Abstract
Arsenic trioxide (As2O3) is a highly effective therapeutic against acute promyelocytic leukaemia, but its clinical efficacy is burdened by serious cardiac toxicity. The present study was performed to evaluate the effect of omega (ω)-3 fatty acid on As2O3-induced cardiac toxicity in in vivo and in vitro settings. In in vivo experiments, male Wistar rats were orally administered with As2O3 4 mg/kg body weight for a period of 45 days and cardiotoxicity was assessed. As2O3 significantly increased the tissue arsenic deposition, micronuclei frequency and creatine kinase (CK)-MB activity. There were a rise in lipid peroxidation and a decline in reduced glutathione, glutathione peroxidase, glutathione-S-transferase, superoxide dismutase and catalase in heart tissue of arsenic-administered rats. The cardioprotective role of ω-3 fatty acid was assessed by combination treatment with As2O3. ω-3 fatty acid co-administration with As2O3 significantly alleviated these changes. In in vitro study using H9c2 cardiomyocytes, As2O3 treatment induced alterations in cell viability, lactate dehydrogenase (LDH) release, lipid peroxidation, cellular calcium levels and mitochondrial membrane potential (∆Ψm). ω-3 fatty acid co-treatment significantly increased cardiomyocyte viability, reduced LDH release, lipid peroxidation and intracellular calcium concentration and improved the ∆Ψm. These findings suggested that the ω-3 fatty acid has the potential to protect against As2O3-induced cardiotoxicity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Arsenic is a metalloid that exists in inorganic as well as organic forms. Inorganic arsenic is the most toxic form, and the International Agency for Research on Cancer has classified it as a group 1 carcinogen [1]. However, arsenic compounds have also been used for medical purposes. Arsenic trioxide (As2O3) is a potent anticancer agent with substantial efficacy against acute promyelocytic leukaemia [2]. As2O3 exerts its anticancer activity through the induction of apoptosis mediated by reactive oxygen species (ROS), oxidative stress and excessive intracellular calcium influx [3]. Although generally considered as a relatively safe therapeutic strategy, the clinical usefulness of As2O3 has been limited by its cardiotoxicity characterized by QT prolongation, ventricular tachycardia, arrhythmia, torsades de pointes and sudden cardiac death [4]. Due to these limitations, many patients are prevented from receiving this highly efficacious treatment.
An alternative approach to block the adverse effects posed by As2O3 on cardiac functioning would be to use agents that can mitigate the cardiotoxic effects. This would allow the patients to exploit the full therapeutic potential of As2O3. Omega-3 fatty acids (n-3) are long-chain polyunsaturated fatty acid (PUFA) commonly having 18, 20 or 22 carbon atoms in chain length. The most common source of n-3 PUFA is fish oil which is high in long-chain fatty acids such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Consumption of omega (ω)-3 fatty acid, specifically EPA and DHA, decreases the risk of heart failure and attenuates pathological cardiac remodelling in response to pressure overload [5]. Dietary supplementation with EPA and DHA can also influence cardiac mitochondrial function and energetics through the alteration of membrane phospholipids [6]. Evidence from cohort studies and clinical trials has indicated that a diet rich in ω-3 fatty acids reduces the plasma level of triglycerides, the incidence of cardiac arrhythmias and sudden cardiac death [7]. In addition, the benefits of fish oil supplements during cancer therapy include increasing the anticancer drug efficacy [8] and reducing the cardiac side effects of various chemotherapeutic treatments [9].
Previous studies have shown that fish oil dietary supplements are of benefit to patients undergoing cancer therapy [10]; however, it is still unclear whether EPA or DHA has a beneficial effect on As2O3 therapy, especially in terms of reducing its cardiotoxicity. Many chemical compounds were tried along with As2O3 to reduce the toxic side effects, but with limited success. Therefore, the present study was performed in vitro and in vivo to identify the protective efficacy of ω-3 fatty acid against As2O3-induced cardiac toxicity.
Materials and Methods
Chemicals and Reagents
Arsenic trioxide, sodium pyruvate, thiobarbituric acid, Triton X-100, phenazine methosulphate, nitroblue tetrazolium (NBT), bovine serum albumin, cis-4,7,10,13,16,19-docosahexaenoic acid and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma-Aldrich, Bangalore, India. ω-3 fatty acid (Maxepa® (60 % EPA and 40 % DHA)), l-aspartate, α-oxoglutarate, 2,4-dinitrophenylhydrazine, nicotinamide adenine dinucleotide (reduced), thiobarbituric acid, 5,5′-dithiobis-nitrobenzoic acid, nicotinamide adenine dinucleotide phosphate (NADPH), reduced glutathione and 1-chloro-2,4-dinitrobenzene (CDNB) were purchased from Merck Specialties Pvt. Ltd., Mumbai, India. Dulbecco’s modified Eagle’s medium (DMEM) was purchased from Gibco-BRL, Invitrogen Corporation, Carlsbad, CA, USA. Foetal bovine serum (FBS) and other supplements were purchased from Himedia Pvt. Ltd., Mumbai, India.
