has been shown to improve the clinical damage caused by oxidative stress. Additional research is required to determine the actual efficacy of these therapeutic interventions.
References
Aboubakr M, Elsayd F, Soliman A, Fadl SE, El-Shafey A, Abdelhiee EY (2020) L-Carnitine and vitamin E ameliorate cardiotoxicity induced by tilmicosin in rats. Environ Sci Pollut Res Int 27:23026–23034. https://doi.org/10.1007/s11356-020-08919-6
Adeshara KA, Diwan AG, Tupe RS (2016) Diabetes and complications: cellular signaling pathways, current understanding and targeted therapies. Curr Drug Targets 17:1309–1328. https://doi.org/10.2174/1389450117666151209124007
Ammar HO, Shamma RN, Elbatanony RSE, Khater B (2020) Antioxidants in cancer therapy: recent trends in application of nanotechnology for enhanced delivery. Sci Pharm 88:5. https://doi.org/10.3390/scipharm88010005
Astuti IY, Yupitawati A, Nurulita NA (2021) Anti-aging activity of tetrahydrocurcumin, Centella asiatica extract, and its mixture. Adv Tradit Med 21:57–63. https://doi.org/10.1007/s13596-020-00532-9
Awa H, Futamura A, Higashiguchi T, Ito A, Mori N, Murai M, Ohara H, Chihara T, Kaneko T (2017) Effects of combined treatment with branched-chain amino acids, citric acid, L-carnitine, coenzyme Q10, zinc, and various vitamins in tumor-bearing mice. Biol Pharm Bull 40:266–271. https://doi.org/10.1248/bpb.b16-00638
Ay M, Luo J, Langley M, Jin H, Anantharam V, Kanthasamy A, Kanthasamy AG (2017) Molecular mechanisms underlying protective effects of quercetin against mitochondrial dysfunction and progressive dopaminergic neurodegeneration in cell culture and MitoPark transgenic mouse models of Parkinson’s Disease. J Neurochem 141:766–782. https://doi.org/10.1111/jnc.14033
Bai J, Wang Q, Qi J, Yu H, Wang C, Wang X, Ren Y, Yang F (2019) Promoting effect of baicalin on nitric oxide production in CMECs via activating the PI3K-AKT-eNOS pathway attenuates myocardial ischemia-reperfusion injury. Phytomedicine 63:153035. https://doi.org/10.1016/j.phymed.2019.153035
Barbalace MC, Malaguti M, Giusti L, Lucacchini A, Hrelia S, Angeloni C (2019) Anti-inflammatory activities of marine algae in neurodegenerative diseases. Int J Mol Sci 20:3061. https://doi.org/10.3390/ijms20123061
Bosch-Morell F, Villagrasa V, Ortega T, Acero N, Muñoz-Mingarro D, González-Rosende ME, Castillo E, Sanahuja MA, Soriano P, Martínez-Solís I (2020) Medicinal plants and natural products as neuroprotective agents in age-related macular degeneration. Neural Regen Res 15:2207–2216. https://doi.org/10.4103/1673-5374.284978
Bose C, Alves I, Singh P, Palade PT, Carvalho E, Børsheim E, Jun SR, Cheema A, Boerma M, Awasthi S, Singh SP (2020) Sulforaphane prevents age-associated cardiac and muscular dysfunction through Nrf2 signaling. Aging Cell 19:e13261. https://doi.org/10.1111/acel.13261
Brimson JM, Prasanth MI, Malar DS, Brimson S, Tencomnao T (2020) Rhinacanthus nasutus “tea” infusions and the medicinal benefits of the constituent phytochemicals. Nutrients 12:3776. https://doi.org/10.3390/nu12123776
Brunetti G, Di Rosa G, Scuto M, Leri M, Stefani M, Schmitz-Linneweber C, Calabrese V, Saul N (2020) Healthspan maintenance and prevention of Parkinson’s-like phenotypes with hydroxytyrosol and oleuropein aglycone in C. elegans. Int J Mol Sci 21:2588. https://doi.org/10.3390/ijms21072588
Bumrungpert A, Lilitchan S, Tuntipopipat S, Tirawanchai N, Komindr S (2018) Ferulic acid supplementation improves lipid profiles, oxidative stress, and inflammatory status in hyperlipidemic subjects: a randomized, double-blind, placebo-controlled clinical trial. Nutrients 10:713. https://doi.org/10.3390/nu10060713
