Abstract
Purpose
Breast cancer patients receiving hormonal therapies face risks of relapse, increased rates of cardiovascular events, and toxicities of therapy such as aromatase inhibitor (AI)-associated musculoskeletal symptoms (AIMSS). C-reactive protein (CRP), a marker for inflammation, is associated with breast cancer outcomes. We evaluated whether the olive-derived polyphenol hydroxytyrosol combined with omega-3 fatty acids and curcumin would reduce CRP and musculoskeletal symptoms in breast cancer patients receiving adjuvant hormonal therapies.
Experimental design
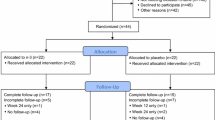
This prospective, multicenter, open-label, single arm, clinical trial enrolled post-menopausal breast cancer patients (n = 45) with elevated C-reactive protein (CRP) taking predominantly aromatase inhibitors to receive a combination of hydroxytyrosol, omega-3 fatty acids, and curcumin for 1 month. CRP, other inflammation-associated cytokines, and pain scores on the Brief Pain Inventory were measured before therapy, at the end of therapy and 1 month after completion of therapy.
Results
CRP levels declined during the therapy [from 8.2 ± 6.4 mg/L at baseline to 5.3 ± 3.2 mg/L (p = 0.014) at 30 days of treatment], and remained decreased during the additional 1 month off therapy. Subjects with the highest baseline CRP levels had the greatest decrease with the therapy. Pain scores also decreased during the therapy. There were no significant adverse events.
Conclusions
The combination of hydroxytyrosol, omega-3 fatty acids, and curcumin reduced inflammation as indicated by a reduction in CRP and reduced pain in patients with aromatase-induced musculoskeletal symptoms. Longer studies comparing this combination to other anti-inflammatories in larger groups of patients with clinical outcome endpoints are warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast cancer patients face numerous medical challenges. Recurrences as late as 15–20 years from diagnosis remain a risk [1, 2] and as this risk recedes, cardiovascular events assume increased importance [3]. Toxicities of therapy such as aromatase inhibitor (AI)-associated musculoskeletal symptoms (AIMSS), manifesting as arthralgias and myalgias in > 50% of those treated, frequently compromise quality of life, limit compliance, and prompt early drug discontinuation [4,5,6,7,8,9,10] which has been associated with increased mortality [11]. A number of lifestyle modifications—exercise [12,13,14], weight loss, and dietary enhancements [15, 16]—have been proposed to improve survival and ameliorate these complications, but long-term adherence may be challenging to achieve [17], indicating the need for additional convenient, well-tolerated interventions aimed at the underlying pathogenesis.
Chronic inflammation contributes to carcinogenesis [18] and promotion of existing breast cancer [19, 20] and is a factor in complications related to breast cancer therapy [21,22,23]. Chronic inflammation-induced carcinogenesis results in part from generation of DNA-damaging reactive oxygen and nitrogen species under the influence of inflammatory cytokines, while the inflammatory microenvironment of established cancers promotes tumor growth and progression, epithelial-mesenchymal transition, angiogenesis, metastases, and resistance to therapy and immune attack [24,25,26,27]. Although the underlying mechanisms of aromatase-associated arthralgias are debated, inflammation is likely a contributing factor [22, 28], particularly at the local joint level [29,30,31].
An emerging understanding of the inflammatory state suggests that disparate pathogenic events converge on a common cascade of inflammatory cytokines [32] originating with inflammasome-mediated activation of IL-1β and subsequently interleukin-6 (IL-6). One well-described downstream inflammatory marker is C-reactive protein (CRP) generated in the liver in response to IL-6 produced by macrophages and T cells [33]. While it is unclear whether CRP itself mediates the pathogenic effects of inflammation, elevated levels of CRP are associated with increased risk of cardiovascular disease and overall mortality [34, 35], and are correlated with decreased survival in patients with metastatic cancer [36]. The Health, Eating, Activity and Lifestyle (HEAL) study demonstrated that elevated circulating CRP concentrations measured 31 months after diagnosis of breast cancer were related to a reduction in overall survival, regardless of age, tumor stage, race, and body mass index [37]. Furthermore, in a prospective cohort of Danish women with invasive breast cancer, elevated CRP levels at diagnosis had a negative impact on overall and disease-free survival, more so as the CRP levels increased [38]. A meta-analysis also concluded that higher CRP levels carried a poorer prognosis in breast cancer [39]. Other recent reports have reached similar conclusions and it has also been suggested that therapies whose effect can be measured by a reduction in CRP may improve outcome of breast cancer [25, 40].
Although there are a number of agents that reduce inflammation (NSAIDS, statins, corticosteroids, DMARDS), all carry risks of serious toxicity and may not adequately address the underlying inflammatory state [41]. Certain diets, however, such as the Mediterranean diet can achieve lower levels of inflammation, and are associated with lower risk of breast cancer in post-menopausal women [42] and reductions in cardiovascular events. The high extra virgin olive oil (EVOO) content of the Mediterranean diet [43] is one of the components thought to be responsible for this benefit. Indeed, in the PREDIMED study [44], a trial of patients at high cardiovascular risk who were randomized to a Mediterranean diet supplemented with extra-virgin olive oil, a Mediterranean diet supplemented with mixed nuts, or a control low fat diet, major cardiovascular events were reduced in those receiving the olive oil or nuts. CRP and IL-6 were significantly reduced by the Mediterranean diet with olive oil [45].