Animals
Male Wistar rats weighing 200–220 g were purchased from the Small Animal Breeding Station of Government Veterinary College, Mannuthy, Kerala, India, and acclimatized for 1 week. All the animals were maintained under standard laboratory conditions of temperature (25 ± 2 °C) and 12-h light and dark cycles throughout the experimental period. The rats were provided with laboratory chow (Hindustan Lever Ltd., India) and water ad libitum. Experiments were performed as per the guidelines of Institutional Animal Ethical Committee, School of Biosciences, Mahatma Gandhi University, Kerala, India (B1662009/2).
Experimental Protocol
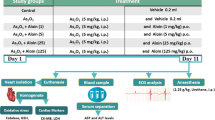
The rats were divided into four groups with six rats in each group: normal control—received normal water; ω-3 fatty acid control—received ω-3 fatty acid (50 mg/kg) body weight (b.wt); As2O3 alone administered—received 4 mg/kg b.wt of As2O3; and the combined administration of As2O3 (4 mg/kg b.wt) and ω-3 fatty acid (50 mg/kg b.wt). Two concentrations (25 and 50 mg/kg b.wt) of ω-3 fatty acid were used for combination treatment with As2O3, and the 50 mg/kg b.wt of ω-3 fatty acid was selected as the most effective dose, based on the dose–response study. One per cent Tween-80 solution was used as vehicle for ω-3 fatty acid administration. Experimental groups were daily administered As2O3 and ω-3 fatty acid for a period of 45 days through oral intubation [3]. The As2O3 dose in in vivo study was selected based on our preliminary report in which we had identified that the As2O3 concentration 4 mg/kg b.wt is the minimal dose that induced cardiotoxic events in experimental rats [11].
At the end of the experimental period, blood collected by cardiac puncture was used for micronuclei (MN) assay and for the determination of creatine kinase-MB (CK-MB). Heart was removed immediately, washed in ice-cold 0.15 M NaCl, blotted on a filter paper, then weighed and homogenized by using Teflon glass homogenizer (1/10th weight/volume) in ice-cold Tris–HCl buffer (0.2 M, pH 7.4). The homogenate was centrifuged at 10000×g for 20 min at 4 °C, and the supernatant was used for the estimations of lipid peroxidation, reduced glutathione (GSH), catalase (CAT), glutathione peroxidase (GPx), superoxide dismutase (SOD) and glutathione-S-transferase (GST).
Detection of Arsenic Accumulation in Heart Tissue
At the end of the experimental period, a portion of the rat’s heart was dissected out, digested by thermal acid microwave digestion and diluted with double-distilled water. Total arsenic deposition in heart tissue was analysed according to the Association of Official Analytical Chemists (AOAC) method by using a standard inductively coupled plasma-optical emission spectroscopy (Optima 2000 DV ICP-OES, Perkin Elmer, Inc. USA).
Serum Enzyme Analysis
CK-MB, the enzyme for cardiotoxicity, was detected by kinetic method (Span Diagnostic Ltd., India) using semi auto analyser—BCA201 (Recorders & Medicare Systems (P) Ltd. Chandigarh, India).
Cytokinesis Block Micronuclei Assay
The lymphocytes were cultured in RPMI 1640 medium containing 15 % foetal calf serum. They were then stimulated with phytohaemagglutinin and incubated for 72 h at 37 °C. Slides were prepared and examined at 100× magnification. The numbers of MN not less than 1000 binucleated cells were scored, and the distributions of MN among binucleated cells were counted [12].
Tissue Antioxidant Enzyme Analysis
The lipid peroxidation was determined by measuring the production of malondialdehyde (MDA), a thiobarbituric acid reacting substance (TBARS), using 1,1,3,3-tetramethoxypropane as standard [13]. Reduced GSH was determined according to the method of Ellman [14] based on the formation of a yellow-coloured complex with DTNB. GPx was determined by measuring the decrease in GSH content after incubating the sample in the presence of H2O2 and NaN3 [15]. GST activity was determined from the rate of increase in conjugate formation between reduced glutathione and CDNB [16]. SOD was measured by the method of Kakkar et al. [17]. Tissue CAT was determined from the rate of decomposition of H2O2 [18].
Histopathology
Heart sections of 5–6 µm were stained with haematoxylin and eosin and examined for histopathological changes using microscope (Motic AE 21, Wetzlar, Germany). The microphotographs were taken using Moticam 1000 (Burtons Medical Equipment Ltd., Kent, UK) at the original magnification of 100×. Subsequently, a blinded pathologist examined microscopic slides from each group (n = 6). Interstitial oedema, fibre separations, capillary congestion and microhaemorrhages in myocardial tissue were scored from 0 to 4 as follows: 0 = no lesion; 1+ = lesions involving <25 % of the myocardium; 2+ = lesions involving 25–50 %; 3+ = lesions involving 50–75 %; and 4+ = lesions involving 75–100 %.
