Abstract
Gut microbiota have a variety of health and nutritional benefits in their host organisms. Their beneficial effects are related to gastrointestinal diseases, immunomodulation, intestinal microbial balance, and antimicrobial properties. However, their properties also involve an influence on the bioavailability of certain drugs and food components in the body. Phytoestrogens are nonsteroidal secondary metabolites with estrogenic activity. They are found in various foods, especially soy, and are used for the treatment of estrogen-associated diseases such as menopause, cardiovascular diseases, breast cancer, and osteoporosis. Some metabolites produced by gut microbiota from phytoestrogens have even stronger effects due to their higher lipophilicity, which leads to a better absorption and a higher affinity with estrogen receptors. The crucial metabolism of phytoestrogens takes place in the gastrointestinal tract where the gut microbiota are present. Probiotics are live microorganisms that can confer health benefits to the host when administered in adequate amounts. They are present in milk products and dietary supplements, and are capable of restoring the gut microbial communities when ingested. Most of the probiotics are bacteria and thus their intake can enhance the metabolism of phytoestrogens and, therefore, enhance their pharmacological effects. In this review, we summarize the influence of gut microbiota on the metabolism of phytoestrogens and their beneficial effects on the host.

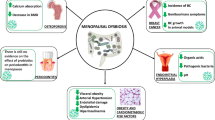
Graphical Abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Throughout history, plants have been used as medicines. Even today, their beneficial effects are recognized and used in modern medicine (Athni and Athni 2019). Plants have diverse chemical compositions, which are responsible for their varied pharmacological activity. A lot of those chemical compositions are FDA approved, and one-third of the new molecular entities are from natural origin (Patridge et al. 2016). Phytoestrogens are nonsteroidal plant secondary metabolites with estrogenic activity, which are present in a wide variety of foods such as flax seeds, soy, and other legumes; they show physiological similarity to 17-β-estradiol, the primary female sex hormone (Rietjens et al. 2017).
One of the crucial factors that may affect for the activity of medicinal plants is the metabolism of their active ingredients, which takes place in the gastrointestinal tract and in the liver. In the gastrointestinal tract, there is a complex community of bacteria (gut microbiota) that are essential for the host’s health. They contribute to normal gastrointestinal function and play a fundamental role in preserving immune and metabolic homeostasis, and other functions as offering protection against pathogens (Thursby and Juge 2017). An absence of intestinal bacteria is associated with reductions in important metabolic contributions to vitamin K, folate, and short-chain fatty acids (Canny and McCormick 2008).
Lactic acid bacteria (LAB) are the most common inhabitants of the human gastrointestinal tract and include lactobacilli, lactococci, streptococci, enterococci, leuconostoc, and pediococci. Other important genera in gut microbiota are the bifidobacteria. These genera differ in their morphology, pH, salt tolerance, optimal temperature, habitats, and pathogenic potential (Pessione 2012). Gut microbiota play a key role in the metabolism of phytoestrogens and their transformation to therapeutically active substances. Phytoestrogens are not produced by the endocrine system but are consumed in food and, therefore, they are also called dietary estrogens. Isoflavones, ellagitannins, and lignans are the main groups of phytoestrogens, and are metabolized by intestinal bacteria into equol, urolithins, and enterolignans, respectively (Gaya et al. 2016a). Probiotics are living microorganisms that are found in milk dietary supplements and can modulate the balance of the microbiota by repopulating the gut. Their colonization in the gut can contribute to the metabolism of phytoestrogens; thus, the consumption of phytoestrogen-rich foods, together with selected probiotic bacteria, may increase the production of bioactive metabolites (Landete et al. 2017).
Due to the structural similarity to the endogenous estrogen 17-β-estradiol (1), phytoestrogens interact with estrogen receptors (ER) causing estrogenic and antiestrogenic effects (Peirotén et al. 2019b). A wide range of beneficial health effects have been attributed to phytoestrogens, with evidence from molecular and cellular experiments, animal studies, and human clinical trials showing that phytoestrogens may potentially confer health benefits related to cardiovascular diseases, cancer, osteoporosis, and menopausal symptoms (Desmawati and Sulastri 2019; Tham et al. 1998). However, the phytoestrogens may cause disruption of the endocrine system, indicating that they may have the potential to cause side effects as well (Patisaul and Jefferson 2010).

Health Benefits and Chemical Properties of Phytoestrogens
Phytoestrogens are common phytochemical molecules that are widely distributed in the plant kingdom. They are present in our daily diet in foods such as fruits (grapes, plum, pear, and apple), vegetables (soybeans, kidney beans, cabbage, spinach, hops, garlic, and onion), wine, and tea; also, they have been identified in a number of botanical dietary supplements (Bacciottini et al. 2007). The intake of phytoestrogens through food is varied in different regions of the world due to changes in local diet. For example, in Asian countries where fermented soy products are part of the traditional diet, isoflavone intake levels may aggregate to about 15–50 mg of isoflavones per day, but in the Western countries, the isoflavone intake can be less than 2 mg isoflavones per day (Eisenbrand and Senate Commission on Food Safety of the German Research 2007). It was found that Asian populations historically have lower risks of cardiovascular disease, menopausal symptoms, breast cancer (and other hormone-dependent cancers), diabetes, and obesity than Western populations (Adlercreutz and Mazur 1997).