The PREDIMED study provided approximately 1 L per week of EVOO which carries with it excess fats and calories and, in populations that do not routinely use olive oil, possibly diminished adherence. Among the components of EVOO most likely to affect inflammation are the polyphenols (including oleuropein, tyrosol, and the most common, hydroxytyrosol [46,47,48]. Administration of olive fruit or olive leaf extracts containing hydroxytyrosol or purified hydroxytyrosol to mice and humans has a number of anti-oxidant and anti-inflammatory effects [29, 48,49,50]. Further, hydroxytyrosol reduced osteoarthritis (OA) related genes in human chondrocytes displaying OA-like features [51]. Therefore, we hypothesized that hydroxytyrosol administration to breast cancer patients would reduce inflammatory mediators associated with malignancy, cardiovascular disease and the musculoskeletal complications of therapy and would ultimately be associated with a better survival and quality of life. Further, as there has been considerable interest in the benefits of other natural products which also reduce chronic inflammation such as omega-3 fatty acids and curcumin [52,53,54], we developed an encapsulated combination of olive fruit-derived hydroxytyrosol, omega-3 fatty acids, and curcumin (PureVida™) and conducted a prospective, short-term study to assess its effects on inflammation measured by CRP levels and pain in breast cancer patients receiving adjuvant hormonal therapy.
Methods
Study design
This was a prospective, multi-center, open-label, single-arm clinical trial. The protocol was approved by the individual institutional review boards/research ethics committees of the seven participating centers. The study was registered in www.clinicaltrials.gov (NCT01819948) and conducted in compliance with the Declaration of Helsinki and the Good Clinical Practice Guidelines.
Participant selection
Eligible participants were post-menopausal women with histopathological diagnosis of AJCC Stage 0-IIIA, ER + and/or PR + breast cancer with gross total resection, more than 12 months from their initial surgery for breast cancer, at least 6 months since last chemotherapy, with no evidence of disease, ECOG 0–1, receiving adjuvant hormonal therapy (letrozole, anastrazole, exemestane or tamoxifen) at a stable dose for at least 3 months at trial entry. They were excluded if average intake of aspirin was > 80 mg/day, ibuprofen > 800 mg/day or naproxen > 500 mg/day, any intake of celecoxib or other COX-2 inhibitors, presence of known autoimmune disease or inflammatory disorder, or any condition requiring the use of systemic corticosteroids or bisphosphonates. All participants signed informed consent approved by their respective institution’s ethics review board before study entry. After enrollment, all participants agreed to abstain from any dietary supplements, olives or extra virgin olive oil for 1 month prior to trial enrollment and during the trial.
Interventions and measurements
Prior to initiating the study investigational product, subjects underwent two consecutive blood draws within 5 ± 2 days of each other. CRP was measured using the standard high-sensitivity assay at each institution. Only those with an average CRP > 3.9 mg/L (the cutoff at which Pierce and al. [37] found an association between CRP level and survival) were enrolled into the study. Subsequently, all enrolled patients took three capsules of PureVida™ (subsequently referred to as EPA/DHA/hydroxytyrosol/curcumin) per day for 30 days (two in the morning during breakfast and one in the evening during dinner). Each active capsule contained 460 mg of fish oil (EPA and DHA), 125 mg of Hytolive® powder (12.5 mg of natural hydroxytyrosol), and 50 mg extract of curcumin (47.5 mg curcuminoids). At 30 and 60 days (i.e., at the completion of the treatment period and then 1 month after completing the treatment period), two blood draws within 5 ± 2 days of each other were obtained. CRP, IL-6, SAA, IFNγ, TNFα, IL-10, IL-15, TGFβ, IGF-1, total cholesterol, HDL, LDL and triglycerides were measured in each sample.
To evaluate the impact of the EPA/DHA/hydroxytyrosol/curcumin on pain, the Brief Pain Intensity Score (BPI-SF) [55] was used. This is a general pain scale for cancer patients and has been used for non-cancer pain. It was selected as there are no well-validated measures specifically designed for arthralgia. The BPI-SF is a 14-item questionnaire that asks subjects to rate pain over the last 24 h (worst, least, average and actual pain) and the degree to which it interferes with daily activities on a 0–10 scale, where higher scores indicate more pain. This is one of the most widely used and reliable scales to assess pain changes in cancer patients. Subjects were asked to complete this questionnaire at baseline and at week 2 and 4 during therapy to evaluate possible changes in their pain.
Endpoints and statistical analysis
The primary endpoint was the change in CRP levels associated with EPA/DHA/hydroxytyrosol/curcumin administration. A significant decrease was defined as a decrease of mean CRP levels of at least 1.3 mg/L using the average of the two blood draws prior to study product administration as the baseline. To achieve statistical significance (p < 0.05), we estimated that 45 subjects would be required to provide a statistical power of 80% to detect a significant CRP decrease. The mean of the results of the two pre-treatment blood samples was compared with the mean of the results of the two post-treatment samples, applying the Student’s t test. If at any point of the study, one of the two CRP levels was missing, then the remaining available determination was used for the analysis.
