Abstract
The inoculation of diazotrophic endophytic bacteria in micropropagated sugarcane plants has been utilized in studies on the association between plants and diazotrophic bacteria, allowing us to evaluate the potential of BNF and growth promotion. The objective of this study was to evaluate the effects of inoculation (alone and in a mixture) of different strains of endophytic bacteria from the sugarcane variety RB867515, collected in northeast Brazil, on sugarcane growth at the initial growth stage (45 and 120 days after inoculation—DAI). For this purpose, two experiments were carried out in a greenhouse at the Agronomic Institute of Pernambuco (IPA), located in the city of Goiania, PE, Brazil, in a completely randomized design. The first experiment, with micropropagated seedlings grown in tubes at 45 DAI, was composed of uninoculated plants, plants inoculated in vitro with three individual endophytic bacterial isolates, and plants inoculated in vitro with a mixture of all three bacterial isolates. The second experiment, at 150 DAI, consisted of inoculated plants transplanted to pots with nonsterile soil without nitrogen fertilization and uninoculated plants with nitrogen fertilization equivalent to 80 kg of N ha−1. The variables analyzed were the shoot and root dry weight, tillering and N content accumulated in the plant. At 45 DAI, there was no significant difference between the inoculated plants and the uninoculated control. The inoculation of nitrogen-fixing bacteria native to the northeast region in micropropagated seedlings of sugarcane variety RB867515 grown in pots promoted plant development and presented similar performance to the nitrogen treatment at 150 DAI.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Currently, Brazil plays an important global role in the production and trade of agricultural commodities, which grows each year through the adoption of new technologies and increased chemical inputs. Among the main Brazilian agricultural crops, sugarcane (Saccharum spp. L.) is one of the most important, with Brazil being the largest producer in the world. According to the National Supply Company (CONAB), the area planted in the 2017/18 season was approximately 8.6 million hectares, with an estimated production for the 2018/2019 season of 625.96 million tons (CONAB 2018). To obtain the current levels of production, it is estimated that in 2017, 34.4 million tons of fertilizers were used, of which 26.3 million tons were imported (National Association for the Diffusion of Fertilizers and Correctives—ANDA 2018).
In the 2018/19 season, sugarcane production will decrease by − 1.2% in relation to that in the previous season (CONAB 2018). Brazil’s production is forecast down by 4.7 million tons to 34.2 million based on the expectation that more sugarcane will be diverted towards ethanol production due to weak prices resulting from burdensome global supplies (USDA 2017). N is one of the nutrients with the lowest rate of utilization by sugarcane, and approximately 50% of all fertilizer applied is lost by leaching in the form of nitrate (NO3-) (Spatzal 2015). The development of technologies to increase crop productivity is strategic for the sustainability of the sugarcane production system in terms of production costs and environmental damages associated with its use (Siqueira Neto et al. 2016).
Some alternatives have been sought to reduce the environmental impact of nitrogen fertilizer (e.g., rotation of crops, alternating nitrogen-fixing plants with others that do not have this natural capacity, green manure and use of inoculants), and one of them is biological nitrogen fixation (BNF), which is a major pathway of external N input to unmanaged terrestrial ecosystems (Houlton and Morford 2015). This process is mediated by microorganisms called diazotrophs. These microorganisms are able to grow in medium free of combined nitrogen, using the gaseous form (N2) as a nitrogen source, which is reduced to ammonia (NH3) through the enzymatic complex nitrogenase and assimilated as amino acids by plants (Franche et al. 2009).
Some research institutions have conducted studies with the purpose of developing inoculants for grasses with the ability to stimulate increased productivity and/or dry matter and N accumulation by the plant. There are reports in the literature that inoculants with diazotrophic bacteria promote an increase in plant development, including productivity, in sugarcane varieties RB867515 and RB72454 showing similarity to the addition of 120 kg ha−1 N (Schultz et al. 2012). The stimulation of plant development by some species of bacteria can be realized through synthesis of phytohormones and secondary metabolites and through the availability of nutrients such as phosphorus by its solubilization, and nitrogen by the biological fixation of atmospheric nitrogen (Compant et al. 2010; Gamalero and Glick 2015).