Camandola S, Plick N, Mattson MP (2019) Impact of coffee and cacao purine metabolites on neuroplasticity and neurodegenerative disease. Neurochem Res 44:214–227. https://doi.org/10.1007/s11064-018-2492-0
Campbell MS, Ouyang A, I M K, Charnigo RJ, Westgate PM, Fleenor BS, (2019) Influence of enhanced bioavailable curcumin on obesity-associated cardiovascular disease risk factors and arterial function: a double-blinded, randomized, controlled trial. Nutrition 62:135–139. https://doi.org/10.1016/j.nut.2019.01.002
Castejón ML, Montoya T, Alarcón-de-la-Lastra C, Sánchez-Hidalgo M (2020) Potential protective role exerted by secoiridoids from Olea europaea L. in cancer, cardiovascular, neurodegenerative, aging-related, and immunoinflammatory diseases. Antioxidants 9:149. https://doi.org/10.3390/antiox9020149
Castro NP, Rangel MC, Merchant AS, MacKinnon G, Cuttitta F, Salomon DS, Kim YS (2019) Sulforaphane suppresses the growth of triple-negative breast cancer stem-like cells in vitro and in vivo. Cancer Prev Res 12:147–158. https://doi.org/10.1158/1940-6207.CAPR-18-0241
Chainoglou E, Hadjipavlou-Litina D (2020) Curcumin in health and diseases: Alzheimer’s disease and curcumin analogues, derivatives, and hybrids. Int J Mol Sci 21:1975. https://doi.org/10.3390/ijms21061975
Chen Y, Meng J, Li H, Wei H, Bi F, Liu S, Tang K, Guo H, Liu W (2019) Resveratrol exhibits an effect on attenuating retina inflammatory condition and damage of diabetic retinopathy via PON1. Exp Eye Res 181:356–366. https://doi.org/10.1016/j.exer.2018.11.023
Chen X, Li X, Xu X, Li L, Liang N, Zhang L, Lv J, Wu YC, Yin H (2021) Ferroptosis and cardiovascular disease: role of free radical-induced lipid peroxidation. Free Radic Res. https://doi.org/10.1080/10715762.2021.1876856
Chudzińska M, Rogowicz D, Wołowiec Ł, Banach J, Sielski S, Bujak R, Sinkiewicz A, Grześk G (2020) Resveratrol and cardiovascular system-the unfulfilled hopes. Ir J Med Sci. https://doi.org/10.1007/s11845-020-02441-x
da Fonseca LJS, Nunes-Souza V, Goulart MOF, Rabelo LA (2019) Oxidative stress in rheumatoid arthritis: what the future might hold regarding novel biomarkers and add-on therapies. Oxid Med Cell Longev 2019:7536805. https://doi.org/10.1155/2019/7536805
Di Tano M, Raucci F, Vernieri C, Caffa I, Buono R, Fanti M, Brandhorst S, Curigliano G, Nencioni A, de Braud F, Longo VD (2020) Synergistic effect of fasting-mimicking diet and vitamin C against KRAS mutated cancers. Nat Commun 11:2332. https://doi.org/10.1038/s41467-020-16243-3
Du G, Song J, Du L, Zhang L, Qiang G, Wang S, Yang X, Fang L (2020) Chemical and pharmacological research on the polyphenol acids isolated from Danshen: a review of salvianolic acids. Adv Pharmacol 87:1–41. https://doi.org/10.1016/bs.apha.2019.12.004
Dydjow-Bendek D, Zagoźdźon P (2020) Total dietary fats, fatty acids, and omega-3/omega-6 ratio as risk factors of breast cancer in the Polish population - a case-control study. In Vivo 34:423–431. https://doi.org/10.21873/invivo.11791
Elfawy HA, Das B (2019) Crosstalk between mitochondrial dysfunction, oxidative stress, and age-related neurodegenerative disease: etiologies and therapeutic strategies. Life Sci 218:165–184. https://doi.org/10.1016/j.lfs.2018.12.029
Eriksdotter M, Vedin I, Falahati F, Freund-Levi Y, Hjorth E, Faxen-Irving G, Wahlund LO, Schultzberg M, Basun H, Cederholm T, Palmblad J (2015) Plasma fatty acid profiles in relation to cognition and gender in Alzheimer’s disease patients during oral omega-3 fatty acid supplementation: the omegAD study. J Alzheimers Dis 48:805–812. https://doi.org/10.3233/JAD-150102
