Abstract
Background and aims
Diazotrophic endophytic bacteria colonizing cereal plants have a tremendous scope to increase crop yields by supporting low input sustainable agricultural demands of nitrogen. Present work was aimed at studying diversity and functional attributes of nitrogen fixing bacterial endophytes from cereal plants.
Methods
Diazotrophic endophytic bacteria from cereal plants were enriched on nitrogen-free medium and their diversity analyzed by PCR-denaturing gradient gel electrophoresis. Evaluation of plant growth promoting traits of individual isolates and their effect on wheat plants was carried out in plant-soil system.
Results
DGGE analysis and band sequencing showed diazotrophic community to be similar in different plant parts but different than total endophytes. Thirty-one nitrogen fixing endophytic bacteria affiliated to Actinobacteria, Proteobacteria and Firmicutes representing 14 genera were isolated, where Arthrobacter, Rhizobium, and Bacillus spp. were more cosmopolitan. Cross-colonization of the endophytes monitored by green fluorescent protein tagging showed that they are not plant specific. All the bacterial isolates showed presence of nifH gene, siderophore production whereas 81% and 48% isolates showed IAA and P-solubilization, respectively. Biocontrol activity was seen only in Streptomyces spp. which inhibited the growth of Rhizoctonia solani. Pot experiments conducted with wheat plant inoculations showed good growth promotion and correlated with IAA and siderophore production by the isolates.
Conclusion
Diverse endophytic nitrogen fixing bacteria colonize cereal plants non-specifically and possess other plant beneficial traits which help in plant growth promotion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rhizosphere, the soil immediately surrounding the roots and the internal tissues of the plants are colonized by a specially enriched population of bacteria. Many of them are helpful in improving plant health, reducing susceptibility towards disease and relieving abiotic stress (Compant et al. 2010). Endophytes are organisms which colonize and thrive inside the plant tissues during their life cycle and their community varies with plant type, age and stress. From the complex microbial community associated with the exterior surfaces of plants, only a small group is able to enter in to the plant parts and successfully thrive in the interior of the plants without inducing a defense response. The study of plant-associated bacteria is important not only for understanding the ecological role of such bacteria in their interaction with plants but also for the biotechnological application of these bacteria for the promotion of plant growth and agricultural yields (Kuklinsky-Sobral et al. 2004).
Diazotrophic (nitrogen fixing) endophytic bacteria have tremendous potential in increasing nitrogen availability to plants particularly in the case of cereals which unlike legumes do not form symbiotic nitrogen fixing organs like nodules with the bacterial partner. The non-nodulating endophytic bacteria penetrate the cortex area of plant but further entry in to the endodermis requires cell wall degrading enzymes such as cellulases and polygalacturonases, presence of these being confirmed by genome analysis of several endophytic bacteria. Along with the cell wall degrading enzymes, lipopolysaccharide, flagella, pili, and twitching motility also help in mobility and colonization of the above-ground parts of the host plants. Colonization in xylem vessel by bacteria leads to their spread in stems and leaves, where the range of cultivable population is 103–104 CFU g−1 fresh weight of tissue (Compant et al. 2010). Plant growth promoting endophytes are important as inoculants for enhancing plant growth and decreasing the severity of disease incidence in crops (Kang et al. 2007). Endophytic bacteria entering in plants and seeds are more important for site restoration compared to the rhizobacteria which require application every year to maintain their effects (Chanway et al. 2000).
The present study was aimed at studying the diversity of diazotrophic endophytic bacteria from various Poaceae plants by culture-independent as well as culturing methods. The cultured bacteria were studied for their ability to reenter inside another host plant apart from which they were isolated and assayed for their effect on plant growth.
Materials and methods
Isolation of diazotrophic endophytic bacteria
Isolation of diazotrophic endophytic bacteria was carried out from stems and leaves of various Poaceae family plants (maize, wheat, pearl millet, sorghum, and rice) of Gujarat (India) region. Epiphytic bacteria were removed by washing plant parts under running tap water followed by surface sterilization process with 70% ethanol for 1 min, 2% sodium hypochlorite for 1 min and rinsed five times with sterile distilled water. The effectiveness of surface sterilization was checked by inoculating the final rinse water onto Luria-Bertani (LB) agar. One gram of surface sterilized sample was macerated in 1 ml of 0.9% NaCl (N saline) solution. Tissue extract was serially diluted in N saline and aliquots of various dilutions (undiluted, 10−1 to 10−5) were added into semi-solid nitrogen-free base (NFb) medium tubes and also spread on NFb solid medium and incubated at 35 °C for 7 d. Bacterial colonies were purified on JNFb medium by repeated subculturing (Döbereiner et al. 1995).