To test this hypothesis, we used the inoculation of endophytic diazotrophic bacteria isolated from northeast Brazil, originating from the same variety, RB867515 (homologous), which shows high potential for biological nitrogen fixation. This hypothesis was tested using different bacteria that were inoculated alone and mixed onto sugarcane to supply the N necessary for the development of the crop as well as to promote growth.
Materials and methods
Location of the experiment
The experiments were conducted at the Plant Tissue Culture Laboratory (LCTP) and in a greenhouse at the Itapirema Experimental Station (7°38′33.33″S and 34°56′50.80″ W at an altitude of 13 m), located in the city of Goiana, Zona da Mata Norte of Pernambuco (Brazil), both belonging to the Agronomic Institute of Pernambuco (IPA).
Plant material
The plant material used was the commercial variety RB867515, the most cultivated in Brazil. This variety has fast growth, better performance in soils of light texture and medium fertility, in addition to a medium tillering ability, good sprouting in cane plants and first ratoons, a high sucrose content, good productivity and erect growth (RIDESA 2010), and is widespread in northeastern Brazil.
In vitro micropropagation of sugarcane
The micropropagated sugarcane seedlings were provided by the Santa Tereza Power Plant at the initial stage of propagation. Sugarcane seedlings were micropropagated according to a method described by Hendre et al. (1983) using the apical meristem. This methodology uses Murashige and Skoog (MS) medium (Murashige and Skoog 1962) modified in relation to the hormonal concentration to promote callus multiplication (phase I), shoot multiplication (phase II) and root multiplication (phase III) for 80 to 90 days.
The plants micropropagated in the rooting phase were transferred to flasks individually with 20 mL of MS medium, modified by Reis et al. (1999). 48 h after transfer of the plants, the flasks that did not show contamination were selected to receive bacterial inoculum.
Isolation and identification of endophytic bacteria
The isolation of endophytic bacteria from plant samples (stem and roots) was done according to the method described by Döbereiner et al. (1995).
Bacterial DNA extraction was done as per Sambrook et al. (2001). After quantification, the DNA amplification of each isolate was performed by PCR (Polymerase Chain Reaction) with primers fD1 (5′AGA GTTTGAT CCTGGCTCAG 3′) and rD1 (5′AAGGAGGTGATC CAGCC 3′) (Weisburg et al. 1991). The amplified PCR products were sequenced and analyzed on the CLUSTALW software using the GenBank database. Phylogenetic trees were designed on the MEGA software (Tamura et al. 2013) v. 6 integrated with the Clustral W software.
Preparation of the inoculum
The solubilization of inorganic phosphate was tested by the bacterial isolates in insoluble phosphate medium according to the method described by Verma et al. (2001) and Rodriguez et al. (2000). The solubilization halo diameter (translucent area around the colony) was observed after 7 days of incubation and indicated positive response of the isolate to solubilization of calcium phosphate. Data were used to calculate the solubilization index (SI) by the ratio between the halo diameter and the colony diameter.
IAA quantification was performed according to Kuss et al. (2007). The isolates that formed a red color in the period of 30 min were evaluated under spectrophotometer at 520 nm wavelength. The concentration of indole compounds was estimated according to the equation Y = 0.0121X − 0.0075 (R2 = 0.9995) by a standard curve previously prepared with non-inoculated sterile culture medium and the IAA concentrations of: 0.5, 10, 30, 50, 70, 90 and 100 μg mL.
The nitrogen-reducing activity by the nitrogenase complex was measured according to the method described by Boddey et al. (1990). The amount of ethylene produced in the samples was analyzed by gas chromatograph with flame ionization detector. The acetylene reduction activity is given by the nmol of ethylene produced per hour of incubation.
Evaluation of micropropagated sugarcane seedlings at 45 days after inoculation (DAI)
The first experiment was conducted in a greenhouse at the Agronomic Institute of Pernambuco (IPA) in tubes filled with sterilized commercial substrate Basaplant®. In this trial, individual and mixed inoculations were evaluated with the following treatments:
Four types of inoculation: individual inoculation (Rhz), individual inoculation (Ps1), individual inoculation (Ps2) and mixed inoculation (Ps1, Ps2 and Rhz) without nitrogen fertilization. The inoculation of in vitro micropropagated seedlings was according to the method described by Reis (2004). Each vial containing five seedlings was inoculated with 0.1 mL of bacterial suspension in the final rooting phase and maintained for up to 7 days at 25 °C under artificial light and 12 h photoperiod.