The secondary endpoint was the “worst pain” item (item 2) of the BPI-SF. The effect of the EPA/DHA/hydroxytyrosol/curcumin on pain was evaluated comparing the patients’ self-reported pain severity index and worst pain assessments performed at baseline, 2 weeks and 4 weeks after starting study product administration. To this purpose, a paired sample t test was performed. Statistical significance was defined as p value < 0.05. Data about possible adverse events related to the study investigational product was evaluated according to the criteria of the National Cancer Institute (NCI-CTCAE) version 4.
Results
Baseline characteristics of the study participants
From September 2012 to June 2015, 212 participants signed consent for the study. Out of these, 49 had a mean basal CRP > 3.9 mg/L and fulfilled all other eligibility criteria. Three of these did not start the treatment and were excluded, one stopped treatment due to an unplanned surgery, and 45 patients completed treatment as established per protocol. The participant characteristics are shown in Table 1. Subjects had a median age of 57 years (range 40–81) and were 34.8 months from their diagnosis of breast cancer. Median body mass index was in the upper range of the overweight category. Sixty-seven [67] percent were receiving an adjuvant aromatase inhibitor and 33% were receiving tamoxifen.
CRP levels decrease with EPA/DHA/hydroxytyrosol/curcumin
CRP values were available at baseline and day 30 (end of treatment) for 45 participants (Table 2). Mean (± SD) CRP at baseline was 8.2 ± 6.4 mg/L. On day 30, mean CRP was 5.3 ± 3.2 mg/L (p = 0.014), for a mean decrease in CRP of 2.8 mg/L (mean decrease 18.5%). At day 60, 39 of the patients had available CRP values, with a mean value of 5.7 ± 4.3, representing a sustained decrease in CRP (p = 0.064 Student’s t, p < 0.02, Wilcoxon test). One subject had particularly high CRP levels (mean 41.9 mg/L) at baseline in the absence of infection. To determine whether this individual’s data affected the overall results of the study, we re-evaluated mean and median CRP levels with their data removed. Again, there was a statistically significant decrease in mean CRP [7.4 ± 3.9 mg/L at baseline and 5.3 ± 3.2 mg/L at day 30 (mean decrease 1.9 ± 4.5 mg/L, p = 0.006)] and median CRP (5.7 mg/L at baseline and 5.1 mg/L at day 30 (decrease of 1.2 mg/L, p = 0.003). The percentage decrease in CRP was the greatest in those individuals with the highest baseline CRP levels (Table 3). For example, for those with baseline CRP > 9.75, there was a 49% decrease in CRP, whereas for those with baseline CRP between 9.75 and 5.85, there was a 26.2% decrease in CRP, between day 0 and day 30.
Pain score decreases while on therapy
Of the 39 patients with available basal BPI worst pain score, 26 completed the inventory after treatment at day 30. At baseline, average ± standard deviation BPI worst pain score was 3.9 ± 3.1. After 30 days of treatment, there was a mean reduction of 1.6 points (p = 0.011), which corresponds to a 21.5% decrease. Average basal pain severity index score was 2.9 ± 2.4, and showed an average decrease after treatment at day 30 of 1.2 points (p = 0.008), corresponding to a 26.6% decrease. All the individual items of the pain severity index, except “average pain”, showed a statistically significant decrease after 30 days of treatment including “lowest pain level” (p = 0.045, 0.9 points drop; 20% decrease) and “current pain” (p = 0.004; 1.5 points drop; 45% decrease (Table 4).
To determine the effect of EPA/DHA/hydroxytyrosol/curcumin in those who had greater levels of pain, we separately analyzed the pain score in those with a reported basal pain score > 4 points (n = 17, 44%) who completed the BPI-score after treatment (n = 14). The average pre-treatment BPI worst pain score was 6.8 ± 1.5 and after 30 days treatment, there was an absolute decrease of 2.8 points (p = 0.005), corresponding to a 38% decrease. The average BPI pain severity index basal score was 5.75 ± 1.53, and showed a reduction for these patients of 2.36 points (p = 0.044), a mean reduction of 38%.
Analysis of inflammatory biomarkers and blood lipids
There was a decrease in IFN-gamma levels of 8.7% (p = 0.056) at day 30 compared with baseline levels; the remaining inflammatory biomarkers (IL-6, SAA, TNFα, IL-10, TGFβ, and IGF-1) were stable over the period of the study (Supplementary Table 1). In the lipid profile, a 7% decrease (p = 0.011) in triglycerides was observed, while HDL did not differ from basal values and there was a nonsignificant increase in LDL (8.8%, p = 0.054).
Adverse events
Adverse events (AEs) are reported for all 46 participants who initiated therapy (Table 5). In general, the EPA/DHA/hydroxytyrosol/curcumin was associated with few adverse events. Constipation and abnormal or a fish taste were the most common. No clinically relevant adverse events were reported, and no subjects withdrew from treatment due to AEs.