Esfandiarei M, Hoxha B, Talley NA, Anderson MR, Alkhouli MF, Squire MA, Eckman DM, Babu JR, Lopaschuk GD, Broderick TL (2019) Beneficial effects of resveratrol and exercise training on cardiac and aortic function and structure in the 3xTg mouse model of Alzheimer’s disease. Drug Des Devel Ther 3:1197–1211. https://doi.org/10.2147/DDDT.S196119
Fernández-Vega B, Nicieza J, Álvarez-Barrios A, Álvarez L, García M, Fernández-Vega C, Vega JA, González-Iglesias H (2020) The use of vitamins and coenzyme Q10 for the treatment of vascular occlusion diseases affecting the retina. Nutrients 12:723. https://doi.org/10.3390/nu12030723
Ferreira HB, Melo T, Paiva A, Domingues MDR (2021) Insights in the role of lipids, oxidative stress and inflammation in rheumatoid arthritis unveiled by new trends in lipidomic investigations. Antioxidants 10:45. https://doi.org/10.3390/antiox10010045
Ghezzi P (2020) Environmental risk factors and their footprints in vivo - a proposal for the classification of oxidative stress biomarkers. Redox Biol 34:101442. https://doi.org/10.1016/j.redox.2020.101442
Giannaccare G, Pellegrini M, Sebastiani S, Bernabei F, Roda M, Taroni L, Versura P, Campos EC (2019) Efficacy of omega-3 fatty acid supplementation for treatment of dry eye disease: a meta-analysis of randomized clinical trials. Cornea 38:565–573. https://doi.org/10.1097/ICO.0000000000001884
Gonçalves AC, Nunes AR, Alves G, Silva LR (2021) Serotonin and melatonin: plant sources, analytical methods, and human health benefits. Rev Bras Farmacogn 31:162–175. https://doi.org/10.1007/s43450-021-00141-w
Griffiths WJ, Wang Y (2019) Oxysterol research: a brief review. Biochem Soc Trans 47:517–526. https://doi.org/10.1042/BST20180135
Gu Y, Han J, Jiang C, Zhang Y (2020) Biomarkers, oxidative stress and autophagy in skin aging. Ageing Res Rev 59:101036. https://doi.org/10.1016/j.arr.2020.101036
Gupta N, Verma K, Nalla S, Kulshreshtha A, Lall R, Prasad S (2020) Free radicals as a double-edged sword: the cancer preventive and therapeutic roles of curcumin. Molecules 25:5390. https://doi.org/10.3390/molecules25225390
Hano C, Tungmunnithum D (2020) Plant polyphenols, more than just simple natural antioxidants: oxidative stress, aging and age-related diseases. Medicines 7:26. https://doi.org/10.3390/medicines7050026
Hardy K (2021) Paleomedicine and the evolutionary context of medicinal plant use. Rev Bras Farmacogn 31:1–15. https://doi.org/10.1007/s43450-020-00107-4
Hatono M, Ikeda H, Suzuki Y, Kajiwara Y, Kawada K, Tsukioki T, Kochi M, Suzawa K, Iwamoto T, Yamamoto H, Shien T, Yamane M, Taira N, Doihara H, Toyooka S (2021) Effect of isoflavones on breast cancer cell development and their impact on breast cancer treatments. Breast Cancer Res Treat 185:307–316. https://doi.org/10.1007/s10549-020-05957-z
Huang X, Li N, Pu Y, Zhang T, Wang B (2019) Neuroprotective effects of ginseng phytochemicals: recent perspectives. Molecules 24:2939. https://doi.org/10.1007/s10549-020-05957-z
Ingles DP, Cruz Rodriguez JB, Garcia H (2020) Supplemental vitamins and minerals for cardiovascular disease prevention and treatment. Curr Cardiol Rep 22:22. https://doi.org/10.1007/s11886-020-1270-1
Jacob J, Amalraj A, Raj KKJ, Divya C, Kunnumakkara AB, Gopi S (2018) A novel bioavailable hydrogenated curcuminoids formulation (CuroWhiteTM) improves symptoms and diagnostic indicators in rheumatoid arthritis patients - a randomized, double blind and placebo controlled study. J Tradit Complement Med 9:346–352. https://doi.org/10.1016/j.jtcme.2018.06.001