In Vitro Studies
Preparation of Drugs
As2O3 was prepared as a 10 mM aqueous solution and diluted in complete growth medium to the working concentration of 10 μΜ before use. Stock solutions of ω-3 fatty acids (140 mM) were prepared in 99 % ethanol, stored in oxygen-free light-protected vials at −70 °C and diluted in complete growth medium with 10 % FBS before use in such a way that the final concentration of ethanol was less than 0.07 % (v/v). On the day of experiment, test solutions were prepared by diluting the stock solutions in DMEM medium containing 10 % (v/v) FBS and 1X antibiotic–antimycotics to give different concentrations.
Cell Culture and Treatment
In vitro experiments were performed with cardiomyocytes H9c2 (National Centre for Cell Science, Pune, India). H9c2 cells were cultured in DMEM and grown as a monolayer containing hydroxyl ethyl piperazine ethane sulphonic acid and sodium bicarbonate supplemented with 10 % FBS and 1X antibiotic–antimycotics. Cells were maintained in a tissue culture flask and kept in a humidified incubator (5 % CO2 in air at 37 °C) with a medium change every 2–3 days. When the cells reached 70–80 % confluence, they were harvested with trypsin–EDTA (ethylene diamine tetra acetate) and seeded into a new tissue culture flask.
The experimental groups consisted of (a) control cells; (b) cells treated with ω-3 fatty acid (100 µM); (c) cells treated with As2O3 (10 μM); and (d) cells treated with combination of As2O3 (10 μM) and ω-3 fatty acids (100 μM) for 24 h. As2O3 (10 µM) concentration used in this in vitro investigation was equivalent to the anticancer dosage [19]. All the in vitro experiments were performed as triplicate sets.
MTT Assay
The cell viability was assessed by MTT assay, which determines the metabolically active mitochondria of cells. Cells were seeded in 96-well plates (Greiner, Frickenhausen, Germany) with 5 × 103 cells/100 µL and incubated for 24 h at 37 °C. The cells were then treated with As2O3 (10 μM), DHA (50, 100 and 150 μM) and ethanol (0.07 % v/v) and incubated for another 24 h at 37 °C in 5 % CO2 atmosphere. The assay was performed by the addition of premixed MTT reagent, to a final concentration of 10 % of total volume (final concentration 0.5 mg/ml), to culture wells containing various concentrations of the test substance, and incubated for further 4 h. During 4-h incubation, living cells converted the tetrazolium component of the dye solution into a formazan product. The supernatant was removed and the solubilization/stop solution was then added to the culture wells to solubilize the formazan product. The absorbance at 570 nm was recorded using a 96-well plate reader (Bio-Rad, Hercules, CA, USA). The average absorbance of control samples was normalized to 100 %.
LDH Release
LDH, a stable cytosolic enzyme, is rapidly released into the culture medium after disruption of the plasma membrane. Release of LDH was measured (to test for the loss of plasma membrane integrity) by using an in vitro toxicology lactic dehydrogenase assay-based kit (Sigma-Aldrich, Inc., St. Louis, USA). Briefly, cells were seeded at 1 × 106 cells/cm2 and incubated with 10 μM concentration of As2O3 and 100 μM of DHA for 24 h. Then, the plates were centrifuged at 250×g for 4 min to pellet, and the assay was performed as per manufacturer’s instructions. LDH activity was assessed by measuring the density of the sample medium at 490 nm.
Lipid Peroxidation
Lipid peroxidation was used as a surrogate for detecting the formation of reactive oxygen species. H9c2 cells were treated with 10 μM concentration of As2O3 for 24 h to six-well plates to determine the production of (ROS). Furthermore, cells were treated with 10 μM concentration of As2O3 in combination with 100 μM of DHA to determine the protective effect of DHA against lipid peroxides. Cells were removed from the flask using trypsin and centrifuged at 800×g. The pelleted cells were rinsed with cold PBS and pelleted. These cells were resuspended in methanol/H2O (2:0.8) (V:V). To extract the lipids, chloroform was added to the samples, vortexed for 30 s and kept at room temperature until the organic and aqueous phases separated (about 5 min). The chloroform layer containing the lipids was collected, and the lipid hydroperoxides in each of the samples were determined by PeroxiDetect kit (Sigma-Aldrich, Inc., St. Louis, USA), according to the manufacturer’s directions (560 nm).
Detection of Intracellular Calcium
H9c2 cells were incubated with As2O3 and DHA for 24 h. After treatment, the cells (~5 × 106) were centrifuged at 1000–2000×g for 10 min at 4 °C. The cell pellet was homogenized in 0.5–1 ml cold 100 mM Tris buffer (pH 7.5) and centrifuged at 10,000×g for 15 min at 4 °C. Cellular calcium levels were determined by calcium assay kit (Cayman Chemical Company, Ann Arbor, USA) according to the manufacturer’s instructions.
Mitochondrial Membrane Potential Assay
The mitochondrial membrane potential (∆Ψm) was assayed using JC-1 (5,5′,6,6′-tetrachloro-1,1′,3,3-tetraethyl benzimidazolyl carbocyanine iodide) mitochondrial potential sensor according to the manufacturer’s directions (JC-1 mitochondrial membrane potential assay kit, Cayman Chemical Company, Ann Arbor, USA). Briefly, H9c2 cells were cultured for 24 h in 24-well plates (Greiner, Germany), and the cells were treated with 10 μM concentration of As2O3 and 100 μM of DHA for 24 h. The treated cells were washed with PBS and incubated for 30 min in medium containing JC-1 at a concentration of 50 μl/ml. The cells were then examined and photographed using a fluorescence microscope (Eclipse Ni-E, Nikon Instruments Inc., Melville, USA).