Discussion
This study was intended to provide proof of principal that a combination of olive fruit-derived hydroxytyrosol, omega-3 fatty acids, and curcumin could reduce pathologic inflammation as suggested by CRP levels and improve pain in breast cancer patients who were stable on adjuvant hormonal therapy. The results of our study show a significant decrease in pain and CRP levels after continuous administration of this combination. After a 30-day treatment period, women with baseline CRP levels > 3.9 mg/L had a decrease of 2.8 mg/L (p < 0.014) between day 0 and day 30 (mean decrease from the basal level was 18.5%) which persisted at day 60 despite stopping the capsules 30 days prior. The BPI “worst pain” score decreased by 21.5% after 30 days.
CRP was chosen as the major endpoint for this study because it can be routinely measured in hospital laboratories and is consistently associated with worse outcomes from malignancy and cardiovascular disease. Although it is acknowledged that CRP is not likely the effector in these processes, it is likely a surrogate marker for those that are. For example, CRP is downstream of IL-6 which itself has protumorigenic activities [56] and it is downstream of IL-1β which may be a driver of cardiovascular disease [33]. Although we did not detect differences in peripheral blood IL-6 or IL-1β, it is possible that levels could vary in tumor or vascular microenvironments.
The inclusion criteria required a CRP level > 3.9 mg/L at baseline and subjects to be more than 1 year from their initial surgery for breast cancer to test the effect of EPA/DHA/hydroxytyrosol/curcumin in subjects with clinically relevant levels of inflammation, but also far enough from their cancer diagnosis and therapy that it was unlikely acute inflammation was driving the elevated CRP. We note that there is precedence for this choice. Pierce [37], using data from the Health, Eating, Activity, and Lifestyle (HEAL) Study of stage 0 to IIIA breast cancer patients, found that elevated CRP (> 3.9 mg/L) measured 31 months after diagnosis was associated with reduced overall survival. Further their data suggested a threshold effect on survival, rather than a dose–response relationship. Others have found CRP levels in this range of similar relevance for outcome. Allin [38] analyzing CRP levels at the time of diagnosis of breast cancer found that statistically significant differences in outcomes were most marked in the highest tertile of CRP levels (> 3.24 mg/L).
Although our primary focus has been on the benefits of olive fruit-derived hydroxytyrosol, the combination used in this study was developed to include omega-3 fatty acids and curcumin. There is evidence to suggest that each of these ingredients taken individually might have anti-inflammatory properties in vivo. Omega-3 fatty acids carry out their anti-inflammatory effect through several mechanisms [57], including arachidonic acid replacement in phospholipid membranes, direct inhibition of phospholipases and synthesis of anti-inflammatory metabolites, and reduction of circulating inflammatory biomarkers [57,58,59,60]. Curcumin has been shown to diminish CRP in some cases [61], most likely through inactivation of the NFκB which down-regulates TNF-α, interleukins (IL-1, IL-2, IL-6, IL-8, IL-12) and chemokines. Nonetheless, we believe that the most important component of the EPA/DHA/hydroxytyrosol/curcumin is the hydroxytyrosol which has a number of anti-oxidant and anti-inflammatory effects [48, 62] and has also been reported to have direct anti-proliferative effects [63]. It has previously been shown to reduce CRP in rheumatoid arthritis patients [64]; however, Crespo [65] administered hydroxytyrosol 5 and 25 mg by mouth daily for a week to normal volunteers and surprisingly observed a non-significant increase in CRP; however, their healthy volunteers had normal CRP levels and it might be difficult to demonstrate a decrease in such a scenario and during only a week of therapy. Further, we used a higher dose (37.5 mg total per day) of hydroxytyrosol. Lopez-Huertas [66] administered 45 mg of hydroxytyrosol to healthy volunteers with mild hyperlipidemia, and observed a numerical, but not statistically significant, decrease in CRP at 8 (but not 4) weeks. Again, the mean CRP level was lower than the level for our study participants. We observed the greatest decrease in CRP in subjects who had the highest baseline level suggesting the benefit may mainly occur in scenarios with the greater amount of inflammation.