Jorat MV, Tabrizi R, Mirhosseini N, Lankarani KB, Akbari M, Heydari ST, Mottaghi R, Asemi Z (2018) The effects of coenzyme Q10 supplementation on lipid profiles among patients with coronary artery disease: a systematic review and meta-analysis of randomized controlled trials. Lipids Health Dis 17:230. https://doi.org/10.1186/s12944-018-0876-4
Kim J, Kim J, Lee YI, Almurayshid A, Jung JY, Lee JH (2020) Effect of a topical antioxidant serum containing vitamin C, vitamin E, and ferulic acid after Q-switched 1064-nm Nd:YAG laser for treatment of environment-induced skin pigmentation. J Cosmet Dermatol 19:2576–2582. https://doi.org/10.1111/jocd.13323
Klaunig JE (2018) Oxidative Stress and Cancer. Curr Pharm Des 24:4771–4778. https://doi.org/10.2174/1381612825666190215121712
Ko HJ, Kim JH, Lee GS, Shin T (2020) Sulforaphane controls the release of paracrine factors by keratinocytes and thus mitigates particulate matter-induced premature skin aging by suppressing melanogenesis and maintaining collagen homeostasis. Phytomedicine 77:153276. https://doi.org/10.1016/j.phymed.2020.153276
Korac B, Kalezic A, Pekovic-Vaughan V, Korac A, Jankovic A (2021) Redox changes in obesity, metabolic syndrome, and diabetes. Redox Biol 42:101887. https://doi.org/10.1016/j.redox.2021.101887
Kostyuk V, Potapovich A, Albuhaydar AR, Mayer W, De Luca C, Korkina L (2018) Natural substances for prevention of skin photoaging: screening systems in the development of sunscreen and rejuvenation cosmetics. Rejuvenation Res 21:91–101. https://doi.org/10.1089/rej.2017.1931
Kour G, Haq SA, Bajaj BK, Gupta PN, Ahmed Z (2021) Phytochemical add-on therapy to DMARDs therapy in rheumatoid arthritis: in vitro and in vivo bases, clinical evidence and future trends. Pharmacol Res 169:105618. https://doi.org/10.1016/j.phrs.2021.105618
Kumar CS, Thangam R, Mary SA, Kannan PR, Arun G, Madhan B (2020) Targeted delivery and apoptosis induction of trans-resveratrol-ferulic acid loaded chitosan coated folic acid conjugate solid lipid nanoparticles in colon cancer cells. Carbohydr Polym 231:115682. https://doi.org/10.1016/j.carbpol.2019.115682
Li S, Yang H, Chen X (2019) Protective effects of sulforaphane on diabetic retinopathy: activation of the Nrf2 pathway and inhibition of NLRP3 inflammasome formation. Exp Anim 68:221–231. https://doi.org/10.1538/expanim.18-0146
Liguori I, Russo G, Aran L, Bulli G, Curcio F, Della-Morte D, Gargiulo G, Testa G, Cacciatore F, Bonaduce D, Abete P (2018) Oxidative stress, aging, and diseases. Clin Interv Aging 13:757–772. https://doi.org/10.2147/CIA.S158513
Liu YH, Lin YS, Huang YW, Fang SU, Lin SY, Hou WC (2016) Protective effects of minor components of curcuminoids on hydrogen peroxide-treated human HaCaT keratinocytes. J Agric Food Chem 64:3598–3608. https://doi.org/10.1021/acs.jafc.6b01196
Liu M, Teng CP, Win KY, Chen Y, Zhang X, Yang DP, Li Z, Ye E (2019) Polymeric encapsulation of turmeric extract for bioimaging and antimicrobial applications. Macromol Rapid Commun 40:1800216. https://doi.org/10.1002/marc.201800216
Ma L, Tang L, Yi Q (2019) Salvianolic acids: potential source of natural drugs for the treatment of fibrosis disease and cancer. Front Pharmacol 10:97. https://doi.org/10.3389/fphar.2019.00097
Madore C, Leyrolle Q, Morel L, Rossitto M, Greenhalgh AD, Delpech JC, Martinat M, Bosch-Bouju C, Bourel J, Rani B, Lacabanne C, Thomazeau A, Hopperton KE, Beccari S, Sere A, Aubert A, De Smedt-Peyrusse V, Lecours C, Bisht K, Fourgeaud L, Gregoire S, Bretillon L, Acar N, Grant NJ, Badaut J, Gressens P, Sierra A, Butovsky O, Tremblay ME, Bazinet RP, Joffre C, Nadjar A, Layé S (2020) Essential omega-3 fatty acids tune microglial phagocytosis of synaptic elements in the mouse developing brain. Nat Commun 11:6133. https://doi.org/10.1038/s41467-020-19861-z