Control treatment was without inoculation and without nitrogen fertilization.
The experimental design was a randomized complete block design with 15 replicates. The plants were kept in tubes (acclimatized) for 45 days. The root system and the shoots were separated, dried in an oven at 65 °C until reaching constant mass, and weighed in a semi-analytical balance to determine the dry weight (g) of root (DWR) and shoot (DWS) for evaluation of the effect of inoculation.
Samples of the shoot dry weight were passed through a Wiley mill (2 mm) to determine the total nitrogen accumulated in the plant tissues by the method of Kjeldahl (Alves et al. 1994).
Evaluation of sugarcane seedlings at 150 days after inoculation (DAI)
The second experiment with 150 DAI was conducted in greenhouse with the same seedlings transferred and used in the 45DAI experiment. This experiment was carried out in pots containing 8 kg of non-sterile soil.
The site chosen for soil collection was an area of sugarcane cultivation at the Itapirema Experimental Station, IPA (Goiânia/PE—Brazil). The attributes of the soil used in the experiment are mentioned in Table 1. The soil received NK fertilization according to the recommendation of the crop and based on the chemical analysis thereof. Fertilization was carried out with the equivalent of 80 kg of N ha−1 urea for nitrogen treatment, in addition to 1 mL of Hoagland’s micronutrient solution (Sarruge 1975) per pot.
Three bacterial isolates were selected based on their performance presented in a previous work under controlled conditions and for having positive characteristics presented during in vitro evaluation, such as capacity to grow in medium free of a nitrogen source, to produce IAA and to solubilize inorganic phosphate.
The experiment was conducted in a factorial scheme (5X1), composed of Ps1, Ps2 and Rhz alone and in a mixture (Ps1, Ps2 and Rhz) (inoculated in the in vitro phase of the plant) without nitrogen fertilization, and a control treatment with ten replicates per treatment. The control treatment received nitrogen fertilization without inoculation with diazotrophic bacteria.
The inoculation efficiency was evaluated at 150 days after inoculation. The root dry weight, shoot and the number of tillers were evaluated, and the total nitrogen accumulated in the plant tissues was determined by the Kjeldahl method (Alves et al. 1994).
Statistical analysis
The design was completely randomized. Data were subjected to analysis of variance (ANOVA) by Tukey’s test (p < 0.05) through the SASM-Agro program.
Results
Identification and PGP characters of endophytes
The consensus sequences of each isolate were compared to sequences from the GenBank public database through the BLAST program (NCBI—www.ncbi.nih.gov). The 16S rRNA phylogenetic tree was dominated by families Enterobacteriaceae, Pseudomonadaceae and Rhizobiaceae. This analysis showed identity rates ranging from 77 to 99%.
Three endophytic bacterial isolates from sugarcane varieties RB867515, collected from three regions of northeastern Brazil (Paraiba-PB, Pernambuco-PE e Alagoas-AL), Rhizobium etli (Rhz), Pseudomonas sp. (Ps1) and Pseudomonas sp. (Ps2), in addition to the ability to fix N2 in vitro, showed good indole acetic acid (IAA) production (Rhz = 117.75 µg mL; Ps1 = 148.69 µg mL; Ps2 = 138.89 µg mL), inorganic phosphate solubilization (Rhz = solubilization index 3.18; Ps1 = solubilization index 2.73; solubilization index 2.50) and acetylene reduction activity (Rhz = 4.37 nmol C2H4 h−1;Ps1 = 2.15 nmol C2H4 h−1; Ps2 = 2.67 nmol C2H4 h−1). This corroborates postulated information that many diazotrophic bacteria are ubiquitous, performing, in addition to BNF, solubilization of insoluble phosphates and IAA production (Zaidi et al. 2009).