We also designed the study to enroll subjects receiving adjuvant hormonal therapy. It was our initial intention to enroll predominantly patients receiving aromatase inhibitors, but we did allow those on tamoxifen as well as this would not likely effect the primary endpoint and because breast cancer patients can have numerous other causes for pain (such as breast or chest wall pain, osteoarthritis, osteoporotic fractures, peripheral neuropathy, and others). AIMSS is a major problem for up to 50% of patients undergoing adjuvant therapy with aromatase inhibitor (AI) [6]. It may impact quality of life and be a cause of non-adherence or discontinuation [10]. Attempts to reduce AIMSS have included treatment with vitamin D supplementation [67], glucosamine together with chondroitin [68], vitamin D3 [69], other supplements with omega 3 fatty acids [70], switching therapies [71], exercise adherence [72], acupuncture [73] or duloxetine [74], but no clear treatment has emerged. In some studies, the observed reduction of pain was not statistically significant or clinically meaningful. In other studies, adverse events were significant barriers. Moreover, none of these studies has reported a decrease in CRP. We observed that EPA/DHA/hydroxytyrosol/curcumin showed a reduction in pain scores. Using BPI “worst pain” scores, women reported a 1.6 points reduction (p = 0.011), and 1.2 points reduction (p = 0.008) in the BPI pain severity index. Moreover, women receiving EPA/DHA/hydroxytyrosol/curcumin who had a baseline BPI pain score > 4 (a level often used as an inclusion criteria for other studies of interventions for AIMSS), reported a reduction of worst pain score of 2.8 points (p = 0.005), with a BPI pain severity index decrease of 2.36 points (p = 0.04). These are among the largest decreases among all the mentioned studies, except for duloxetine, which led to serious adverse events in up to 20% of the patients. This larger decrease reflects that a deeper effect is achieved in patients with higher basal pain scores. The magnitude of this decrease was greater than 2 points, which is considered to be clinically significant by the Initiative on Methods, Measurements and Pain Assessment in Clinical Trials consensus committee [75]. That this is an important subgroup for focused study is suggested by the observation that those who start with baseline pain are more likely to discontinue AI therapy sooner [76]. Although the mechanisms that produce AI-induced arthralgia are not fully understood, local inflammation likely plays a contributory role [22, 28]. Different studies have shown that patients receiving AIs have muscle tendon thickening [29] and hand/wrist intraarticular effusions [30] that are not present in control groups of women not receiving AIs. Thus, the reduction in inflammation as measured by a decrease in CRP achieved in this study might play a role in the reduction of pain from AIs.
One limitation of our study was the lack of a placebo-controlled design which was not possible due to patient and site unwillingness to participate in such a study. We, therefore, used patients as their own control. To reduce the regression-to-the-mean effect, we assessed blood levels at two time points pre- and post-therapy and averaged the CRP levels from the two blood draws. We also did not independently confirm compliance with the study therapy by a method such as urinary levels of hydroxytyrosol. Acknowledging its pilot nature, we believe that this study provides promising initial results in terms of reduction of CRP and decrease in pain scores that warrant larger clinical studies with longer periods of EPA/DHA/hydroxytyrosol/curcumin administration and standard clinical endpoints such as disease specific survival and overall survival.
References
Saphner T, Tormey DC, Gray R. Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol. 1996;14:2738–46.
Esserman LJ, Moore DH, Tsing PJ, Chu PW, Yau C, Ozanne E, Chung RE, Tandon VJ, Park JW, Baehner FL, Kreps S, Tutt AN, Gillett CE, Benz CC. Biologic markers determine both the risk and the timing of recurrence in breast cancer. Breast Cancer Res Treat. 2011;129(2):607–16.
Gernaat SAM, Ho PJ, Rijnberg N, Emaus MJ, Baak LM, Hartman M, Grobbee DE, Verkooijen HM. Risk of death from cardiovascular disease following breast cancer: a systematic review. Breast Cancer Res Treat. 2017;164(3):537–55.
Murphy CC, Bartholomew LK, Carpentier MY, et al. Adherence to adjuvant hormonal therapy among breast cancer patients in clinical practice: a systematic review. Breast Cancer Res Treat. 2012;134:459–78.
Sestak I, Cuzick J, Sapunar F, et al. Risk factors for joint symptoms in patients enrolled in the ATAC trial: a retrospective, exploratory analysis. Lancet Oncol. 2008;9:866–72.
Crew KD, Greenlee H, Capodice J, et al. Prevalence of joint symptoms in postmenopausal women taking aromatase inhibitors for early-stage breast cancer. J Clin Oncol. 2007;25:3877–83.
Henry NL, Giles JT, Ang D, et al. Prospective characterization of musculoskeletal symptoms in early stage breast cancer patients treated with aromatase inhibitors. Breast Cancer Res Treat. 2008;111:365–72.
Hershman DL, Kushi LH, Shao T, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8769 early-stage breast cancer patients. J Clin Oncol. 2010;28:4120–8.
Chim K, Xie SX, Stricker CT, et al. Joint pain severity predicts premature discontinuation of aromatase inhibitors in breast cancer patients. BMC Cancer. 2013;13:401.
Kadakia K, Snyder C, Kidwell K. Patient-reported outcomes and early discontinuation in aromatase inhibitor-treated postmenopausal women with early stage breast cancer. Oncologist. 2016;21(5):539–46.
Hershman DL, Shao T, Kushi LH. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat. 2011;126:529–37.
Adraskela K, Veisaki E, Koutsilieris M, Philippou A. Physical exercise positively influences breast cancer evolution. Clin Breast Cancer. 2017;17:408–17.
Kraschnewski JL, Schmitz KH. Exercise in the prevention and treatment of breast cancer: What clinicians need to tell their patients. Curr Sports Med Rep. 2017;16(4):263–7.
Nyrop KA, Callahan LF, Cleveland RJ, Arbeeva LL, Hackney BS, Muss HB. Randomized Controlled Trial of a Home-Based Walking Program to Reduce Moderate to Severe Aromatase Inhibitor-Associated Arthralgia in Breast Cancer Patients. Oncologist. 2017;22:1238–49.
Pierce JP, Stefanick ML, Flatt SW, Natarajan L, Sternfeld B, Madlensky L, Al-Delaimy WK, Thomson CA, Kealey S, Hajek R, Parker BA, Newman VA, Caan B, Rock CL. Greater survival after breast cancer in physically active women with high vegetable-fruit intake regardless of obesity. J Clin Oncol. 2007;25(17):2345–51.