Makuch S, Więcek K, Woźniak M (2021) The immunomodulatory and anti-inflammatory effect of curcumin on immune cell populations, cytokines, and in vivo models of rheumatoid arthritis. Pharmaceuticals 14:309. https://doi.org/10.3390/ph14040309
Marrazzo P, Angeloni C, Hrelia S (2019) Combined treatment with three natural antioxidants enhances neuroprotection in a SH-SY5Y 3D culture model. Antioxidants 8:420. https://doi.org/10.3390/antiox8100420
Martínez N, Herrera M, Frías L, Provencio M, Pérez-Carrión R, Díaz V, Morse M, Crespo MC (2019) A combination of hydroxytyrosol, omega-3 fatty acids and curcumin improves pain and inflammation among early stage breast cancer patients receiving adjuvant hormonal therapy: results of a pilot study. Clin Transl Oncol 21:489–498. https://doi.org/10.1007/s12094-018-1950-0
Mazzolani F, Togni S (2013) Oral administration of a curcumin-phospholipid delivery system for the treatment of central serous chorioretinopathy: a 12-month follow-up study. Clin Ophthalmol 7:939–945. https://doi.org/10.2147/OPTH.S45820
Michalak M, Pierzak M, Kręcisz B, Suliga E (2021) Bioactive compounds for skin health: a review. Nutrients 13:203. https://doi.org/10.3390/nu13010203
Montuschi P, Barnes PJ, Roberts LJ (2004) Isoprostanes: markers and mediators of oxidative stress. FASEB J 18:1791–1800. https://doi.org/10.1096/fj.04-2330rev
Moon SJ, Jhun J, Ryu J, Kwon JY, Kim SY, Jung K, Cho ML, Min JK (2021) The anti-arthritis effect of sulforaphane, an activator of Nrf2, is associated with inhibition of both B cell differentiation and the production of inflammatory cytokines. PLoS ONE 16:2-e0245986. https://doi.org/10.1371/journal.pone.0245986
Muñoz-Bernal ÓA, Coria-Oliveros AJ, de la Rosa LA, Rodrigo-García J, Del Rocío M-R, Sayago-Ayerdi SG, Alvarez-Parrilla E (2021) Cardioprotective effect of red wine and grape pomace. Food Res Int 140:110069. https://doi.org/10.1016/j.foodres.2020.110069
Musial C, Siedlecka-Kroplewska K, Kmiec Z, Gorska-Ponikowska M (2021) Modulation of autophagy in cancer cells by dietary polyphenols. Antioxidants 10:123. https://doi.org/10.3390/antiox10010123
Nelson KM, Dahlin JL, Bisson J, Graham J, Pauli GF, Walters MA (2017) The essential medicinal chemistry of curcumin: miniperspective. J Med Chem 60:1620–1637. https://doi.org/10.1021/acs.jmedchem.6b00975
Niazvand F, Orazizadeh M, Khorsandi L, Abbaspour M, Mansouri E, Khodadadi A (2019) Effects of quercetin-loaded nanoparticles on mcf-7 human breast cancer cells. Medicina 55:114. https://doi.org/10.3390/medicina55040114
Nita M, Grzybowski A (2016) The role of the reactive oxygen species and oxidative stress in the pathomechanism of the age-related ocular diseases and other pathologies of the anterior and posterior eye segments in adults. Oxid Med Cell Longev 3164734. https://doi.org/10.1155/2016/3164734
Oh KS, Oh BK, Mun J, Seo HW, Lee BH (2011) Salvianolic acid A suppress lipopolysaccharide-induced NF-κB signaling pathway by targeting IKKβ. Int Immunopharmacol 11:1901–1906. https://doi.org/10.1016/j.intimp.2011.07.022
Pereira L, Valado A (2021) The seaweed diet in prevention and treatment of the neurodegenerative diseases. Mar Drugs 19:128. https://doi.org/10.3390/md19030128
Poulose SM, Miller MG, Scott T, Shukitt-Hale B (2017) Nutritional factors affecting adult neurogenesis and cognitive function. Adv Nutr 8:804–811. https://doi.org/10.3945/an.117.016261