Evaluation of micropropagated sugarcane seedlings at 45 days after inoculation (DAI)
The results observed in this first evaluation showed no significant difference between the treatments inoculated with the three strains of diazotrophic bacteria (Ps1, Ps2 and Rhz), alone and mixed, and the uninoculated control by Tukey’s test (p < 0.05).
Table 2 presents a summary of the different growth parameters evaluated in the sugarcane plants subjected to the five different treatments. The bacterial inoculation did not promote an increase in shoot dry weight compared with the control (Table 2).
Regarding root dry weight, the treatments inoculated with Pseudomonas (Ps1 and Ps2) were statistically inferior to the uninoculated control and the Rhizobium sp. (Rhz) treatment (Table 2).
As for the number of tillers, there was no significant difference between the treatments by Tukey’s test (p < 0.05), although plants that received the mixed inoculation did not present tillering (Table 2). The tillering of sugarcane begins approximately 40 days after planting, which may justify the result.
The results of the evaluation of the N content in shoots showed that the bacteria did not promote a significant difference between the treatments with and without inoculation (p < 0.05).
Evaluation of sugarcane seedlings at 150 days after inoculation (DAI)
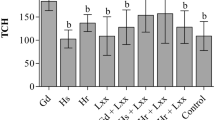
In the second experiment, conducted in pots with nonsterilized soil, the N treatment (nitrogen control) showed a significant difference on the basis of Tukey’s test (p < 0.05) in the four parameters evaluated: shoot and root dry weight, tillering and N content. The plants inoculated with Ps1, Ps2, Rhz and (Ps1, Ps2 and Rhz) did not differ statistically from the plants with nitrogen fertilization in the shoot dry weights (Table 3). This shows that the inoculation had a positive effect on the development of plants.
A lower accumulation of root dry weight was observed with the inoculation treatment, especially in the (Ps1, Ps2 and Rhz) mixture, which promoted a negative effect on root biomass (Table 3).
Regarding the number of tillers, the plants treated with Rhz and the Ps1 + Ps2 + Rhz mixture did not show tillering (Table 3); however, treatments Ps1 and Ps2 favored tillering but did not differ significantly in relation to the nitrogen control. The tillering observed in these treatments may have been caused by ethylene.
Regarding the nitrogen content in the shoots of plants, the results showed that inoculation with Rhz did not present a significant difference in relation to the nitrogen treatment. Notwithstanding, the inoculation with the mixture of strains Ps1, Ps2 and Rhz showed a significant difference on the basis of Tukey’s test (p < 0.05), presenting a slight increase in N accumulation in the shoots compared to that in the nitrogen treatment (Table 3).
The results regarding the nitrogen content in shoots can show the photosynthetic capacity gained by the crop through the treatments.
These results show a different behavior for BNF in the different strains tested in the sugarcane variety RB867515. Inoculation with Rhz was equivalent to the nitrogen control, favoring a greater accumulation of shoot dry weight and a greater accumulation of N in the plants (Table 3).
On the other hand, due to factors intrinsic to the bacterium, we observed that tillering was satisfactory in the plants treated with Ps1 and Ps2. These results are probably related to the complexity of the biological N2 fixation process in sugarcane plants, which involves a range of factors, such as the genetic characteristics of the cultivars and the bacteria associated with them.
All tested isolates have potential mechanisms for promoting plant growth, and one or more of the mechanisms may be responsible for the higher growth of sugarcane under the conditions tested.
Discussion
Sugarcane is able to associate with a great diversity of diazotrophic plant growth-promoting bacteria and reap benefits that are directly or indirectly linked to the nitrogenated nutrition of the plant. These effects are related to greater root growth, higher N absorption and BNF and protection of plants by induction in the phytohormones and indoleacetic acid production (Fukami et al. 2018; Oliveira et al. 2016; Patel and Archana 2017).
The effect of bacterial inoculation is highly dependent on the plant genotype, on soil characteristics, and on many other biotic and abiotic factors (Da Silva et al. 2012). The strains used in this study are homologous because they were isolated from the same crop into which they were subsequently inoculated, and successfully colonized the roots (Boddey and Dobereiner 1982).