Statistical Analysis
All parameters were compared by one-way analysis of variance (ANOVA) followed LSD post hoc multiple comparison test to assess differences between groups. The significance of the results was considered when p values were <0.05. Two-way ANOVA was also performed to test for interactions between As2O3 and ω-3 fatty acid treatment. Data are expressed as means ± standard deviations (SD). Statistical analysis was performed with the SPSS 18.0 statistical package for Windows (SPSS/PC+ version 18, SPSS Inc., Chicago, IL, USA).
Results
Arsenic Deposition in Heart
Identification of the arsenic concentration in tissues will help to understand about its accumulation in tissues. As shown in Fig. 1, arsenic deposition in heart tissue of rats treated with As2O3 was increased (p < 0.05) with respect to the control group. Co-treatment with ω-3 fatty acid resulted in a significant decrease (p < 0.05) in arsenic deposition. Combination treatment showed a significant interaction between ω-3 fatty acid and As2O3 on arsenic deposition (p < 0.001).
Effect on myocardial enzyme
CK-MB is a marker enzyme for cardiac toxicity. CK-MB was significantly (p < 0.05) increased in arsenic-administered rats. ω-3 fatty acid co-administration with As2O3 significantly reduced (p < 0.05) the CK-MB release (Fig. 2). Also there was a significant interaction between ω-3 fatty acid and As2O3 on CK-MB release which was identified by two-way ANOVA (p < 0.001).
Effect on Lipid Peroxidation and Antioxidant Defence System
Lipid peroxidation was used to study the As2O3-induced oxidative degradation of lipids. A significant (p < 0.05) increase in lipid peroxidation was observed in As2O3-treated rats. Co-administration of ω-3 fatty acid significantly reduced (p < 0.05) the lipid peroxidation (Table 1). Detoxification of arsenic was assessed by screening the body’s thiol containing nonenzymatic antioxidant GSH and GSH-dependent antioxidant enzymes (GPx and GST). As2O3 administration significantly (p < 0.05) reduced the GSH, GPx and GST when compared to the control group (Table 2). ω-3 fatty acid co-treatment resulted in a significant (p < 0.05) maintenance of GSH, GPx and GST activity. The antiperoxidative enzymes (SOD and CAT) were significantly decreased (p < 0.05) in As2O3-treated rats, compared with control groups. Co-treatment with ω-3 fatty acid significantly corrected (p < 0.05) the SOD and CAT (Table 2). In two-way ANOVA, significant interaction (p < 0.001) between ω-3 fatty acid and As2O3 treatments on lipid peroxidation, GSH, GPx, GST, SOD and CAT was identified.
Cytokinesis Block Micronuclei Assay
In order to evaluate the genetic damage, the MN frequency of various experimental groups was measured. Figure 3 depicts the frequency of MN which is significantly increased (p < 0.05) in As2O3-administered rats. ω-3 fatty acid co-treatment significantly (p < 0.05) reduced the MN frequency induced by As2O3. A significant (p < 0.001) interaction between ω-3 fatty acid and As2O3 treatment on MN frequency was observed in two-way ANOVA.
Histopathology
The heart tissue samples from As2O3-treated group showed characteristic features of cardiotoxicity manifested in the form of cardiac structural abnormalities, i.e. fibre swelling-near epicardium and endocardium, interstitial oedema, fibre separations, capillary congestion and microhaemorrhages (Fig. 4c). Statistical analysis of the histological scores showed a significant (p < 0.05) level of tissue damage in As2O3-treated rats heart tissue (Table 3). The cardiac tissue of As2O3+ ω-3 fatty acid group showed only minor changes (p < 0.05). Hence, the treatment was found to be effective in preserving the normal cellular morphology (Fig. 4d). Histopathological scoring analysis represents a significant reduction (p < 0.05) in cardiac structural aberrations with ω-3 fatty acid co-treatment (Table 3). Both the control groups showed normal myocardial architecture (Fig. 4a, b).
Protective Effect of DHA on As2O3-Induced Cytotoxicity
In combination treatment with 10 µM As2O3, DHA at different concentrations (50, 100 and 150 µM) showed significant cytoprotective effect on H9c2 cells. The protective effect of different concentrations of DHA was in the following order: DHA 100 μM > DHA 50 μM > DHA 150 μM (Fig. 5). LDH is a cytosolic enzyme and an effective indicator of cellular toxicity. In order to study membrane integrity, LDH leakage of various experimental groups was measured. The LDH release was significantly increased in As2O3 (10 µM)-treated cardiomyocytes, indicating cellular necrosis. Co-treatment with the DHA (100 µM) significantly protected the H9c2 cells (Fig. 6). A significant (p < 0.001) interaction between DHA and As2O3 on LDH release was noticed in two-way ANOVA.