Qusa MH, Siddique AB, Nazzal S, El Sayed KA (2019) Novel olive oil phenolic (-)-oleocanthal (+)-xylitol-based solid dispersion formulations with potent oral anti-breast cancer activities. Int J Pharm 569:118596. https://doi.org/10.1016/j.ijpharm.2019.118596
Rada P, Rojo AI, Evrard-Todeschi N, Innamorato NG, Cotte A, Jaworski T, Tobón-Velasco JC, Devijver H, García-Mayoral MF, Van Leuven F, Hayes JD, Bertho G, Cuadrado A (2012) Structural and functional characterization of Nrf2 degradation by the glycogen synthase kinase 3/β-TrCP axis. Mol Cell Biol 32:3486–3499. https://doi.org/10.1128/MCB.00180-12
Ramalingam M, Kim H, Lee Y, Lee YI (2018) Phytochemical and pharmacological role of liquiritigenin and isoliquiritigenin from radix glycyrrhizae in human health and disease models. Front Aging Neurosci 10:348. https://doi.org/10.3389/fnagi.2018.00348
Rinnerthaler M, Bischof J, Streubel MK, Trost A, Richter K (2015) Oxidative stress in aging human skin. Biomolecules 5:545–589. https://doi.org/10.3390/biom5020545
Rodríguez-López P, Lozano-Sanchez J, Borrás-Linares I, Emanuelli T, Menéndez JA, Segura-Carretero A (2020) Structure–biological activity relationships of extra-virgin olive oil phenolic compounds: health properties and bioavailability. Antioxidants 9:685. https://doi.org/10.3390/antiox9080685
Rodríguez-Morató J, Xicota L, Fitó M, Farré M, Dierssen M, de la Torre R (2015) Potential role of olive oil phenolic compounds in the prevention of neurodegenerative diseases. Molecules 20:4655–4680. https://doi.org/10.3390/molecules20034655
Salama SA, Arab HH, Omar HA, Gad HS, Abd-Allah GM, Maghrabi IA, Al Robaian MM (2018) L-carnitine mitigates UVA-induced skin tissue injury in rats through downregulation of oxidative stress, p38/c-Fos signaling, and the proinflammatory cytokines. Chem Biol Interact 285:40–47. https://doi.org/10.1016/j.cbi.2018.02.034
Samarghandian S, Azimi-Nezhad M, Farkhondeh T, Samini F (2017) Anti-oxidative effects of curcumin on immobilization-induced oxidative stress in rat brain, liver and kidney. Biomed Pharmacother 87:223–229. https://doi.org/10.1016/j.biopha.2016.12.105
Schwartz SG, Wang X, Chavis P, Kuriyan AE, Abariga SA (2020) Vitamin A and fish oils for preventing the progression of retinitis pigmentosa. Cochrane Database Syst Rev 6:6-CD008428. https://doi.org/10.1002/14651858.CD008428.pub3
Seen S, Tong L (2018) Dry eye disease and oxidative stress. Acta Ophthalmol 96:e412–e420. https://doi.org/10.1111/aos.13526
Singh NA, Mandal AK, Khan ZA (2016) Potential neuroprotective properties of epigallocatechin-3-gallate (EGCG). Nutr J 15:60. https://doi.org/10.1186/s12937-016-0179-4
Singh A, Kukreti R, Saso L, Kukreti S (2019) Oxidative stress: a key modulator in neurodegenerative diseases. Molecules 24:1583. https://doi.org/10.3390/molecules24081583
Son SM (2012) Reactive oxygen and nitrogen species in pathogenesis of vascular complications of diabetes. Diabetes Metab J 36:190–198. https://doi.org/10.4093/dmj.2012.36.3.190
Srinivas US, Tan BWQ, Vellayappan BA, Jeyasekharan AD (2019) ROS and the DNA damage response in cancer. Redox Biol 25:101084. https://doi.org/10.1016/j.redox.2018.101084
Stojanov S, Kreft S (2020) Gut microbiota and the metabolism of phytoestrogens. Rev Bras Farmacogn 30:145–154. https://doi.org/10.1007/s43450-020-00049-x
Sun Q, Kang RR, Chen KG, Liu K, Ma Z, Liu C, Deng Y, Liu W, Xu B (2021) Sirtuin 3 is required for the protective effect of resveratrol on manganese-induced disruption of mitochondrial biogenesis in primary cultured neurons. J Neurochem 156:121–135. https://doi.org/10.1111/jnc.15095