There are many different phytoestrogens found in plants, but the most common ones occurring in the diet and in food supplements, are the isoflavones daidzein (2), formononetin (3), genistein (4), and biochanin (5). The ellagitannins are a diverse class of hydrolyzable tannins that originated from vegetables and medicinal plants, as hexahydroxydiphenic acid (6), that undergo metabolic transformations ending in urolithins. Plant lignan, sometimes called lignan precursors, include pinoresinol (7), lariciresinol (8), secoisolariciresinol (9), and matairesinol (10) (Rietjens et al. 2017). Enterolignans, as enterodiol (11) and enterolactone (12), are formed from plant lignans by intestinal bacteria and displayed phytoestrogenic activity (Lampe 2003).

The effect of phytoestrogens on human health is largely mediated by gut microbiota, which metabolizes them into bioactive derivatives with better bioavailability and receptor affinity, which leads to beneficial effects on the host’s health due to higher anti-inflammatory, antineoplastic, estrogenic/antiestrogenic, and apoptotic actions than their precursors (Bravo et al. 2017). This pharmacological potential is probably due to the antioxidant activity of these metabolites to interact with important cellular processes in which key enzymes such as cyclooxygenase, lipoxygenase, phospholipase A2, NADH-oxidase, and glutathione reductase are involved (Amarowicz and Pegg 2019). The capacity of these polyphenols to interact with oxygen-activated species, since they are strong scavengers of lipid radicals, gives them a potential preventive effect against DNA damage and lipid peroxidation. Furthermore, the antioxidant activity of enterolignans has been suggested to contribute to the decrease of atherosclerosis, hyperglycemia, and hypercholesterolemia (Peirotén et al. 2019b).
Phytoestrogens and Menopausal Symptoms
Menopause is characterized by changes in women’s hormone balances. In menopause, the levels of estrogen decrease, which triggers uncomfortable symptoms such as hot flashes, night sweats, sleep disturbances, and vaginal dryness. These symptoms are less common in Asian women compared with the women in Europe and America (Freeman and Sherif 2007). Due to the similarities of phytoestrogens to 17-β-estradiol (1), they can imitate estrogen effects in the host. The use of phytoestrogens for alleviating menopausal symptoms has shown positive results. A meta-analysis of 543 potentially relevant studies where the efficacy of phytoestrogens was examined for the relief of menopausal symptoms showed that these phytochemicals reduce the frequency of hot flashes in menopausal women without serious side effects (Chen et al. 2015). Furthermore, gut microbiota influences the activity of phytoestrogens through the synthesis of β-glucuronidase, an enzyme responsible for estrogen deconjugation which enables it to bind to its receptors, and thus reduce the menopausal symptoms (Baker et al. 2017).
Phytoestrogens and Cardiovascular Diseases
The female sex hormones serve as antihypertensive molecules antagonizing the prohypertensive effects of testosterone. Phytoestrogens mediate their effects through the ER, which are found in many tissues of the human body, including vascular tissues. Phytoestrogens maintain endothelial integrity and decrease vascular permeability; they also increase nitrogen oxide, prostaglandin I2, and/or endothelium-derived hyperpolarizing factor release leading to endothelium-dependent vasodilation (Gencel et al. 2012). Daily administration of soy proteins containing isoflavones to post-menopausal woman showed positive vasodilatory effects (Steinberg et al. 2003). Bioavailability of phytoestrogens in the human body is greatly influenced by gut flora. Some phytoestrogen metabolites manifest similar or different cardiovascular activities; these compounds are associated with cardiovascular risk factors, in particular blood pressure, abdominal obesity, and serum lipids, triglycerides, glucose, and inflammatory markers (Frankenfeld 2016). Consumption of yogurt and dietary lignans was associated in improvement of some cardiovascular health parameters such as lowering the levels of total cholesterol and low-density lipoprotein cholesterol in Mediterranean population (Creus-Cuadros et al. 2017). Bifidobacterium has the ability to metabolize daidzein (2) to equol (13), which has increased absorption, bioavailability, and greater affinity for ER; thus, a superior efficacy compared with its precursor daidzein. Binding of equol (13) to ER leads to inhibition of the production of endothelin-1, a vasoconstrictor peptide that causes vasodilation (Gencel et al. 2012; Lye et al. 2009).
Phytoestrogens and Breast Cancer
Breast cancer is the second most common cause of cancer-related deaths in women. Estrogen and progesterone induce the growth of malignant cells through binding of the ER. There are two types of ER in our body, i.e., ERα and ERβ. Phytoestrogens can also bind to ER, and some have a preferential affinity for ERβ, which can inhibit the transcriptional growth-promoting activity of ERα. Their anticancer effects are also related to inhibition of cyclin D1, and expression of cyclin-dependent kinase inhibitors (p21, p27, and p57) and tumor suppressor genes (APC, ATM, PTEN, SERPINB5) (Basu and Maier 2018). However, the binding of phytoestrogens to ERβ is dose-dependent, and only saturating doses show inhibitory effects on breast cancer cell growth (Bilal et al. 2014).
A meta-analyses of the protective function of lignans and enterolignans against breast cancer showed that a high lignan intake, as well as its resulting enterolignan concentration, in postmenopausal women is associated with a significant reduction in the risk of breast cancer (Buck et al. 2010).