Community DNA extraction and PCR amplification
Total bacterial endophytic community DNA was extracted from roots, stems and leaves of maize, wheat, pearl millet, sorghum, and rice plants according to Garbeva et al. (2001). For isolation of community DNA of culturable nitrogen fixing endophytes, macerated tissue samples as prepared above were grown in NFb semi-solid medium and bacterial mass from each tube was transferred to new semi-solid NFb medium tube after 7 d of incubation. This process was repeated several times and finally transferred to NFb broth and incubated under static conditions, from which community DNA was extracted using a similar protocol as for total bacterial endophytic community DNA extraction. For DGGE analysis, 16S rRNA gene was amplified from total as well as enriched culturable diazotrophic community using 341F forward primer having a GC clamp and 534R reverse primer as described by Subrahmanyam et al. (2011).
Denaturing gradient gel electrophoresis (DGGE) and DNA sequence analysis
DGGE analysis was carried out in duplicates on two independent gels of 10% polyacrylamide with a gradient of 40 to 70% denaturant (100% denaturant contained 7 M urea and 40% formamide) using D-code system (Bio-Rad, Hercules, CA, USA), in 1X TAE buffer, at constant voltage 50 V for 16 h at 65 °C (Sharaff and Archana 2015). One of the two gels was stained with silver staining and the other by SYBR green (Invitrogen, UK). Imaging was done by AlphaEase 4.0 software (Alpha innotech, USA). The diversity indices were obtained using the GelCompar II (Applied Maths NV). Selected DNA bands were carefully cut out from the gel stained with SYBR green (for ease of reamplification) and placed in 100 μl sterile distilled water at 4 °C for overnight. PCR amplification of the eluted bands was carried out using primers 341F without the GC clamp and 534R. Amplified PCR products were purified and subjected to Sanger sequencing (SciGenom Labs, India). The resulting sequence was analyzed by BLASTn in NCBI database (Altschul et al. 1990).
Bacterial identification by biochemical characterization and 16S rRNA gene sequencing
Biochemical characteristics of endophytic bacteria were determined for each colony type and included gram staining, catalase, Hugh Leifson oxidase test, MR-VP, indole test etc. performed as mentioned in the Bergey’s Manual of Determinative Bacteriology (Holt et al. 1994). The isolates were tested for carbohydrate fermentation with 10 sugars (Himedia, India): glucose, galactose, fructose, xylose, mannitol, maltose, meso-erythritol, sucrose, inositol, lactose. In this test, 1% proteose peptone water with Andrade’s indicator was used for detection of carbohydrate fermentation and inverted Durham tubes for the detection of gas production. A 1% sugar solution was sterilized at 10 psi for 20 min and added separately into the medium. Overnight grown cultures were used as inocula and test result recorded after incubation at 35 °C for 24–48 h.
For identification of bacteria based on 16S rRNA gene sequence, genomic DNA extraction from pure cultures was carried out as described Sambrook and Russell (2001). Universal primers of eubacteria 27F and 1107R were used for amplification of 16S rRNA gene. The polymerase chain reactions (PCR) were carried out in 25 μl systems consisting of 50 ng DNA template, 0.5 μl of 10 μM each primer, 1 μl of 2.5 mM dNTPs each, 0.5 U Taq DNA polymerase and 2.5 μl 10X Taq buffer. Amplification was carried out at initial denaturation at 95 °C for 5 min, followed by 30 cycles consisting of initial denaturation 94 °C for 45 s, annealing temperature 58 °C for 30 s, elongation 72 °C for 1 min, final elongation carried out at 72 °C for 10 min (Chaturvedi and Archana 2012). Amplified PCR products were analyzed on 1% agarose gel. Sequencing of 16S rRNA gene amplicons was performed by Xcelris Labs (India). The sequences were analyzed by the Sequence Match tool at Ribosomal Database Project (RDP) (Wang et al. 2007) and Nucleotide BLASTn in NCBI database (Altschul et al. 1990). A phylogenetic tree was constructed by neighbor-joining algorithm with Jukes-Cantor model method of MEGA 6 (Tamura et al. 2013).