Protective effect of DHA on As2O3-induced cytotoxicity: Data represented as mean ± SD, n = 3. aStatistically significant difference when compared to the control group at p < 0.05 level. bStatistically significant difference when compared to the As2O3 −10 μM at p < 0.05 level. cStatistically significant difference when compared to As2O3 −10 μM at p < 0.001 level
Protective Effect of DHA on As2O3-Induced Lipid Peroxidation
Oxidative degradation of lipids with As2O3 and DHA treatment in H9c2 cardiomyocytes was assessed with lipid peroxidation assay. In H9c2 cardiomyocytes, 10 µM of As2O3 treatment for 24 h significantly induced lipid peroxidation. The co-treatment with DHA (100 µM) significantly (p < 0.05) attenuated the lipid peroxy radical production. In two-way ANOVA, a significant (p < 0.001) interaction between DHA and As2O3 treatment on lipid peroxidation was observed (Fig. 7).
Effect of DHA on As2O3-Altered Intracellular Calcium
Intracellular Ca2+ that regulates cardiac rhythm and signalling activities was assessed in H9c2 cardiomyocytes. As2O3 (10 µM) treatment for a period of 24 h significantly increased the intracellular calcium concentration in H9c2 cells. DHA co-treatment with As2O3 maintained the intracellular concentration to near normal level (Fig. 8). A significant (p < 0.01) interaction between DHA and As2O3 on cellular calcium concentration was identified in H9c2 cardiomyocytes by two-way ANOVA.
Mitochondrial Membrane Potential by JC-1 Staining in H9c2
Exposure of H9c2 cells to As2O3 produced significant alteration in the mitochondrial ∆Ψm as indicated by the change in fluorescence of the JC-1 probe. In healthy control cells with high mitochondrial ∆Ψm, JC-1 complexes (J-aggregates) were identified in intense red fluorescence. As2O3 caused an increase in the intensity of green fluorescence when compared to control H9c2 cells. The ω-3 fatty acid DHA co-treated cells showed significantly improved mitochondrial ∆Ψm (p < 0.05) (Fig. 9).
Mitochondrial membrane potential by JC-1 staining in H9c2: As2O3-induced loss of membrane potential and its maintenance with the co-treatment with DHA in H9c2 cells for a period of 24-h treatment. Healthy cells with high ΔΨm in red fluorescence and apoptotic or unhealthy cells with low ΔΨm in green fluorescence. Original magnification ×200
Discussion
Our findings showed that the administration of As2O3 increased the deposition of arsenic in heart tissue. Arsenic deposited in tissues is capable of binding to thiol or sulfhydryl groups of proteins, thereby inactivating them. This includes the proteins mainly involved in cellular energy pathways and DNA synthesis and repair [20]. The cytoskeleton has also been suggested as a potential cellular target for arsenic because of its major constituent tubulin which has relatively high sulfhydryl content [21]. In the present study, co-administration of ω-3 fatty acid significantly reduced the arsenic deposition in heart tissue possibly due to its effective action on antioxidant defence status [22, 23].
In the current investigation, a significant increase in CK-MB, a well-known diagnostic marker of cardiac toxicity, was observed with As2O3 administration. Yeh et al. [24] reported that the treatment with arsenic showed significant increase in serum CK-MB and cardiac lipid peroxidation. So the increased release of marker enzyme from the damaged myocardium into systemic circulation may be due to the arsenic-induced myocardial toxicity. ω-3 fatty acid co-treatment was found to decrease the CK-MB level, possibly by reducing the As2O3-induced cellular toxic effects.
Micronucleus is the small nucleus that forms whenever a chromosome or a fragment of a chromosome is not incorporated into one of the daughter nuclei during cell division. A significantly elevated rate of MN frequency was observed in arsenic-treated groups. The arsenic–protein binding theory of carcinogenesis describes events that initiate with arsenic–SH binding at various enzymatic sites, followed by downstream events that include enzymes that alter signal transcription and cellular redox status, cause DNA methylation and impaired DNA repair and induce chromosomal aberrations [25]. The reduction in MN frequency on treatment with ω-3 fatty acid observed in our study may be due to its antigenotoxicity. Li et al. [26] reported that the ω-3 fatty acid showed antigenotoxic activity by reducing the anticancer drug cyclophosphamide-induced MN formation.
The ability of glutathione to react with electrophiles directly or as a cofactor (for enzymes GPx and GST) plays an important role in arsenic detoxification and the arsenic-induced oxidative stress [27]. In the current investigation, GSH, GPx and GST concentrations were reduced and the lipid peroxidation was increased with As2O3 treatment. The arsenic-induced oxidative stress observed in our study may directly be by the depletion of GSH or independently by inhibition of GPx and GST or by generation of free radicals higher than the normal level. DHA treatment may help to enhance the upregulation of intracellular GSH and suppress the accumulation of intracellular peroxides [28]. The protective treatment with ω-3 fatty acid significantly reduced the arsenic-induced lipid peroxidation and increased the GSH concentration. This may be mediated by the activity of GPx, which converts H2O2 into water and therefore reduced the peroxidative damage. Decreased activities of the antiperoxidative enzymes may lead to formation of ROS and bring about a number of reactions harmful to the cellular and subcellular membranes in the heart tissue [29]. In the present study, the decreased CAT and SOD activity indicates that the cells ability to detoxify H2O2 was impaired due to the increased arsenic exposure. Fish oil containing n-3 fatty acid supplementation in mice has shown to enhance the activities and mRNA levels of CAT, GPx and SOD in hepatic and other tissues of mice [23]. In the current study, ω-3 fatty acid co-supplementation decreased the arsenic-induced ROS and lipid peroxidation by increasing the SOD and CAT concentration.