Phytoestrogens and Osteoporosis
Estrogen deficiency is known to cause significant alterations in bone metabolism and is a major risk factor for osteoporosis in postmenopausal women. Estrogen is responsible for suppressing osteoclast activity, a large multinucleate bone cell which absorbs bone tissue during growth and healing, and thereby preventing bone resorption. On the other hand, osteoblasts are responsible for the formation of bone tissue. Both ERα and ERβ are found in osteoblasts and are responsible for bone mineralization (Setchell and Lydeking-Olsen 2003). In postmenopausal women, the deficiency of estrogen level is triggering the osteoclast activity and thus promotes bone fractions, e.g., osteoporosis. However, the osteoclast activity is greater due to the low estrogen levels. Phytoestrogens have shown potential in preventing and treating osteoporosis. In one randomized controlled trial, the use of red clover extract which is rich in isoflavone aglycones together with probiotics enhancing isoflavones bioavailability over a 1-year period showed that intake of red clover extract, administrated twice a day, potently attenuated bone mineral density loss caused by estrogen deficiency (Lambert et al. 2017).
Phytoestrogens and Diabetes
Diabetes is a major health problem around the globe. Its prevalence estimated by the International Diabetes Federation is that 382 million adults were diagnosed and 5.1 million deaths occurred in 2013 due to diabetes (Talaei and Pan 2015). It is accepted that the diet plays crucial role in the development and progression of diabetes. The high intake of refined sugars is related to many diseases and diabetes is one of them. However, the food can also lower the high blood glucose level and thus alleviate the symptoms or even prevent the occurrence of diabetes. Flaxseed is highly nutritious food rich in essential fatty acids, fibers, and lignans. The anti-inflammatory, antioxidative, and lipid-modulating properties of lignans are associated with reduced blood glucose in subjects with type 2 diabetes and prediabetes (Parikh et al. 2019). Gut microbiota can improve the antidiabetic effects of lignans through their metabolism and converting them to enterodiol (11) and enterolactone (12). Two cohort studies indicated that lignan metabolites are associated with lower risk of type 2 diabetes in US women (Sun et al. 2014). The combination of flaxseed and yogurt has also improved the hyperglycemic symptoms in patients with type 2 diabetes. Particularly, the daily consumption of 200 g flaxseed-enriched yogurt for 8 weeks resulted in lowering their hemoglobin A1c concentration (Hasaniani et al. 2019).
Gut Microbiota and Metabolism of Phytoestrogens
The three main structural types of phytoestrogens (isoflavones, ellagitannins, and lignans) are metabolized by gut microbiota into equol, urolithins, and enterolignans, respectively.
Isoflavone Metabolism
Isoflavones are flavonoids present in various plants. Almost all isoflavones present in plants and food are glycosides, which are less estrogenic than their aglycones, and their absorption rate in the human intestine is lower due to their higher molecular weight and greater hydrophilicity (Hur et al. 2000). In order to improve the estrogenic effects and the bioavailability of isoflavones, they have to release their sugar component. This is accomplished by the enzyme β-glucosidase, which is present in some LAB and other gut bacteria and, thus, improving the positive health effects of isoflavones (Landete et al. 2016). Lactobacillus delbrueckii was found to be involved in the biotransformation of the isoflavone genistin (14) to its aglyconic form, genistein (4), but was not able to metabolize daidzein (2) and formononetin (3), which are already aglycons (Iqbal and Zhu 2009). Lactobacillus rhamnosus JCM 2771 was also found to be involved in the production of daidzein (2) and genistein (4) from daidzin (15). The increased concentration of L. rhamnosus JCM 2771 was also associated with higher concentration of equol (13) (Tamura et al. 2011) (Scheme 1). The intermediate metabolite between daidzein (2) and equol (13) is dihydrodaidzein (16), which can undergo on two metabolic pathways and produce O-desmethylangolensin (17) or tetrahydrodaidzein (18). Likewise, genistein (4) is further metabolized to dihydrogenistein (19), then to 6-OH-O-desmethylangolensin (20) and 4-hydroxyphenyl-2-propionic acid (21).
Facultative anaerobe bacteria found in the human intestine showed positive results in the metabolism of isoflavones: almost all the strains from the genera Bifidobacterium and Lactobacillus, along with Enterococcus faecalis and Streptococcus bovis, metabolized genistin (14) and daidzin (15) to daidzein (2) and genistein (4). The exceptions were Bifidobacterium bifidum, Lactobacillus brevis, Lactobacillus ermentum, and Lactobacillus reuteri that could not appreciably convert isoflavone glucosides to their aglycones (Tsuchihashi et al. 2008). In another study, soy isoflavones were given to seventeen postmenopausal women for period of 1 week in order to determine the gut microbiota influence on isoflavones. The results from urinary excretion revealed that only 4 subjects were equol (13) producers and all subjects produced O-desmethylangolensin (17). In addition, the proportions of Bifidobacterium and Eubacterium were significantly greater in equol vs nonequol producers (Nakatsu et al. 2014). The β-glucosidase activity from three strains of Lactobacillus acidophilus, two of Lactobacillus casei, and one of Bifidobacterium was tested. All of the six bacteria were incubated in soymilk, and the β-glucosidase activity was determined based on aglycone formation. L. acidophilus 4461 showed the highest aglycone formation (76.9%) after 24 h of incubation (Otieno et al. 2006). Another L. acidophilus strain (L10), demonstrated highest aglycone formation after 36 h of incubation, whereas in L. casei L26 and Bifidobacterium lactis B94, the highest aglycone formation was achieved after 12 h and 24 h, respectively (Donkor and Shah 2007). The reason for the time-dependent enzyme activity is due to the cell number. The enzyme activity depends on the cell growth, greater cell count results in greater enzyme activity. Addition of specific substances can increase the growth of some bacterial species and thus improve their metabolic effects. The addition of 2% lactose to soymilk and incubating 11 LAB strains has shown enhanced biotransformation of isoflavone glucosides to the more bioactive isoflavone aglycones. Lack of lactose in soymilk slows the growth of LAB and, therefore, decreases the isoflavone metabolism (Ding and Shah 2010).