Hamer J, Warner E. Lifestyle modifications for patients with breast cancer to improve prognosis and optimize overall health. CMAJ. 2017;189(7):E268–74.
Irwin ML, McTiernan A, Bernstein L, Gilliland FD, Baumgartner R, Baumgartner K, Ballard-Barbash R. Physical activity levels among breast cancer patients. Med Sci Sports Exerc. 2004;36:1484–91.
Chan DS, Bandera EV, Greenwood DC, Norat T. Circulating C-Reactive Protein and Breast Cancer Risk-Systematic Literature Review and Meta-analysis of Prospective Cohort Studies. Cancer Epidemiol Biomarkers Prev. 2015;24(10):1439–49.
Suman S, Sharma PK, Rai G, Mishra S, Arora D, Gupta P, Shukla Y. Current perspectives of molecular pathways involved in chronic inflammation-mediated breast cancer. Biochem Biophys Res Commun. 2016;472(3):401–9.
Allen MD, Jones LJ. The role of inflammation in progression of breast cancer: Friend or foe? (Review). Int J Oncol. 2015;47(3):797–805.
Pecoraro M, Del Pizzo M, Marzocco S, Sorrentino R, Ciccarelli M, Iaccarino G, Pinto A, Popolo A. Inflammatory mediators in a short-time mouse model of doxorubicin-induced cardiotoxicity. Toxicol Appl Pharmacol. 2016;293:44–52.
Borrie AE, Kim RB. Molecular basis of aromatase inhibitor associated arthralgia: known and potential candidate genes and associated biomarkers. Expert Opin Drug Metab Toxicol. 2017;13(2):149–56.
Wright F, Hammer M, Paul SM, Aouizerat BE, Kober KM, Conley YP, Cooper BA, Dunn LB, Levine JD. DEramo Melkus G, Miaskowski C. Inflammatory pathway genes associated with inter-individual variability in the trajectories of morning and evening fatigue in patients receiving chemotherapy. Cytokine. 2017;91:187–210.
DeNardo DG, Coussens LM. Inflammation and breast cancer: Balancing immune response—crosstalk between adaptive and innate immune cells during breast cancer progression. Breast Cancer Res. 2007;9:212.
Asegaonkar SB, Asegaonkar BN, Takalkar UV, Advani S, Thorat AP. C-reactive protein and breast cancer: new insights from old molecule. Int J Breast Cancer. 2015;2015:145647.
Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44.
Landskron G, De la Fuente M, Thuwajit P, Thuwajit C, Hermoso MA. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res. 2014;2014:149185.
Baum J, Chen L, Chen J, et al. Arthralgia among women taking aromatase inhibitors: is there a shared inflammatory mechanism with co-morbid fatigue and insomnia? Breast Cancer Res. 2015;17:89.
Morales L, Pans S, Paridaens R, et al. Debilitating musculoskeletal pain and stiffness with letrozole and exemestane: associated tenosynovial changes on magnetic resonance imaging. Breast Cancer Res Treat. 2007;104:87–91.
Dizdar O, Ozcakar L, Malas FU, et al. Sonographic and electrodiagnostic evaluations in patients with aromatase inhibitor-related arthralgia. J Clin Oncol. 2009;27:4955–60.
Morales L, Pans S, Verschueren K, Van Calster B, Paridaens R, Westhovens R, Timmerman D, De Smet L, Vergote I, Christiaens MR, Neven P. Prospective Study to Assess Short-Term Intra-Articular and Tenosynovial Changes in the Aromatase Inhibitor-Associated Arthralgia Syndrome. J Clin Oncol. 2008;26:3147–52.
Karki R, Man SM, Kanneganti TD. Inflammasomes and Cancer. Cancer Immunol Res. 2017;5(2):94–9.
Ridker PM. From C-Reactive protein to interleukin-6 to interleukin-1: moving upstream to identify novel targets for atheroprotection. Circ Res. 2016;118(1):145–56.
Singh TP, Morris DR, Smith S, Moxon JV, Golledge J. Systematic Review and Meta-Analysis of the Association Between C-Reactive Protein and Major Cardiovascular Events in Patients with Peripheral Artery Disease. Eur J Vasc Endovasc Surg. 2017;54(2):220–33.
Ridker PM. Inflammatory biomarkers and risks of myocardial infarction, stroke, diabetes, and total mortality: implications for longevity. Nutr Rev. 2007;65(12 Pt 2):S253–9.
Mahmoud FA, Rivera NI. The role of C-reactive protein as a prognostic indicator in advanced cancer. Curr Oncol Rep. 2002;4:250–5.
Pierce BL, Ballard-Barbash R, Bernstein L, et al. Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. J Clin Oncol. 2009;27(21):3437–44.
Allin KH, Nordestgaard BG, Flyger H, et al. Elevated pre-treatment levels of plasma C-reactive protein are associated with poor prognosis after breast cancer: a cohort study. Breast Cancer Res. 2011;13(3):R55.