Administration of As2O3 produced clear structural changes in myocardium which confirms cardiotoxicity. These histological alterations may be the outcome of As2O3-induced cellular manifestations such as loss of cardiac actin, reduced size, damage to the nuclei, increased lipid peroxidation and oxidative stress [30]. ω-3 fatty acid supplementation ameliorated the oxidative stress and cellular damage by increasing the CAT. This may also be due to the replacement of O2 −, H2O2 and OH• radicals attacked PUFA components in the membrane with new ω-3 fatty acids [22, 31]. In the present study, the co-administration of ω-3 fatty acid reduced the structural abnormalities which could be attributed to its antioxidant property. The dose of ω-3 fatty acid used in our study is similar to that recommended by World Health Organization (WHO) and American Heart Association (AHA) for humans (approximated ratio of 50 mg/kg/day) [32–34].
The reduction in cell viability and increase in LDH release in H9c2 cardiomyocytes in in vitro experiments may be due to arsenic toxicity. The generation of ROS and induction of calcium overload in As2O3-treated cells are the key initial molecular events implicated in the molecular aetiology of apoptosis in myocardium [19]. Lipid peroxidation was significantly increased following As2O3 administration, and this result was consistent with the mechanism of free radical-mediated cardiotoxicity of As2O3 [35]. Our result also showed that co-treatment with ω-3 fatty acid attenuated the increase in peroxy radical production in As2O3-treated cardiomyocytes. This indicated that ω-3 fatty acid might effectively reduce As2O3-induced ROS generation by stabilizing the antioxidant enzymes and therefore improving the cell viability and decreasing the LDH release.
In the current investigation, As2O3 treatment significantly increased the Ca2+ concentration in H9c2 cardiomyocytes. Raghu and Cherian [36] reported that As2O3 treatment induces ROS generation and the induction of calcium overload in cardiac myocytes leading to apoptosis. ROS increases the Ca2+ influx through the L-type calcium channel and leads to cytosolic Ca2+ accumulation. The potent inhibition of the L-type Ca2+ current by the PUFAs should prevent excessive cytosolic Ca2+ fluctuations and the likelihood of fatal outcomes [36]. As2O3-induced elevation in the intracellular Ca2+ concentration was effectively reduced with ω-3 fatty acid, and this seems to be due to its ability to prevent calcium overload by maintaining the activity of L-type calcium channels.
As2O3-induced apoptotic or unhealthy cells having low ΔΨm were identified in green fluorescence with JC1 staining. An increase in mitochondrial Ca2+ concentration depolarizes mitochondrial inner membrane potential, produces ROS and triggers the mitochondrial permeability transition pore (mPTP) opening [37]. This may follow the subsequent release of apoptogenic proteins into the cytosol from the matrix leading to loss of ΔΨm, osmotic swelling, rupture of the outer mitochondrial membrane and cardiomyocyte apoptosis [38]. O’Shea et al [39] reported that dietary supplementation with EPA and DHA increased mitochondrial membrane phospholipid fatty acid composition and delayed mPTP opening in heart. Khairallah et al [40] also reported that supplementation with DHA profoundly altered mitochondrial phospholipid fatty acid composition and delayed Ca2+-induced mPTP opening. In the present study, co-treatment with ω-3 fatty acid significantly improved ΔΨm which might be due to the alteration in the membrane phospholipid fatty acid composition and the reduction in Ca2+ accumulation.
Conclusion
In summary, the findings from the present study reveals that co-administration of ω-3 fatty acid has the potential for protecting and stabilizing cardiac tissue against As2O3-induced cardiotoxicity in vivo and in vitro. ω-3 fatty acid treatment significantly reduced the oxidative stress by improving the antioxidant enzyme activity and controlled intracellular Ca2+ concentration, thereby stabilizing the ΔΨm. However, further studies are warranted for the identification of the precise molecular mechanism of arsenic binding in heart tissue and the protective action of ω-3 fatty acid against arsenic deposition.
References
IARC. (1987). Overall evaluations of carcinogenicity: an updating of IARC Monographs volumes 1 to 42. IARC Monogr Eval Carcinog Risks Hum Suppl., 7, 1–440.
Iland, H. J., & Seymour, J. F. (2013). Role of arsenic trioxide in acute promyelocytic leukemia. Current Treatment Options in Oncology, 14(2), 170–184.
Huan, S. Y., Yang, C. H., & Chen, Y. C. (2000). Arsenic trioxide therapy for relapsed acute promyelocytic leukemia: an useful salvage therapy. Leukaemia & Lymphoma, 38(3–4), 283–293.