Gaya et al. analyzed 92 strains of LAB and bifidobacteria from the genera Lactobacillus, Enterococcus, Lactococcus, and Bifidobacterium for their capacity to metabolize isoflavones. Bifidobacteria was the group showing the greatest ability to metabolize isoflavones, while the percentage of isoflavone metabolites produced by the other genera were 39.1%, 43.5%, and 47.8% for Lactobacillus, Enterococcus, and Lactococcus, respectively (Gaya et al. 2016b). Lactococcus garvieae, isolated from the human intestine, is able to metabolize daidzein (15) to equol (13) after a steady state of growth has been achieved (Uchiyama et al. 2007).
The anti-inflammatory effects of isoflavone metabolites were demonstrated in vitro where four Lactobacillus species (Lactobacillus plantarum DPPMA24W and DPPMASL33, Lactobacillus fermentum DPPMA114, and L. rhamnosus DPPMAAZ1) showed the highest activity for metabolizing isoflavones. These bacteria were incubated at 37 °C in soymilk that was made from organically farmed soybeans, and after 96 h, the medium contained 57 μM daidzein, 140.3 μM genistein, 20.4 μM glycitein, and 37.3 μM equol. Soymilk containing the bacterial strains and the soymilk without the bacteria were tested on intestinal human Caco-2/TC7 cells for anti-inflammatory effects resulting from the isoflavone metabolism. The inflammatory effect was induced by interferon-γ (1000 U/ml) and lipopolysaccharide (100 ng/ml). The results demonstrated that only the soymilk containing the bacteria inhibited cell inflammation and the synthesis of IL-8 after treatment with interleukin-1β (2 ng/ml) (Di Cagno et al. 2010).
Ellagitannin Metabolism
Ellagitannins are a family of bioactive polyphenols contained in a number of edible plants and fruits, such as edible seeds, nuts, raspberries, strawberries, pomegranate, and longan (Ismail et al. 2016). They are composed of hexahydroxydiphenoyl units, galloyl units, and/or sanguisorboyl units bounded to a sugar molecule. The ellagitannins that are transformed by the gut microbiota are the ones that contain a hexahydroxydiphenoyl unit, and they are metabolized to urolithins in the gastrointestinal tract. Urolithins can also be synthesized from ellagic acid, a metabolite of ellagitannins. Ellagitannins and ellagic acid have very low bioavailability, but the urolithins have better absorption and are present in the blood stream as glucuronide and sulfate conjugates. Recent research has shown preliminary evidence for the anti-inflammatory, anticarcinogenic, antiglycative, antioxidant, and antimicrobial effects of urolithins (Espin et al. 2013; Zhang et al. 2019).
The most important urolithins produced by the gut microbiota are urolithin A (22), isourolithin A (23), urolithin B (24), urolithin C (25), urolithin M6 (26), and urolithin M7 (27). Urolithins were found in fecal samples of three healthy volunteers without a history of gastrointestinal disease and without usage of antibiotics in the past 6 months (Piwowarski et al. 2016).

The metabolism of ellagitannins was examined in forty healthy volunteers. The participants were divided into four groups where each group consumed foods containing ellagitannins: strawberries (250 g), red raspberries (225 g), walnuts (35 g), and oak-aged red wine (300 ml). Five urine fractions were collected, and the results demonstrated that ellagitannins were not present in the samples; however, the microbial metabolite urolithin B (24) was detected in all of the subjects. The mean percentage of metabolite excretion was 2.8% (strawberries), 16.6% (walnuts), 3.4% (red raspberries), and 6.5% (oak-aged red wine) (Cerda et al. 2005). The results of a pharmacokinetic observation of twenty healthy volunteers, after consumption of 300 g fresh berries (100 g of strawberry puree, 100 g of frozen raspberries, and 100 g of frozen cloudberries [Rubus chamaemorus]) has shown that urolithin A, B, C, and D glucuronides, methyl-urolithin C, and D glucuronides, plus dimethyl-ellagic acid glucuronide were detected in the urine, while in the control group these metabolites were not found (Puupponen-Pimia et al. 2013).
Gordonibacter urolithinfaciens and Gordonibacter pamelaeae DSM 19378(T) can be isolated from the feces of a healthy human volunteer. It has been shown that in anaerobic conditions during the stationary phase of the bacterial growth, catabolism of ellagic acid has occurred and showed production of pentahydroxy-urolithin (urolithin M-5), tetrahydroxy-urolithin (urolithin M-6), and trihydroxy-urolithin (urolithin C), while dihydroxy-urolithins (urolithin A and isourolithin A) and monohydroxy-urolithin (urolithin B) were not produced (Selma et al. 2014). Another strain of the Gordonibacter genus (CEBAS 4A1, 4A2, 4A3, 4A4) has shown production of isourolithin A (23), but did not produced urolithin A (22) and urolithin B (24) (Selma et al. 2017). The bacteria involved in the metabolism of a specific ellagitannin are still unknown, and further research is necessary to discover the exact mechanism of biotransformation of this type of phytoestrogens.