Positive results in the initial development of the sugarcane variety RB867515 were also observed by Gírio et al. (2015) with the inoculation of diazotrophic bacteria. The variety RB867515 is more demanding on soil fertility (RIDESA 2010), which justifies its response to nitrogen fertilizer and inoculation. Schultz et al. (2012), Pereira et al. (2013) and Gírio et al. (2015) observed that the genotype of this variety is more responsive to the inoculation of growth-promoting bacterial strains.
Since N is directly related to plant growth and development (Sengupta and Gunri 2015), the observed biomass gain, in addition to the efficiency in the process of assimilation of this element, is a possible contribution of biological fixation in some sugarcane varieties (Donato et al. 2003), which would explain the fact that the higher accumulation of shoot dry weight in the inoculated treatments did not differ statistically from the nitrogen control.
The experiment with micropropagated seedlings of sugarcane variety SP 70-1143 (evaluated at 65 days) did not show significant differences between the treatments with inoculation of diazotrophic bacteria and the uninoculated control according to Canuto et al. (2003). According to the author the difficulty in selecting seedlings of the same size among the micropropagated seedlings obtained for the study, hinders the comparison of the tested treatments and their possible effects in a short period of time, especially for long-cycle plants such as sugarcane.
Lesser root development in sugarcane plants of the variety SP813250 that received inoculation with two bacterial strains under greenhouse conditions was observed by Lima et al. (2011); however, Gírio et al. (2015), when using a bacterial inoculant in presprouted seedlings in variety RB867515, observed that the root dry weight did not increase, but there was an increase in root length.
Although the mass of the root system was not favored by the inoculation, it is possible to infer that the growth-promoting bacteria modified the root system architecture (Gosal et al. 2012). However increases in shoot biomass attributed to the effect of rhizobia on nonleguminous plants have been reported by several authors, citing increases in shoot and leaf biomass, height and/or photosynthetic activity (Chi et al. 2005; Singh et al. 2005).
The photosynthetic capacity is strongly influenced by the amount of nitrogen in the leaf (Chapin et al. 1987). Knowing that Rhizobium sp. (Rhz) has the nifH gene, we can assume that there were adequate conditions inside the plant for the nitrogenase to be active and that the nitrogen was supplied to the plant by biological fixation. Similar results were obtained in rice plants when the RI-530 strain of R. leguminosarum significantly increased the percentage of nitrogen in the leaves (Chi et al. 2005).
The beneficial effect of the inoculation of diazotrophic bacteria on plants in early stages has already been observed in tomato and red pepper inoculated with bacteria of the genera Pseudomonas and Serratia, which mainly promoted increased plant vigor (Islam et al. 2013).
The interaction of plants with beneficial microorganisms at the beginning of plant development is of great importance, as reported by several authors (Gírio et al. 2015; Vargas et al. 2012; Santoyo et al. 2016). For plants that go through the nursery stage, for example, the association with growth-promoting bacteria is of great importance since it prepares the plants for transplanting to the field, stimulating the early growth of the seedling and, consequently, reducing its time of acclimatization, which increases productivity, the turnover in the occupation of infrastructure and the efficiency of the use of specialized labor (Gouda et al. 2018; Silveira et al. 2003).
The present work offers a technological package, leading to micropropagated plants inoculated with bacteria that have the capacity and efficiency to fix atmospheric nitrogen, reducing the use of nitrogen fertilizer and helping supply nitrogen to increase and sustain the production of sugarcane without damaging the environment. This is especially important because the inadequate use of nitrogen fertilizers contributes to the intensification of climate change and causes direct damage to plants. These are among researchable priorities for consolidating the great potential of sugarcane for increasing soil C stocks, offsetting anthropogenic CO2, and effectively mitigating global climate change.
Conclusion
The inoculation of nitrogen-fixing bacteria in micropropagated seedlings of sugarcane variety RB867515 promoted plant development and presented similar performance to the nitrogen treatment. When used individually, the bacterial genus Pseudomonas (Ps1 and Ps2) promoted better tillering, while the genus Rhizobium (Rhz) presented higher dry biomass of shoots and N content in relation to the those in treatment with nitrogen fertilization, promoting the growth of sugarcane plants. The isolated bacteria Ps1, Ps2 and Rhz are capable of and efficient in fixing atmospheric nitrogen, while the mixture of the strains (Ps1, Ps2 and Rhz) did not show a good synergism.