Sunagawa Y, Funamoto M, Sono S, Shimizu K, Shimizu S, Genpei M, Miyazaki Y, Katanasaka Y, Morimoto E, Ueno M, Komiyama M, Kakeya H, Wada H, Hasegawa K, Morimoto T (2018) Curcumin and its demethoxy derivatives possess p300 HAT inhibitory activity and suppress hypertrophic responses in cardiomyocytes. J Pharmacol Sci 136:212–217. https://doi.org/10.1016/j.jphs.2017.12.013
Tan CC, Yu JT, Wang HF, Tan MS, Meng XF, Wang C, Jiang T, Zhu XC, Tan L (2014) Efficacy and safety of donepezil, galantamine, rivastigmine, and memantine for the treatment of Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis 41:615–631. https://doi.org/10.3233/JAD-132690
Thota RN, Rosato JI, Dias CB, Burrows TL, Martins RN, Garg ML (2020) Dietary supplementation with curcumin reduce circulating levels of glycogen synthase kinase-3β and islet amyloid polypeptide in adults with high risk of type 2 diabetes and Alzheimer’s disease. Nutrients 12:1032. https://doi.org/10.3390/nu12041032
Tomeh MA, Hadianamrei R, Zhao X (2019) A review of curcumin and its derivatives as anticancer agents. Int J Mol Sci 20:1033. https://doi.org/10.3390/ijms20051033
Varghese MV, Abhilash M, Paul MV, Alex M, Nair RH (2017) Omega-3 fatty acid protects against arsenic trioxide-induced cardiotoxicity in vitro and in vivo. Cardiovasc Toxicol 17:109–119. https://doi.org/10.1007/s12012-016-9361-3
Welcome MO (2020) Blood brain barrier inflammation and potential therapeutic role of phytochemicals. PharmaNutrition 11:100177. https://doi.org/10.1016/j.phanu.2020.100177
Wu D, Xu H, Chen J, Zhang L (2020) Effects of astaxanthin supplementation on oxidative stress. Int J Vitam Nutr Res 90:179–194. https://doi.org/10.1024/0300-9831/a000497
Yu T, Xu YY, Zhang YY, Li KY, Shao Y, Liu G (2018) Plumbagin suppresses the human large cell lung cancer cell lines by inhibiting IL-6/STAT3 signaling in vitro. Int Immunopharmacol 55:290–296. https://doi.org/10.1016/j.intimp.2017.12.021
Zahiruddin S, Basist P, Parveen A, Parveen R, Khan W, Gaurav AS (2020) Ashwagandha in brain disorders: a review of recent developments. J Ethnopharmacol 257:112876. https://doi.org/10.1016/j.jep.2020.112876
Zaki AM, El-Tanbouly DM, Abdelsalam RM, Zaki HF (2018) Plumbagin ameliorates hepatic ischemia-reperfusion injury in rats: role of high mobility group box 1 in inflammation, oxidative stress and apoptosis. Biomed Pharmacother 106:785–793. https://doi.org/10.1016/j.biopha.2018.07.004
Zhang YJ, Gan RY, Li S, Zhou Y, Li AN, Xu DP, Li HB (2015) Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules 20:21138–21156. https://doi.org/10.3390/molecules201219753
Zhou YT, Zhu L, Yuan Y, Ling S, Xu JW (2020) Effects and mechanisms of five Psoralea prenylflavonoids on aging-related diseases. Oxid Med Cell Longev:2128513.https://doi.org/10.1155/2020/2128513
Zhu L, Zhang Z, Xia N, Zhang W, Wei Y, Huang J, Ren Z, Meng F, Yang L (2020) Anti-arthritic activity of ferulic acid in complete Freund’s adjuvant (CFA)-induced arthritis in rats: JAK2 inhibition. Inflammopharmacology 28:463–473. https://doi.org/10.1007/s10787-019-00642-0
Acknowledgements
The first author thanks the Chandigarh University for providing the infrastructure to carry out the research work.
Author information
Authors and Affiliations
Contributions
AD and ZAK both conceived and designed the review paper. AD and VD contributed to the paper realization.
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Devi, A., Dwibedi, V. & Khan, Z.A. Natural Antioxidants in New Age-Related Diseases. Rev. Bras. Farmacogn. 31, 387–407 (2021). https://doi.org/10.1007/s43450-021-00175-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43450-021-00175-0