Lignan Metabolism
Lignans are a group of bioactive phenolic compounds biosynthesized by the dimerization of two substituted phenylpropane units. They are widely distributed in plants and are an important group of phytoestrogens present in our diets (Rodríguez-García et al. 2019). Cereals, soybeans, cruciferous vegetables, and some fruits (particularly apricots and strawberries) possess high amounts of these compounds, e.g., matairesinol (10), pinoresinol (7), lariciresinol (8), sesamin (28), arctigenin (29), and syringaresinol (30), as well as secoisolariciresinol (9) and its glycosylated form, secoisolariciresinol diglucoside (31) (Yoder et al. 2015).

These compounds undergo substantial bacterial metabolism in the intestine and are transformed into more biologically active lignan metabolites, also known as enterolignans. The intestinal microbiota hydrolyzes the sugar from the glycoside molecule and the aglycone is released. This is followed by dehydroxylation and demethylation to give enterodiol (11), which can be further oxidized to produce enterolactone (12). Matairesinol (10) can be directly transformed to enterolactone by demethylation by the gut microbiota (Scheme 2) (Clavel et al. 2006a).
Lignans have different capacities to produce enterolignans as demonstrated by quantitative analyses of lignan transformations to enterolactone (12) and enterodiol (11) (Heinonen et al. 2001). Lignans were extracted from different plants and their transformation was induced by fecal microorganisms from healthy volunteers. The results were (a) 62% of matairesinol (10) was converted to enterolactone; (b) 72% of secoisolariciresinol (9) was converted to enterolactone and enterodiol; 55% of pinoresinol (7) was converted to enterolactone and enterodiol; (c) 4% of syringaresinol (24) was converted to enterolactone and enterodiol, while several other metabolites were also identified; (d) 23% of 7-hydroxymatairesinol was converted to 7-hydroxyenterolactone and enterolactone; (e) 4% of arctigenin (23) was converted to enterolactone. Two additional metabolites were also identified, 3′-demethyl-4′-dehydroxyarctigenin and 3′-demethylarctigenin, an isomer of matairesinol; and (f) isolariciresinol remained mostly unchanged (Heinonen et al. 2001).
There are several gut microbiota species that are capable of metabolizing lignans and transforming them into enterolignans. Hydrolysis of secoisolariciresinol diglucoside can be accomplished by some Bacteroides and Clostridium strains (Clavel et al. 2006b). Three different Lactobacillus strains (Lactobacillus salivarius INIA P183, L. salivarius INIA P448 and Lactobacillus gasseri INIA P508) were capable of metabolizing lignans from flax extracts into enterodiol and enterolactone (Bravo et al. 2017). Twenty Bifidobacterial strains and three Lactobacillus strains (L. gasseri INIA P508, L. salivarius INIA P448, and L. salivarius INIA P183) were tested for their metabolism of lignans. Three bifidobacterial strains (Bifidobacterium bifidum INIA P466, Bifidobacterium catenulatum INIA P732, and Bifidobacterium pseudolongum INIA P2) produced low levels of enterodiol from lignan extracts, while Bifidobacterium pseudocatenulatum INIA P946 was capable of producing secoisolariciresinol (9) from its glycoside (25). B. catenulatum INIA P732 and L. gasseri INIA P508 were the strains that showed the largest percentage of the metabolite enterolactone with concentrations above 2 mM (Peirotén et al. 2019a). L. acidophilus and L. casei have been tested for metabolizing secoisolariciresinol diglucoside present in flaxseed (Linum usitatissimum). L. acidophilus and L. casei were tested on the bioaccessibility of lignan in whole flaxseeds and flaxseed flour submitted to a full in vitro simulation of the digestive process from mouth to large intestinal digestion. Secoisolariciresinol diglucoside is hydrolyzed to secoisolariciresinol and then metabolized to enterodiol and enterolactone (Scheme 2). The results showed that the two tested bacteria influenced the bioaccessibility of lignans and enterolignans (Muñoz et al. 2018).
Serum concentration of enterolactone is affected by the use of antimicrobial drugs. In one cross-sectional health survey studying 2753 Finnish men and women aged 25–64 years, it was shown that the serum concentration of enterolactone was significantly lower in those who had used oral antimicrobials than in nonusers. The results demonstrated that each antimicrobial lowered serum enterolactone concentration by 16% in men and 11% in women. This study showed the importance of intestinal bacteria in the biotransformation and absorption of plant lignans (Kilkkinen et al. 2002).
Conclusion
In this review, the positive health effects of phytoestrogens (isoflavones, ellagitannins, and lignans), their chemical structures, as well as their metabolism induced by gut microflora and probiotics are described. Phytoestrogens can bind to the ER and, therefore, promote estrogenic/antiestrogenic properties. Their effects are mostly useful in postmenopausal women, where hormones are unbalanced. Phytoestrogens have beneficial health effects in alleviating menopausal symptoms, cardiovascular diseases, breast cancer, osteoporosis, and diabetes. However, these beneficial activities on human health mainly depend on their metabolization by gut microbiota present in the small and large intestine, since metabolites (genistein, equol, enterolignans, and certain urolithins) are more likely to produce the beneficial effects due to their greater lipophilicity and better absorption, antioxidant properties, and capacity to bind to ER. Most of the gut bacteria express β-glucosidase, which is involved in the sugar hydrolysis to release the lipophilic active aglycone.