Han Y, Mao F, Wu Y, Fu X, Zhu X, Zhou S, Zhang W, Sun Q, Zhao Y. Prognostic role of C-reactive protein in breast cancer: a systematic review and meta-analysis. Int J Biol Markers. 2011;26(4):209–15.
Guo L, Liu S, Zhang S, Chen Q, Zhang M, Quan P, Lu J, Sun X. C-reactive protein and risk of breast cancer: a systematic review and meta-analysis. Sci Rep. 2015;5:10508.
Kapoor D, Trikha D, Vijayvergiya R, Kaul D, Dhawan V. Conventional therapies fail to target inflammation and immune imbalance in subjects with stable coronary artery disease: a system-based approach. Atherosclerosis. 2014;237(2):623–31.
van den Brandt PA, Schulpen M. Mediterranean diet adherence and risk of postmenopausal breast cancer: results of a cohort study and meta-analysis. Int J Cancer. 2017;140(10):2220–31.
Psaltopoulou T, Kosti RI, Haidopoulos D, Dimopoulos M, Panagiotakos DB. Olive oil intake is inversely related to cancer prevalence: a systematic review and a meta-analysis of 13,800 patients and 23,340 controls in 19 observational studies. Lipids Health Dis. 2011;10:127.
Estruch R, Ros E, Salas-Salvadó J, Covas MI, Corella D, Arós F, Gómez-Gracia E, Ruiz-Gutiérrez V, Fiol M, Lapetra J, Lamuela-Raventos RM, Serra-Majem L, Pintó X, Basora J, Muñoz MA, Sorlí JV, Martínez JA. Martínez-González MA; PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368(14):1279–90.
Casas R, Sacanella E, Urpí-Sardà M, Corella D, Castañer O, Lamuela-Raventos RM, Salas-Salvadó J, Martínez-González MA, Ros E, Estruch R. Long-Term Immunomodulatory Effects of a Mediterranean Diet in Adults at High Risk of Cardiovascular Disease in the PREvención con DIeta MEDiterránea (PREDIMED)Randomized Controlled Trial. J Nutr. 2016;146(9):1684–93.
Braakhuis AJ, Campion P, Bishop KS. Reducing breast cancer recurrence: the role of dietary polyphenolics. Nutrients. 2016;8:547. https://doi.org/10.3390/nu8090547.
Rigacci S, Stefani M. Nutraceutical properties of olive oil polyphenols. an itinerary from cultured cells through animal models to humans. Int J Mol Sci. 2016;17:843. https://doi.org/10.3390/ijms17060843.
Rafehi H, Ververis K, Karagiannis TC. Mechanisms of action of phenolic compounds in olive. J Diet Suppl. 2012;9(2):96–109.
Liu YN, Jung JH, Park H, Kim H. Olive leaf extract suppresses messenger RNA expression of proinflammatory cytokines and enhances insulin receptor substrate 1 expression in the rats with streptozotocin and high-fat diet-induced diabetes. Nutr Res. 2014;34(5):450–7.
Boss A, Kao CH, Murray PM, Marlow G, Barnett MP, Ferguson LR. Human intervention study to assess the effects of supplementation with olive leaf extract on peripheral blood mononuclear cell gene expression. Int J Mol Sci. 2016;17(12):E2019.
Facchini A, Cetrullo S, D’Adamo S, Guidotti S, Minguzzi M, Facchini A, Borzì RM, Flamigni F. Hydroxytyrosol prevents increase of osteoarthritis markers in human chondrocytes treated with hydrogen peroxide or growth-related oncogene α. PLoS One. 2014;9(10):e109724.
Fabian CJ, Kimler BF, Hursting SD. Omega-3 fatty acids for breast cancer prevention and patientship. Breast Cancer Res. 2015;4(17):62.
Fadus MC, Lau C, Bikhchandani J, Lynch HT. Curcumin: an age-old anti-inflammatory and anti-neoplastic agent. J Tradit Complement Med. 2016;7(3):339–46.
Molfino A, Amabile MI, Monti M, Arcieri S, Rossi Fanelli F, Muscaritoli M. The role of docosahexaenoic acid (DHA) in the control of obesity and metabolic derangements in breast cancer. Int J Mol Sci. 2016;17(4):505.
de Andrés Ares J, Cruces Prado LM, Canos Verdecho MA, Penide Villanueva L, Del Valle Hoyos M, Herdman M, Traseira Lugilde S, Velázquez Rivera I. Validation of the short form of the brief pain inventory (BPI-SF) in Spanish patients with non-cancer-related pain. Pain Pract. 2015;15(7):643–53.
Chang Q, Bournazou E, Sansone P, Berishaj M, Gao SP, Daly L, Wels J, Theilen T, Granitto S, Zhang X, Cotari J, Alpaugh ML, de Stanchina E, Manova K, Li M, Bonafe M, Ceccarelli C, Taffurelli M, Santini D, Altan-Bonnet G, Kaplan R, Norton L, Nishimoto N, Huszar D, Lyden D, Bromberg J. The IL-6/JAK/Stat3 feed-forward loop drives tumorigenesis and metastasis. Neoplasia. 2013;15(7):848–62.
Jiang J, Li K, Wang F, Yang B, Fu Y, Zheng J, Li D. Effect of marine-derived n-3 polyunsaturated fatty acids on major eicosanoids: a systematic review and meta-analysis from 18 randomized controlled trials. PLoS One. 2016;11(1):e0147351.