References
Alves BJR, Santos JCF, Urquiaga S, Boddey RM (1994) Métodos de determinação do nitrogênio em solo e planta. In: Hungria M, Araújo RS (eds) Manual de métodos empregados em estudos de microbiologia agrícola. Empresa Brasileira de Pesquisa Agropecuária, Centro Nacional de Pesquisa Agropecuária arroz e feijão, Centro Nacional de Pesquisa da soja – Brasília: EMBRAPA–SPI (EMBRAPA – CNPAF. Documentos, 46). ISSN 0101-9716, pp 449–469
Associação Nacional para Difusão de Adubos (ANDA) (2018) Accessed on line 2018. http://www.anda.org.br/index.php?mpg=03.00.00&ver=por. Accessed 20 Jan 2018
Boddey RM, Dobereiner J (1982) Association of Azospirillum and other diazotrophs with tropical gramineae. Non-symbiotic nitrogen fixation and organic matter in the tropics. Symposium papers vol 1. 12th Int. Congr Soil Sci, New Delhi, pp 28–47
Boddey RM, Boddey LH, Urquiaga S (1990) A técnica de redução de acetileno na medição da fixação biológica de nitrogênio. Embrapa-CNPBS. Documentos, 6 edn. Universidade Rural, Itaguaí/Rio de Janeiro
Canuto EL, Salles JF, Oliveira ALM, Perin L, Reis VM, Baldani JI (2003) Resposta de plantas micropropagadas de cana-de-açúcar à inoculação de bactérias diazotróficas endofíticas. Agronomia 37:67–72
Chapin FS, Bloom AJ, Field CB, Waring RH (1987) Plant responses to multiple environmental factors. Bioscience 37(49–57):132. https://doi.org/10.2307/1310177
Chi F, Shen SH, Cheng HP, Jing YX, Yanni YG, Dazzo FB (2005) Ascending migration of endophytic rhizobia, from roots to leaves, inside rice plants and assessment os benefits to rice growth physiology. Appl Environ Microbiol 71:7271–7278. https://doi.org/10.1128/AEM.71.11.7271-7278.2005
Compainha Nacional de Abastecimento—CONAB (2018) Acompanhamento safra brasileira de cana, v 5—Safra 2018/2019, n.1. Primeiro levantamento, pp 1–62. https://www.conab.gov.br/info-agro/safras/cana. Acessed 18 Jun 2018
Compant S, Clément S, Sessitsch A (2010) Plant growth-promoting bacteria in the rhizo- and endosphere of plants: their role, colonization, mechanisms involved and prospects for utilization. Soil Biol Biochem 42:669–678. https://doi.org/10.1016/j.soilbio.2009.11.024
Da Silva M, Antonio C, de Oliveira P, Xavier G, Rumjanek N, Soares LH, Reis V (2012) Survival of endophytic bacteria in polymer-based inoculants and efficiency of their application to sugarcane. Plant Soil 356:231–243. https://doi.org/10.1007/s11104-012-1242-3
Döbereiner J, Baldani VLD.B, Baldani JI (1995) Como isolar e ientificar bactérias diazotróficas de plantas não leguminosas. Embrapa-CNPAB–SPI, Itaguaí
Donato VMTS, Andrade AG, Souza ES, França JGE (2003) Metabolismo de plantas de cana-de-açúcar cultivadas in vitro sob diferentes concentrações de nitrogênio. Pesq Agropec Bras 38:1373–1379
Franche C, Lindström K, Elmerich C (2009) Nitrogen-fixing bacteria associated with leguminous and non-leguminous plants. Plant Soil 321:35–59. https://doi.org/10.1007/s11104-008-9833-8
Fukami J, Cerezini P, Hungria M (2018) Azospirillum: benefits that go far beyond biological nitrogen fixation. AMB Express 8:1–12. https://doi.org/10.1186/s13568-018-0608-1
Gamalero E, Glick BR (2015) Bacterial modulation of plant ethylene levels. Plant Physiol 169:13–22. https://doi.org/10.1104/pp.15.00284
Gírio LAS, Dias FLF, Reis VM, Urquiaga S, Schultz N, Bolonhezi D, Mutton MA (2015) Bactérias promotoras de crescimento e adubação nitrogenada no crescimento inicial de cana-de-açúcar proveniente de mudas pré-brotadas. Pesq Agropec Bras 50:33–43. https://doi.org/10.1590/S0100-204X2015000100004