Intake of phytoestrogen-rich foods with probiotics can increase the metabolism and bioavailability of phytoestrogens and thus increase their positive effects. The knowledge of the specific mechanism of how probiotics transform phytoestrogens can help to understand the best combinations of probiotics and phytoestrogens and their usage in therapy.
Data Availability
The data that support the findings of this study are openly available.
References
Adlercreutz H, Mazur W (1997) Phyto-oestrogens and western diseases. Ann Med 29:95–120
Amarowicz R, Pegg RB (2019) Natural antioxidants of plant origin. In: Ferreria I, Barros L (eds) Advances in food and nutrition research. Functional food ingredients from plants. Academic, Cambridge, pp 1–81. https://doi.org/10.1016/bs.afnr.2019.02.011
Athni TS, Athni SS (2019) The evolution of modern medicine: garden to pill box. In: Joshee N, Dhekney S, Parajuli P (eds) Medicinal Plants. Springer, Cham, pp 1–16. https://doi.org/10.1007/978-3-030-31269-5_1
Bacciottini L, Falchetti A, Pampaloni B, Bartolini E, Carossino AM, Brandi ML (2007) Phytoestrogens: food or drug? Clin Cases Miner Bone Metab 4:123–130
Baker JM, Nakkash LA, Herbst-Kralovetz MM (2017) Estrogen–gut microbiome axis: physiological and clinical implications. Maturitas 103:45–53
Basu P, Maier C (2018) Phytoestrogens and breast cancer: in vitro anticancer activities of isoflavones, lignans, coumestans, stilbenes and their analogs and derivatives. Biomed Pharmacother 107:1648–1666. https://doi.org/10.1016/j.biopha.2018.08.100
Bilal I, Chowdhury A, Davidson J, Whitehead S (2014) Phytoestrogens and prevention of breast cancer: the contentious debate. World J Clin Oncol 5:705–712
Bravo D, Peiroten A, Alvarez I, Landete JM (2017) Phytoestrogen metabolism by lactic acid bacteria: enterolignan production by Lactobacillus salivarius and Lactobacillus gasseri strains. J Funct Foods 37:373–378
Buck K, Zaineddin AK, Vrieling A, Linseisen J, Chang-Claude J (2010) Meta-analyses of lignans and enterolignans in relation to breast cancer risk. Am J Clin Nutr 92:141–153
Canny GO, McCormick BA (2008) Bacteria in the intestine, helpful residents or enemies from within? Infect Immun 76:3360–3373
Cerda B, Tomas-Barberan FA, Espin JC (2005) Metabolism of antioxidant and chemopreventive ellagitannins from strawberries, raspberries, walnuts, and oak-aged wine in humans: identification of biomarkers and individual variability. J Agric Food Chem 53:227–235
Chen MN, Lin CC, Liu CF (2015) Efficacy of phytoestrogens for menopausal symptoms: a meta-analysis and systematic review. Climacteric 18:260–269
Clavel T, Dore J, Blaut M (2006a) Bioavailability of lignans in human subjects. Nutr Res Rev 19:187–196
Clavel T, Borrmann D, Braune A, Doré J, Blaut M (2006b) Occurrence and activity of human intestinal bacteria involved in the conversion of dietary lignans. Anaerobe 12:140–147
Creus-Cuadros A, Tresserra-Rimbau A, Quifer-Rada P, Martínez-González MA, Corella D, Salas-Salvadó J, Fitó M, Estruch R, Gómez-Gracia E, Lapetra J, Arós F, Fiol M, Ros E, Serra-Majem L, Pintó X, Moreno JJ, Ruiz-Canela M, Sorli JV, Basora J, Schröder H, Lamuela-Raventós RM, PREDIMED Study Investigators (2017) Associations between both lignan and yogurt consumption and cardiovascular risk parameters in an elderly population: observations from a cross-sectional approach in the PREDIMED study. J Acad Nutr Diet 117:609–622. https://doi.org/10.1016/j.jand.2016.11.003
Desmawati D, Sulastri D (2019) Phytoestrogens and their health effect. Open Access Maced J Med Sci 7:495–499. https://doi.org/10.3889/oamjms.2019.086
Di Cagno R, Mazzacane F, Rizello CG, Vincentini O, Silano M, Giuliano G, De Angelis M, Gobbetti M (2010) Synthesis of isoflavone aglycones and equol in soy milks fermented by food-related lactic acid bacteria and their effect on human intestinal Caco-2 cells. J Agric Food Chem 58:10338–10346
Ding WK, Shah NP (2010) Enhancing the biotransformation of isoflavones in soymilk supplemented with lactose using probiotic bacteria during extended fermentation. J Food Sci 75:M140–M149
Donkor ON, Shah NP (2007) Production of β-glucosidase and hydrolysis of isoflavone phytoestrogens by Lactobacillus acidophilus, Bifidobacterium lactis, and Lactobacillus casei in soymilk. J Food Sci 73:M15–M20
Eisenbrand G, Senate Commission on Food Safety of the German Research F (2007) Isoflavones as phytoestrogens in food supplements and dietary foods for special medical purposes. Opinion of the Senate Commission on Food Safety (SKLM) of the German Research Foundation (DFG)-(shortened version). Mol Nutr Food Res 51:1305–1312