Characterization of plant growth promoting (PGP) traits
NifH gene amplification was carried out with PolF and AQER primers (Poly et al. 2001). The PCR reaction mixture was made as described earlier for 16S rRNA amplification and PCR was carried out as follows: an initial denaturation at 94 °C for 5 min followed by 30 cycles of 94 °C for 1 min, 55 °C for 45 s and 72 °C for 1 min with a final extension at 72 °C for 5 min.
Phosphate solubilization was examined on Pikovskaya’s agar plates. Isolates showing positive (zone of clearance) results were further studied for acidification of rock phosphate (RP) containing buffered minimal medium with 100 mM glucose and 50 mM Tris-Cl pH 8.0 (Gyaneshwar et al. 1999). Senegal RP (Sharma et al. 2005) was used as the sole P source and methyl red used as a pH indicator. Overnight grown cultures were spot inoculated on the surface of the solidified medium and incubated at 28 °C for 96 h. RP solubilization was recorded positive when a change in color of the medium from yellow to red surrounding the bacterial colony (due to acid production) was observed. For quantitative analysis of RP solubilization, a similar medium was used except that pH indicator and agar were absent; P released in supernatant was measured at regular time interval by ascorbate method described previously (Gyaneshwar et al. 1999).
Indole acetic acid (IAA) production was checked by growing bacterial cultures in 50 ml of LB broth amended with 5 mM L- tryptophan for 48 h at 28 °C (200 rpm) under shaking condition. IAA production was quantitated spectrophotometrically by using Salkowski’s reagent as described by Ahmad et al. (2008). Pure IAA (Himedia, India) was taken as reference for calibration.
For detection of siderophore production, endophytic bacterial isolates were grown in deferrated LB broth and incubated for 48 h under shaking condition at 180 rpm. Identification of hydroxamate siderophore was carried by taking 0.5 ml culture supernatant and adding 0.5 ml of 6 N H2SO4 and autoclaving the mixture at 15 psi for 30 min. After the reaction mixture cooled down 1 ml sulphanilic acid (1% w/v in 30% acetic acid v/v) was added followed by 0.5 ml of iodine (1.3% iodine in 30% acetic acid). Excess of iodine was removed by addition of 2% (w/v) 1 ml sodium arsenate (Na3AsO4). Then α-naphthylamine (0.3% w/v in 30% acetic acid) was added and incubated the reaction mixture for 30 min, absorbance was taken at 526 nm. Hydroxylamine hydrochloride was used as standard (0.1–1 μg ml−1) for calibration. Catechol-type of siderophore was measured by Arnow test. Isolates were grown in similar condition as above mentioned for hydroxamate-type siderophore. Culture supernatant (1 ml) was mixed with 1 ml of 0.5 N HCl followed by 1 ml of nitrite molybdate reagent (10% each sodium nitrite and sodium molybdate in distilled water), allowed to stand for 5 min and then 1 ml 1 N NaOH was added. The absorbance of the pink color developed was recorded at 510 nm and calibrated against 2, 3-dihydroxybenzoic acid as standard (10–100 μg ml−1) (Khan et al. 2006).
All the bacterial isolates were screened for the hydrogen cyanide production (Lorck 1948). LB agar was amended with glycine 4.4 g l−1 and the bacterial cultures were inoculated on its surface in top agar. A Whatman filter paper No. 1 saturated in solution containing sodium carbonate (2% w/v) and picric acid (0.5% v/v) was attached to the lid of the plate, which was then sealed with parafilm and incubated at 35 °C for 4–5 d. The development of orange to brown color on the filter paper indicated HCN production.
Dual culture test was performed to analyze the antagonistic activity of endophytic isolates against plant pathogenic fungus Rhizoctonia solani. The endophytic isolates were grown for 12–16 h except Streptomyces spp. which were grown for 48 h in LB broth at 28 °C. R. solani was grown for 5 d on potato dextrose agar (PDA) and an agar plug with the mycelia was transferred on to one side of a fresh PDA plate while test cultures were streaked on the opposite side. Plates were incubated at 30 °C for 5 d and observed for zone of inhibition of the fungal mycelia around the bacterial growth.