Gosal SK, Kalia A, Uppal SK, Kumar R, Walia SS, Singh K, Singh H (2012) Assessing the benefits of Azotobacter bacterization in sugarcane: a field appraisal. Sugar Tech 14:61–67. https://doi.org/10.1007/s12355-011-0131-z
Gouda S, Kerry RG, Das G, Paramithiotis S, Shin H, Patra JK (2018) Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiol Res 206:131–140. https://doi.org/10.1016/j.micres.2017.08.016
Hendre RR, Iyor RS, Kotwalm M, Kluspe SS, Mascarenhas AF (1983) Rapid multiplication of sugar cane by tissue culture. Sugar Cane 1:5–8
Houlton BZ, Morford SL (2015) A new synthesis for terrestrial nitrogen inputs. Soil 1:381–397. http://dx.doi.org/10.5194/soil-1-381-2015
Islam MR, Sultana T, Joe MM, Yim W, Cho JC, Sa T (2013) Nitrogen-fixing bacteria with multiple plant growth-promoting activities enhance growth of tomato and red pepper. J Basic Microbiol 53:1004–1015. https://doi.org/10.1002/jobm.201200141
Kuss AV, Kus VV, Lovato T, Flores ML (2007) Fixação de nitrogênio e produção de ácido indol acético in vitro por bactérias diazotróficas endofíticas. Pesquisa Agropecuária Brasileira 42:1459–1465. https://doi.org/10.1590/S0100-204X2007001000013
Lima RC, Kozusny-Andreani DI, Junior RA, Fonseca L (2011) Caracterização fenotípica de bactérias diazotróficas endofíticas isoladas de cana-de-açúcar. Rev Fac Nac Agron 64:5803–5813
Murashigue T, Skoog F (1962) A revised medium for rapid growth and bioassays with Tobacco tissue cultures. Physiol Plant 15:473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Oliveira RP, Schultz N, Monteiro RC, Pereira W, Araújo AP, Urquiaga S, Reis VM (2016) Growth analysis of sugarcane inoculated with diazotrophic bacteria and nitrogen fertilization. Afr J Agric Res 11(30):2786–2795. https://doi.org/10.5897/AJAR2016.11141
Patel JK, Archana G (2017) Diverse culturable diazotrophic endophytic bacteria from Poaceae plants show cross-colonization and plant growth promotion in wheat. Plant Soil 417:99–116. https://doi.org/10.1007/s11104-017-3244-7
Pereira W, Leite JM, Hipólito GS, Santos CLR, Reis VM (2013) Acúmulo de biomassa em variedades de cana-de-açúcar inoculadas com diferenes estirpes de bactérias diazotróficas. Rev Ci Agro 44:363–370. https://doi.org/10.1590/S1806-66902013000200020
Rede Interuniversitária para o Desenvolvimento do Setor Sucroalcooleiro—RIDESA (2010) Catálogo nacional de variedades “RB” de cana-de-açúcar. Curitiba, p 136
Reis VM (2004) Método de inoculação de bactérias diazotróficas em plantas de cana-de-açúcar micropropagadas. EMBRAPA, Comunicado técnico 65, Seropédica/Rio de Janeiro, Brasil. ISSN 1517-8862
Reis VM, Olivares FL, Oliveira ALM, Reis Junior FB, Baldani JI, Döbereiner J (1999) Technical approaches to inoculate micropropagated sugar cane plants with Acetobacter diazotrophicus. Plant Soil 206:205–211
Rodrigues Neto J, Malavolta Júnior VA, Victor O (1986) Meio simples para isolamento e cultivo de Xanthomonas campestris pv. citri tipo B. Summa Phytopathol 12:16
Rodriguez H, Gonzalez T, Selman G (2000) Expresion of a mineral phosphate solubilizing gene from Erwinia herbícola in two rhizobacterial strains. J Biotechnol 84:155–161. https://doi.org/10.1016/S0168-1656(00)00347-3
Sambrook J, Maccallum P, Russel D (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Springs Harbour Press, New York