Barbey, J. T., & Soignet, S. (2001). Prolongation of the QT interval and ventricular tachycardia in patients treated with arsenic trioxide for acute promyelocytic leukemia. Annals of Internal Medicine, 135, 842–843.
Teng, L. L., Shao, L., Zhao, Y. T., Yu, X., Zhang, D. F., & Zhang, H. (2010). The beneficial effect of n-3 polyunsaturated fatty acids on doxorubicin-induced chronic heart failure in rats. Journal of International Medical Research, 38(3), 940–948.
Stanley, W. C., Khairallah, R. J., & Dabkowski, E. R. (2012). Update on lipids and mitochondrial function: impact of dietary n-3 polyunsaturated fatty acids. Curr Opin Clin Nutr Metab Care., 15, 122–126.
Leaf, A., Xiao, Y. F., Kang, J. X., & Billman, G. E. (2003). Prevention of sudden cardiac death by n-3 polyunsaturated fatty acids. Pharmacology & Therapeutics, 98, 355–377.
Hardman, W. E. (2002). Omega-3 fatty acids to augment cancer therapy. Journal of Nutrition, 132, 3508s–3512s.
Merendino, N., Costantini, L., Manzi, L., Molinari, R., D’Eliseo, D., & Velotti, F. (2013). Dietary omega-3 polyunsaturated fatty acid DHA: A potential adjuvant in the treatment of cancer. Biomedicine Research International, 2013, 310186.
Hardman, W. E., Avula, C. P., Fernandes, G., & Cameron, I. L. (2001). Three percent dietary fish oil concentrate increased efficacy of doxorubicin against MDA-MB 231 breast cancer xenografts. Clinical Cancer Research, 7(7), 2041–2049.
Mathews, V. V., Paul, M. V., Abhilash, M., Manju, A., Abhilash, S., & Nair, R. H. (2013). Myocardial toxicity of acute promyelocytic leukaemia drug-arsenic trioxide. European Review for Medical and Pharmacological Sciences, 1, 34–38.
Fenech, M. (1993). The cytokinesis-block micronucleus technique: a detailed description of the method and its application to genotoxicity studies in human populations. Mutation Research, 285, 35–44.
Buege, J. A., & Aust, S. D. (1978). The thiobarbituric acid assay. Methods in Enzymology, 52, 306–307.
Ellman, G. L. (1959). Tissue sulfhydryl groups. Archives of Biochemistry and Biophysics, 82(1), 70–77.
Rotruck, J. T., Pope, A. L., Ganther, H. E., Swanson, A. B., Hafeman, D. G., & Hoekstra, W. G. (1973). Selenium: Biochemical role as a component of glutathione peroxidase. Science, 179, 588–590.
Habig, W. H., Pabst, M. J., & Jakoby, W. B. (1974). Glutathione S-transferase, the first enzymatic step in mercapturic acid formation. Journal of Biological Chemistry, 249(22), 7130–7139.
Kakkar, P., Das, B., & Viswanathan, P. N. (1984). A modified spectrophotometric assay of superoxide dismutase. Indian Journal of Biochemistry Biophysics, 21(2), 130–132.
Aebi, H. (1974). Catalase. In H. U. Bergmeyer (Ed.), Methods of enzymatic analysis (Vol. 2, pp. 673–678). New York: Academic Press.
Zhao, X. Y., Li, G. Y., Liu, Y., Chai, L. M., Chen, J. X., Zhang, Y., et al. (2008). Resveratrol protects against arsenic trioxide-induced cardiotoxicity in vitro and in vivo. British Journal of Pharmacology, 154(1), 105–113.
Ratnaike, R. N. (2003). Acute and chronic arsenic toxicity. Postgraduate Medical Journal, 79(933), 391–396.
Li, Y. M., & Broome, J. D. (1999). Arsenic targets tubulins to induce apoptosis in myeloid leukemia cells. Cancer Research, 59, 776–780.
Mathews, V. V., Paul, M. S., Abhilash, M., Manju, A., Abhilash, S., & Nair, R. H. (2014). Mitigation of hepatotoxic effects of arsenic trioxide through omega-3 fatty acid in rats. Toxicology and Industrial Health, 30(9), 806–813.
Bhattacharya, A., Lawrence, R. A., Krishnan, A., Zaman, K., Sun, D., & Fernandes, G. (2003). Effect of dietary n-3 and n-6 oils with and without food restriction on activity of antioxidant enzymes and lipid peroxidation in livers of cyclophosphamide treated autoimmune-prone NZB/W female mice. Journal of the American College of Nutrition, 22(5), 388–399.
Yeh, J. Y., Cheng, L. C., Ou, B. R., Whanger, D. P., & Chang, L. W. (2002). Differential influences of various arsenic compounds on glutathione redox status and antioxidative enzymes in porcine endothelial cells. Cellular and Molecular Life Sciences, 59(11), 1972–1982.
Flora, S. J. (2011). Arsenic-induced oxidative stress and its reversibility. Free Radical Biology and Medicine, 51(2), 257–281.