Espin JC, Larrosa M, Garcia-Conesa MT, Tomas-Barberan F (2013) Biological significance of urolithins, the gut microbial ellagic acid-derived metabolites: the evidence so far. Evid Based Complement Alternat Med. https://doi.org/10.1155/2013/270418
Frankenfeld CL (2016) Cardiometabolic risk and gut microbial phytoestrogen metabolite phenotypes. Mol Nutr Food Res. https://doi.org/10.1002/mnfr.201500900
Freeman EW, Sherif K (2007) Prevalence of hot flushes and night sweats around the world: a systematic review. Climacteric 10:197–214
Gaya P, Medina M, Sanchez-Jimenez A, Landete JM (2016a) Phytoestrogen metabolism by adult human gut microbiota. Molecules. https://doi.org/10.3390/molecules21081034
Gaya P, Peiroten A, Medina M, Landete JM (2016b) Isoflavone metabolism by a collection of lactic acid bacteria and bifidobacteria with biotechnological interest. Int J Food Sci Nutr 67:117–124
Gencel VB, Benjamin MM, Bahou SN, Khalil RA (2012) Vascular effects of phytoestrogens and alternative menopausal hormone therapy in cardiovascular disease. Mini-Rev Med Chem 12:149–174
Hasaniani N, Rahimlou M, Ramezani Ahmadi A, Mehdizadeh Khalifani A, Alizadeh M (2019) The effect of flaxseed enriched yogurt on the glycemic status and cardiovascular risk factors in patients with type 2 diabetes mellitus: randomized, open-labeled, controlled study. Clin Nutr Res 8:284–295. https://doi.org/10.7762/cnr.2019.8.4.284
Heinonen S, Nurmi T, Liukkonen K, Poutanen K, Wahala K, Deyama T, Nishibe S, Adlercreutz H (2001) In vitro metabolism of plant lignans: new precursors of mammalian lignans enterolactone and enterodiol. J Agric Food Chem 49:3178–3186
Hur HG, Lay JO, Beger RD, Freeman JP, Rafii F (2000) Isolation of human intestinal bacteria metabolizing the natural isoflavone glycosides daidzin and genistin. Arch Microbiol 174:422–428
Iqbal MF, Zhu WY (2009) Characterization of newly isolated Lactobacillus delbrueckii-like strain MF-07 isolated from chicken and its role in isoflavone biotransformation. FEMS Microbiol Lett 291:180–187
Ismail T, Calcabrini C, Diaz AR, Fimognari C, Turrini E, Catanzaro E, Akhtar S, Sestili P (2016) Ellagitannins in cancer chemoprevention and therapy. Toxins. https://doi.org/10.3390/toxins8050151
Kilkkinen A, Pietinen P, Klaukka T, Virtamo J, Korhonen P, Adlercreutz H (2002) Use of oral antimicrobials decreases serum enterolactone concentration. Am J Epidemiol 155:472–477
Lambert MNT, Thybo CB, Lykkeboe S, Rasmussen LM, Frette X, Christensen LP, Jeppesen PB (2017) Combined bioavailable isoflavones and probiotics improve bone status and estrogen metabolism in postmenopausal osteopenic women: a randomized controlled trial. Am J Clin Nutr 106:909–920
Lampe JW (2003) Isoflavonoid and lignan phytoestrogens as dietary biomarkers. J Nutr 133(Suppl 3):956S–964S
Landete JM, Arques J, Medina M, Gaya P, de Las RB, Munoz R (2016) Bioactivation of phytoestrogens: intestinal bacteria and health. Crit Rev Food Sci 56:1826–1843
Landete JM, Gaya P, Rodriguez E, Langa S, Peiroten A, Medina M, Arques JL (2017) Probiotic bacteria for healthier aging: immunomodulation and metabolism of phytoestrogens. Biomed Res. https://doi.org/10.1155/2017/5939818
Lye HS, Kuan CY, Ewe JA, Fung WY, Liong MT (2009) The improvement of hypertension by probiotics: effects on cholesterol, diabetes, renin, and phytoestrogens. Int J Mol Sci 10:3755–3775
Muñoz O, Fuentealba C, Ampuero D, Figuerola F, Estevez AM (2018) The effect of Lactobacillus acidophilus and Lactobacillus casei on the in vitro bioaccessibility of flaxseed lignans (Linum usitatissimum L.). Food Funct 9:2426–2432. https://doi.org/10.1039/c8fo00390d
Nakatsu CH, Armstrong A, Clavijo AP, Martin BR, Barnes S, Weaver CM (2014) Fecal bacterial community changes associated with isoflavone metabolites in postmenopausal women after soy bar consumption. PLoS One. https://doi.org/10.1371/journal.pone.0108924
Otieno DO, Ashton JF, Shah NP (2006) Evaluation of enzymic potential for biotransformation of isoflavone phytoestrogen in soymilk by Bifidobacterium animalis, Lactobacillus acidophilus and Lactobacillus casei. Food Res Int 39:394–407
Parikh M, Maddaford TG, Austria JA, Aliani M, Netticadan T, Pierce GN (2019) Dietary flaxseed as a strategy for improving human health. Nutrients. https://doi.org/10.3390/nu11051171
Patisaul HB, Jefferson W (2010) The pros and cons of phytoestrogens. Front Neuroendocrinol 31:400–419
Patridge E, Gareiss P, Kinch MS, Hoyer D (2016) An analysis of FDA-approved drugs: natural products and their derivatives. Drug Discov Today 21:204–207
Peirotén A, Gaya P, Alvarez I, Bravo D, Landete JM (2019a) Influence of different lignan compounds on enterolignan production by Bifidobacterium and Lactobacillus strains. Int J Food Microbiol 289:17–23. https://doi.org/10.1016/j.ijfoodmicro.2018.08.028