Calder PC. Mechanisms of action of (n-3) fatty acids. J Nutr. 2012;142(3):592S–9S.
Li K, Huang T, Zheng J, Wu K, Li D. Effect of marine-derived n-3 polyunsaturated fatty acids on C-reactive protein, interleukin 6 and tumor necrosis factor α: a meta-analysis. PLoS One. 2014;9(2):e88103.
Rangel-Huerta OD, Aguilera CM, Mesa MD, Gil A. Omega-3 long-chain polyunsaturated fatty acids supplementation on inflammatory biomakers: a systematic review of randomised clinical trials. Br J Nutr. 2012;107(Suppl 2):S159–70.
Chandran B, Goel A. A randomized, pilot study to assess the efficacy and safety of curcumin in patients with active rheumatoid arthritis. Phytother Res. 2012;26:1719–25.
Visioli F, Bernardini E. Extra virgin olive oil’s polyphenols…) with: “Bernardini E and Visioli F. High quality, good health: the case for olive oil. Eur J Lipid Sci Technol. 2017;119:1500505.
Bernini R, Gilardini Montani MS, Merendino N, Romani A, Velotti F. Hydroxytyrosol-derived compounds: a basis for the creation of new pharmacological agents for cancer prevention and therapy. J Med Chem. 2015;58(23):9089–107.
Bitler CM, et al. Olive extract supplement decreases pain and improves daily activities in adults with osteoarthritis and decreases plasma homocysteine in those with rheumatoid arthritis. Nutr Res. 2007;27:470–7.
Crespo MC, Tomé-Carneiro J, Burgos-Ramos E, Loria Kohen V, Espinosa MI, Herranz J, Visioli F. One-week administration of hydroxytyrosol to humans does not activate Phase II enzymes. Pharmacol Res. 2015;95–96:132–7.
Lopez-Huertas E, Fonolla J. Hydroxytyrosol supplementation increases vitamin C levels in vivo. A human volunteer trial. Redox Biol. 2017;11:384–9.
Baum M, Budzar AU, Cuzick J, et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet. 2002;359:2131–9.
Greenlee H, Crew KD. Shao T Phase II study of glucosamine with chondroitin on aromatase inhibitor-associated joint symptoms in women with breast cancer. Support Care Cancer. 2013;21(4):1077–87.
Shapiro AC, Adlis SA. Robien K Randomized, blinded trial of vitamin D3 for treating aromatase inhibitor-associated musculoskeletal symptoms (AIMSS). Breast Cancer Res Treat. 2016;155(3):501–12.
Hershman DL, Unger JM, Crew KD, et al. Randomized multicenter placebo-controlled trial of omega-3 fatty acids for the control of aromatase inhibitor-induced musculoskeletal pain: SWOG S0927. J Clin Oncol. 2015;33:1910–7.
Kidwell KM, Seewald NJ, Snyder CF. Crossover from one aromatase inhibitor (AI) to another in the exemestane and letrozole pharmacogenetics (ELPh) trial. J Clin Oncol. 2016;34(3_suppl):158.
Arem H, Sorkin M, Cartmel B. Exercise adherence in a randomized trial of exercise on aromatase inhibitor arthralgias in breast cancer patients: the hormones and physical exercise (HOPE) study. J Cancer Surviv. 2016;10(4):654–62.
Crew KD, Capodice J, Greenlee H, et al. Randomized, blinded, sham-controlled trial of acupuncture for the management of aromatase inhibitor-associated joint symptoms in women with early stage breast cancer. J Clin Oncol. 2010;28:1154–60.
Henry NL, Unger JM, Schott A. A randomized placebo-controlled phase III study of duloxetine for treatment of aromatase inhibitor (AI)-associated musculoskeletal symptoms in women with early-stage breast cancer: SWOG S1202. In: 39th Breast Cancer Symposium. 2016.
Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105–21.
Henry NL, Speth K, Lintermans A, Kidwell KM, Carlson R, Hayes DF, Neven P. Associations between patient and anthropometric characteristics and aromatase inhibitor discontinuation. Clin Breast Cancer. 2017;17(5):350–355.e4.
Acknowledgements
The authors thank Francesco Visioli, PhD for his advice during study design and management and for his helpful review of the manuscript, and APICES for data collection and statistical analysis.
Funding
PhytoMed Medical Foods, S.L. has privately funded this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Martinez N declares that she has no conflict of interest. Herrera M declares that she has no conflict of interest. Frías L declares that she has no conflict of interest. Provencio M declares that he has no conflict of interest. Pérez Carrión R declares that he has no conflict of interest. Díaz V declares that she has no conflict of interest. Morse M owns stock options in Oliventures. Crespo MC declares that she has no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Martínez, N., Herrera, M., Frías, L. et al. A combination of hydroxytyrosol, omega-3 fatty acids and curcumin improves pain and inflammation among early stage breast cancer patients receiving adjuvant hormonal therapy: results of a pilot study. Clin Transl Oncol 21, 489–498 (2019). https://doi.org/10.1007/s12094-018-1950-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-018-1950-0