All the plant growth promoting traits were replicated three times.
Detection of hydrolytic enzymes
Many endophytic organisms produce the extracellular enzymes which may help the bacteria gain entry inside the host plant, derive the nutrition or help in inhibition of the pathogens. Cellulase, chitinase, pectinase and protease activities were determined qualitatively on appropriate media plates spot inoculated with overnight grown cultures and incubated at 28 °C. Cellulase production was detected by congo red according to Teather and Wood (1982) on carboxymethylcellulose (CMC) agar (0.5% CMC, 1% yeast extract, and 0.05% peptone) plates after incubation for 60 h. Chitinase production was determined on Monreal Reese medium containing colloidal chitin (0.5%) after incubation for 7 d as a zone of clearance (Nagpure and Gupta 2013). Protease and pectinase production was determined by the methods of Pillai and Archana (2008) and Verma et al. (2001), respectively.
The quantitative enzyme assays for cellulase, chitinase, protease and pectinase activity were performed using culture supernatants of cells grown in dilute LB (0.3X) containing individually CMC (1 g l−1), colloidal chitin (10 g l−1), skim milk powder (10 g l−1), or pectin (5 g l−1) respectively after incubation at 28 °C for 5 d under shaking conditions. The enzyme assays were carried out by methods described for cellulase (Saha et al. 2006), chitinase (Nagpure and Gupta 2013), and pectinase (Khatri et al. 2015). Protease assay was carried out by the method of Kembhavi et al. (1993) and the reaction mixture was incubated at 37 °C for 30 min. All the enzyme assays were replicated three times.
Tagging of endophytes with green fluorescent protein (gfp)
Thirteen gram-negative endophytic bacterial isolates were tagged with gfp for confirming their endophytic colonization of wheat plants by transformation with the broad host range plasmid pHC60 carrying the gfp gene and harboring tetracycline resistance (Cheng and Walker 1998; Sharaff and Archana 2016). The plasmid was transformed into E. coli S17.1 (Simon et al. 1983) followed by bi-parental conjugation with the endophytic isolates (Tanaka et al. 2006). Transformants were selected on the basis of growth on JNFb medium supplemented with 40 μg ml−1 tetracycline.
Detection of endophytic presence of isolates by confocal laser scanning microscopy
The endophytic nature of isolates was confirmed by inoculating in wheat plants with gfp tagged endophytes under hydroponics system in Murashige-Skoog medium (Himedia, India) and observing for the presence of bacteria in plant parts by confocal laser scanning microscopy (CLSM). Wheat seeds were surface sterilized by first washing under running tap water and then transferred to 1% HgCl2 for 5 min, treated with 70% ethanol for 5 min followed by repeated washes with sterile distilled water. Seeds were then transferred on 0.8% agar containing petri-plate and germinated under dark at 25 °C for 2–4 d. Germinated seedlings with similar lengths of radicle were soaked for 30 min with overnight grown pure cultures of endophytic bacteria grown individually and diluted appropriately (in N saline) to give 108 CFU ml−1. Inoculated seedlings were transferred for growth into the hydroponics system and cultivated at 25 °C under greenhouse conditions with natural daylight. On 7th d after inoculation plants were harvested and different plants parts were processed immediately for CLSM (LSM 700 Carl Zeiss, GmbH). Bacterial counts (CFU g−1 fresh weight) were determined from surface sterilized plants by plating extracts on NFb medium. The endophytic bacteria that were not tagged with gfp were also processed similarly for CFU g−1 determination for confirming their endophytic nature.
Pot inoculation experiments
The diazotrophic endophyte bacterial isolates were analyzed for their plant growth promotion ability in gnotobiotic condition (sterilized soil and surface sterilized seeds). Bacterized wheat seedlings (dipped in culture suspension of approximately 108–109 CFU ml−1) were prepared for pot inoculation as mentioned above for hydroponics. Each pot received 2 kg of sterile soil and four bacterized seedlings (two pots per treatment); the uninoculated plants were taken as negative control and seedlings inoculated Herbaspirillum seropedicae Z67 taken as positive control. Plants were maintained in a totally random arrangement in greenhouse at 25 °C, under natural daylight (approximately 12 h photoperiod) and relative humidity of 75%. After 30 d of growth, plants were uprooted, surface sterilized and processed for CFU g−1 of fresh weight of tissue for detection endophytic presence of bacteria. Effect of various strains on the following different parameters of wheat plants was determined: chlorophyll content (Arnon 1949), root length, and shoot length, the dry and wet weight of root, stem and leaves. Nitrogen content of the plants was measured using the micro-Kjeldahl method (Villegas et al. 1984). Pot experiments were repeated twice and results compiled from plants of both the experiments.