Peirotén A, Bravo D, Landete JM (2019b) Bacterial metabolism as responsible of beneficial effects of phytoestrogens on human health. Crit Rev Food Sci Nutr. https://doi.org/10.1080/10408398.2019.1622505
Pessione E (2012) Lactic acid bacteria contribution to gut microbiota complexity: lights and shadows. Front Cell Infect Microbiol. https://doi.org/10.3389/fcimb.2012.00086
Piwowarski JP, Granica S, Stefanska J, Kisst AK (2016) Differences in metabolism of ellagitannins by human gut microbiota ex vivo cultures. J Nat Prod 79:3022–3030
Puupponen-Pimia R, Seppanen-Laakso T, Kankainen M, Maukonen J, Torronen R, Kolehmainen M, Leppanen T, Moilanen E, Nohynek L, Aura AM, Poutanen K, Tomas-Barberan FA, Espin JC, Oksman-Caldentey KM (2013) Effects of ellagitannin-rich berries on blood lipids, gut microbiota, and urolithin production in human subjects with symptoms of metabolic syndrome. Mol Nutr Food Res 57:2258–2263
Rietjens I, Louisse J, Beekmann K (2017) The potential health effects of dietary phytoestrogens. Br J Pharmacol 174:1263–1280
Rodríguez-García C, Sánchez-Quesada C, Toledo E, Delgado-Rodríguez M, Gaforio JJ (2019) Naturally lignan-rich foods: a dietary tool for health promotion? Molecules. https://doi.org/10.3390/molecules24050917
Selma MV, Beltran D, Garcia-Villalba R, Espin JC, Tomas-Barberan FA (2014) Description of urolithin production capacity from ellagic acid of two human intestinal Gordonibacter species. Food Funct 5:1779–1784
Selma MV, Beltran D, Luna MC, Romo-Vaquero M, Garcia-Villalba R, Mira A, Espin JC, Tomas-Barberan FA (2017) Isolation of human intestinal bacteria capable of producing the bioactive metabolite isourolithin a from ellagic acid. Front Microbiol. https://doi.org/10.3389/fmicb.2017.01521
Setchell KD, Lydeking-Olsen E (2003) Dietary phytoestrogens and their effect on bone: evidence from in vitro and in vivo, human observational, and dietary intervention studies. Am J Clin Nutr 78:593S–609S
Steinberg FM, Guthrie NL, Villablanca AC, Kumar K, Murray MJ (2003) Soy protein with isoflavones has favorable effects on endothelial function that are independent of lipid and antioxidant effects in healthy postmenopausal women. Am J Clin Nutr 78:123–130
Sun Q, Wedick NM, Pan A, Townsend MK, Cassidy A, Franke AA, Rimm EB, Hu FB, van Dam RM (2014) Gut microbiota metabolites of dietary lignans and risk of type 2 diabetes: a prospective investigation in two cohorts of U.S. women. Diabetes Care 37:1287–1295
Talaei M, Pan A (2015) Role of phytoestrogens in prevention and management of type 2 diabetes. World J Diabetes 6:271–283
Tamura M, Hori S, Nakagawa H (2011) Lactobacillus rhamnosus JCM 2771: impact on metabolism of isoflavonoids in the fecal flora from a male equol producer. Curr Microbiol 62:1632–1637
Tham DM, Gardner CD, Haskell WL (1998) Clinical review 97: potential health benefits of dietary phytoestrogens: a review of the clinical, epidemiological, and mechanistic evidence. J Clin Endocrinol Metab 83:2223–2235
Thursby E, Juge N (2017) Introduction to the human gut microbiota. Biochem J 474:1823–1836
Tsuchihashi R, Sakamoto S, Kodera M, Nohara T, Kinjo J (2008) Microbial metabolism of soy isoflavones by human intestinal bacterial strains. J Nat Med Tokyo 62:456–460
Uchiyama S, Ueno T, Suzuki T (2007) Identification of a newly isolated equol-producing lactic acid bacterium from the human feces. J Intest Microbiol 21:217–220
Yoder SC, Lancaster SM, Hullar MAJ, Lampe JW (2015) Gut microbial metabolism of plant lignans: influence on human health. In: Tuohy K, Rio DD (eds) Diet-microbe interactions in the gut effects on human health and disease. Academic, Cambridge, pp 103–117
Zhang S, Maghout TA, Cao H, Pelzl L, Salker MS, Veldhoen M, Cheng A, Lang F, Singh Y (2019) Gut bacterial metabolite urolithin A (UA) mitigates Ca2+ entry in T cells by regulating miR-10a-5p. Front Immunol. https://doi.org/10.3389/fimmu.2019.01737
Acknowledgments
The authors are grateful to Mr. Karsten Fatur for his proof reading as a native speaker and a scientist.
Funding
For this research, the authors did not receive any extra founding. Research was founded by the organizations, where the authors are affiliated: University of Ljubljana, Faculty of Pharmacy, Ljubljana, Slovenia and Jožef Stefan Institute, Department of Biotechnology, Ljubljana, Slovenia.
Author information
Authors and Affiliations
Contributions
SS (PhD student) wrote the first draft of the manuscript. SK revised the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Stojanov, S., Kreft, S. Gut Microbiota and the Metabolism of Phytoestrogens. Rev. Bras. Farmacogn. 30, 145–154 (2020). https://doi.org/10.1007/s43450-020-00049-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43450-020-00049-x