Santoyo G, Moreno-Hagelsieb G, Orozco-Mosqueda MDC, Glick BR (2016) Plant growth-promoting bacterial endophytes. Microbiol Res 183:92–99. https://doi.org/10.1016/j.micres.2015.11.008
Sarruge JR (1975) Soluções nutritivas para crescimento de plantas. Summa Phytopathol 1:231–234
Schultz N, Morais RF, Silva JA, Baptista RB, Oliveira RP, Leite JM, Pereira W, Carneiro Júnior JB, Alves BJR, Baldani JI, Boddey RM, Urquiaga S, Reis VM (2012) Avaliação agronômica de variedades de cana-de-açúcar inoculadas com bactérias diazotróficas e adubadas com nitrogênio. Pesq Agropec bras 47:261–268. https://doi.org/10.1590/S0100-204X2012000200015
Sengupta A, Gunri SK (2015) Microbial intervention in agriculture: Na overview. Afr J Microbiol Res 9:1215–1226. https://doi.org/10.5897/AJMR2014.7325
Silveira APD, Silva LR, Azevedo IC, Oliveira E, Meletti LMM (2003) Desempenho de fungos micorrízicos arbusculares na produção de mudas de maracujazeiro-amarelo, em diferentes substratos. Bragantia 62:89–99. https://doi.org/10.1590/S0006-87052003000100012
Singh RK, Mishra RPN, Jaiswal HK (2005) Role of rhizobial endophytes as nitrogen fixer in promoting plant growth and productivity of Indian cultivated upland rice (Oryza sativa L.) plants. In: Wang YP, Lin M, Tian ZX, Elmerich C, Newton WE (eds) Biological nitrogen fixation, sustainable agriculture and the environment. Springer, Amsterdam, pp 289–291
Siqueira Neto M, Galdos MV, Feigl BJ, Cerri CEP, Cerri CC (2016) Direct N2O emission factors for synthetic N-fertilizer and organic residues applied on sugarcane for bioethanol production in Central-Southern Brazil. GCB Bioenergy 8(2):269–280. https://doi.org/10.1111/gcbb.12251
Spatzal T (2015) The center of biological nitrogen fixation: FeMo-Cofactor. Z Anorg Allg Chem 641:10–17
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729. https://doi.org/10.1093/molbev/mst197
United States Department of Agriculture—USDA (2017) Sugar: world markets and trade. Foreign agricultural service. https://apps.fas.usda.gov/psdonline/circulars/sugar.pdf. Acessed 03 July 2018
Vargas L, Carvalho TLG, Ferreira PCG, Baldani VLD, Baldani JI, Hemerly AS (2012) Early responses of rice (Oryza sativa L.) seedlings to inoculation with beneficial diazotrophic bacteria are dependente on plant and bacterial genotypes. Plant Soil 356:127–137. https://doi.org/10.1007/s11104-012-1274-8
Verma SC, Ladha JK, Tripathi K (2001) Evaluation of plant growth promoting and colonization ability of endophytic diazotrophic from deep water rice. J Biotechnol 91:127–141. https://doi.org/10.1016/S0168-1656(01)00333-9
Weisburg WG, Barns SM, Pelletier DA, Gene-Trak DJL (1991) 16S Ribosomal DNA amplification for phylogenetic study. J Bacteriol 173:697–703
Zaidi A, Khan MS, Ahemad M, Oves M, Wani PA (2009) Recent advances in plant growth promotion by phosphate-solubilizing microbes. In: Khan MS, Zaidi A, Musarrat J (eds) Microbial strategies for crop improvemen. Springer, Dordrecht, pp 23–50
Funding
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq grant number 310030/2015-3), and MCSB obtained a scholarship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
do Carmo Silva Barreto, M., do Vale Barreto Figueiredo, M., Silva, M.V. et al. Inoculation of endophlytic diazotrophic bacteria in micropropagated seedlings of sugarcane (Saccharum officinarum sp.). Environmental Sustainability 2, 5–12 (2019). https://doi.org/10.1007/s42398-019-00044-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42398-019-00044-6