Data analysis
Data are expressed as a mean along with standard deviation and the number of replicates mentioned at each experiment. Statistical analyses for plant inoculation experiments were performed in Microsoft Excel. The variance in data was analyzed using Levene’s test which was then followed by the Student’s t-test to determine significant differences between the means. Principal component analysis was carried out using PAST software (Hammer et al. 2001).
Results
Diversity of endophytic diazotrophic bacteria from Poaceae plants
The diversity of diazotrophic community residing in the different plants analyzed using 16S rRNA DGGE of bacterial population enriched in NFb medium was compared with total endophytic bacterial community (directly obtained from plant tissues without any enrichment). There was a good congruence of band patterns and diversity indices on the two duplicate gels regardless of being stained with SYBR green (Fig. 1) or silver staining (data not shown). As seen, band patterns of different plants showed considerable variation with respect to both total as well as diazotrophic communities. Shannon-Wiener diversity indices of total endophytic bacterial community were found to be high in rice (root-1.04 and stem-0.99), maize (root-0.76, stem-0.92 and leaves-0.77) and pearl millet (root-0.82) whereas enriched diazotrophic communities of rice (root-1.13) and maize (stem-0.70 and leaves-0.63) showed higher diversity as compared to the other plants (Table 1, calculated from gels depicted in Fig. 1). Sequence analysis of selected bands from the total community showed best matches with uncultured Bacillus and Proteobacteria clones, and Serratia strains while the selected DGGE bands of the diazotrophic community enriched in NFb medium showed best correspondence with uncultured Brevundimonas and Rhizobium clones, Sphingomonas, Pseudomonas and Agrobacterium strains (Table 2).
16S rRNA DGGE community fingerprints of endophytic bacteria from Poaceae plants detected by SYBR green staining. T represents total eubacterial community and D represents diazotrophic endophytic eubacterial populations. R- root; S- stem; L- leaves. Lane M- Marker consisting of mixture consisted of a 16S rRNA amplicons from Staphylococcus sp. (I), Pseudomonas sp. (II), Ralstonia sp. (III), Brevundimonas sp. (IV), Streptomyces sp. (V)
Isolation and phylogenetic characterization of diazotrophic endophytic bacteria
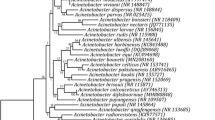
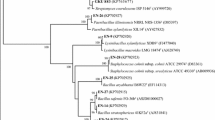
Upon plating plant extracts on NFb medium, it was noticed that the cultivable bacteria able to grow on this medium were in a range of 103–105 CFU g−1 of leaves and stems sample of the plants. A total of 31 endophytic diazotrophic bacteria were isolated as pure cultures from five Poaceae family cereal plants selected on the basis of the differences in phenotype on NFb medium as well as their ability to be repeatedly cultured on NFb medium indicating their diazotrophic nature. Biochemical characterization of all isolates is given in Table S1 (Supplemental material). Analysis of 16S rRNA gene sequence in NCBI and RDP databases shows affiliation of the isolates to phyla Proteobacteria (53%) [α-Proteobacteria (20%), β- Proteobacteria (23%), γ- Proteobacteria (10%)], Actinobacteria (37%) and Firmicutes (10%) (Fig. 2). The 16S rRNA gene sequences obtained are deposited in GenBank (Accession numbers KR921473- KR921502, KT123190).