Li, M., Zhu, Q., Hu, C., Giesy, J. P., Kong, Z., & Cui, Y. (2011). Protective effects of eicosapentaenoic acid on genotoxicity and oxidative stress of cyclophosphamide in mice. Environmental Toxicology, 26(3), 217–223.
Thompson, J. A., White, C. C., Cox, D. P., Chan, J. Y., Kavanagh, T. J., Fausto, N., & Franklin, C. C. (2009). Distinct Nrf1/2-independent mechanisms mediate As 3+-induced glutamatecysteine ligase subunit gene expression in murine hepatocytes. Free Radical Biology and Medicine, 46, 1614–1625.
Komatsu, W., Ishihara, K., Murata, M., Saito, H., & Shinohara, K. (2003). Docosahexaenoic acid suppresses nitric oxide production and inducible nitric oxide synthase expression in interferon-gamma plus lipopolysaccharide-stimulated murine macrophages by inhibiting the oxidative stress. Free Radical Biology and Medicine, 34(8), 1006–1016.
Kumar, S. H. S., & Anandan, R. (2007). Biochemical studies on the cardioprotective effect of glutamine on tissue antioxidant defense system in isoprenaline-induced myocardial infarction in rats. Journal of Clinical Biochemistry and Nutrition, 40(1), 49–55.
Hays, A. M., Lantz, R. C., Rodgers, L. S., Sollome, J. J., Vaillancourt, R. R., Andrew, A. S., et al. (2008). Arsenic-induced decreases in the vascular matrix. Toxicologic Pathology, 36, 805–817.
Iraz, M., Erdogan, H., Ozyurt, B., Ozugurlu, F., Ozgocmen, S., & Fadillioglu, E. (2005). Brief communication: omega-3 essential fatty acid supplementation and erythrocyte oxidant/antioxidant status in rats. Annals of Clinical and Laboratory Science, 35(2), 169–173.
World Health Organization (2015) Interim summary of conclusions and dietary recommendations on total fat. http://www.who.int/nutrition/topics/FFA_summary_rec_conclusion.pdf. Accessed December 18, 2015.
American Heart Association, “Fish 101,” (2014).http://www.heart.org/HEARTORG/GettingHealthy/NutritionCenter/Fish-101_UCM_305986_Article.jsp. Accessed December 18, 2015.
Fappi, A., Godoy, T. S., Maximino, J. R., Rizzato, V. R., de Neves, J. C., Chadi, G., & Zanoteli, E. (2014). The effects of omega-3 fatty acid supplementation on dexamethasone-induced muscle atrophy. Biomedicine Research International, 2014, 961438.
Zhao, X., Feng, T., Chen, H., Shan, H., Zhang, Y., Lu, Y., & Yang, B. (2008). Arsenic trioxide-induced apoptosis in H9c2 cardiomyocytes: implications in cardiotoxicity. Basic & Clinical Pharmacology & Toxicology, 102(5), 419–425.
Raghu, K. G., & Cherian, O. L. (2009). Characterization of cytotoxicity induced by arsenic trioxide (a potent anti-APL drug) in rat cardiac myocytes. Journal of Trace Elements in Medicine and Biology, 23, 61–68.
Crow, M. T., Mani, K., Nam, Y. J., & Kitsis, R. N. (2004). The mitochondrial death pathway and cardiac myocyte apoptosis. Circulation Research, 95, 957–970.
Halestrap, A. P. (2009). What is the mitochondrial permeability transition pore? Journal of Molecular and Cellular Cardiology, 46, 821–831.
O’Shea, K. M., Khairallah, R. J., Sparagna, G. C., Xu, W., Hecker, P. A., Robillard-Frayne, I., et al. (2009). Dietary omega-3 fatty acids alter cardiac mitochondrial phospholipid composition and delay Ca2+-induced permeability transition. Journal of Molecular and Cellular Cardiology, 47(6), 819–827.
Khairallah, R. J., Sparagna, G. C., Khanna, N., O’Shea, K. M., Hecker, P. A., Kristian, T., et al. (2010). Dietary supplementation with docosahexaenoic acid, but not eicosapentaenoic acid, dramatically alters cardiac mitochondrial phospholipid fatty acid composition and prevents permeability transition. Biochimica et Biophysica Acta, 1797(8), 1555–1562.
Acknowledgments
This work was supported by University Grants Commission, New Delhi (F. No.: 39-683/2010SR), and awarded the research fellowship in sciences for meritorious student to Mr. Mathews V. Varghese (F. No.: 4-1/2006 (BSR)/11-29/2008(BSR)). We are grateful to Prof. C.C Kartha, Professor of Eminence, Cardiovascular Disease Biology, Rajiv Gandhi Centre for Biotechnology, Trivandrum, for providing laboratory facilities during in vitro studies.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Varghese, M.V., Abhilash, M., Paul, M.V.S. et al. Omega-3 Fatty Acid Protects Against Arsenic Trioxide-Induced Cardiotoxicity In Vitro and In Vivo. Cardiovasc Toxicol 17, 109–119 (2017). https://doi.org/10.1007/s12012-016-9361-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-016-9361-3