Dendrogram showing phylogenetic relationship of diazotrophic endophytic bacterial isolates from Poaceae plants using 16S rRNA gene partial sequences. The neighbor-joining method was used with Jukes-Cantor model algorithm. Numbers at the nodes indicate the bootstrap value analyzed with 1000 replicates
Endophytic colonization of wheat plants by diazotrophic bacterial isolates
For the confirmation of the endophytic presence of bacteria, thirteen isolates (Ralstonia sp. M1, Acinetobacter sp. M5, Ralstonia sp. MS1, Pantoea sp. MS3, Rhizobium sp. W3, Rhizobium sp. SS2, Rhizobium sp. R2, Brevudimonas sp. R3, Achromobacter sp. RS1, RS3, RS4, RS5, RS8 along with the positive control H. seropedicae Z67) were tagged with gfp containing plasmid pHC60 and inoculated on wheat plants. On the 7th d after inoculation, different parts of the plants were found to show the endophytic presence of bacteria by confocal microscopy. Fig. 3 shows representative images of root endophytic presence of few isolates. Depending on the position of the root observed, its thickness varied from 50 to 130 μm and bacteria were generally detected at 10 to 30 μm depths from root surface indicating their endophytic colonization. This was further confirmed by CFU count (on tetracycline containing NFb medium) which ranged from 103 to 105 CFU g−1 of fresh weight of tissue for all the isolates studied. In control uninoculated plants, no cells were detected by microscopy as well as plating.
Plant growth-promoting traits in different isolates
All endophytic bacterial isolates used in this study showed positive nifH gene amplification (~320 bp). The nifH gene of endophytic strains (Pantoea sp. MS3, Arthrobacter sp. W1, Bacillus sp. S1, and Rhizobium sp. R2) was confirmed by sequencing and analyzed using BLASTn which showed the matching with the nitrogenase reductase gene of Pseudomonas stutzeri (CP002622).
Among all isolates, only Streptomyces spp. (SS1, SS5, and SS8) showed the inhibition of R. solani. Antagonistic effect of Streptomyces spp. was observed by inhibition zone >30 mm after 7 d. All isolates were negative for HCN production. Of all 31 isolates, fifteen endophytic bacterial strains showed the zone of clearance on Pikovskaya’s medium for phosphate solubilization (Table S2). When examined on rock phosphate containing medium only four isolates showed positive result (Staphylococcus sp. M4 J, Staphylococcus sp. M4, Acinetobacter sp. M5, and Ralstonia sp. MS1) and Acinetobacter sp. M5 alone showed the detectable level of Pi release in buffered minimal medium with a release of was 0.294 μmole (pH 4.3) of P after 96 h.
In this study, 81% of endophytic bacterial isolates produced IAA. Many strains showed high IAA production above 100 μg ml−1 (Table S2). Siderophore production analysis on CAS medium was difficult since only a small number of isolates were able to grow on this medium. A majority of isolates showed the mixed type of siderophore production with maximum production seen in case of Achromobacter spp. isolated from rice and Methylobacterium spp. from pearl millet (Table S2). It was observed that 81%, 84%, 35%, and 13% isolates were positive for cellulase, pectinase, chitinase, and protease respectively (Fig. 4a). Highest cellulase activity was detected in the case of Aeromicrobium sp. R1. Protease activity was high found in Arthrobacter sp. M6 while chitinase activity was found high in Staphylococcus sp. M4, Acinetobacter sp. M5 and Pantoea sp. MS3 (Table S2).
Diazotrophic endophytic bacterial isolates were analyzed for total 8 PGP traits but none of the isolates was found positive for all the PGP traits. There were total 8 isolates positive for 6 PGP traits, 8 isolates for 5 PGP traits, 10 isolates for 4 PGP traits, 2 isolates for 3 PGP traits and 3 isolates for 2 PGP traits (Fig. 4b).
Effect of endophyte inoculation on wheat plants
Diazotrophic endophytic bacteria showed a range of effects on wheat plant growth in 30 d pot inoculation experiments. Majority of endophytes showed the improvement in dry mass of stem, root, leaves and increase the chlorophyll content (Table 3). The effectiveness of the isolates on the plant growth was compared with positive control H. seropedicae Z67 and with uninoculated controls. Total nitrogen content of aerial part of plants was also increased significantly over uninoculated controls. Significant plant growth promotion in above and below ground parts of the wheat was observed with the Streptomyces spp. compared to the other isolates (Table 3). Endophytic colonization was confirmed by counting the CFU from surface sterilized plant parts after 30 d of inoculation. Colonization capacity was varied in the different parts of the plants. Higher colonization was observed in roots and stems (102–107 CFU g−1 of fresh tissue) as compared to leaves (102–104 CFU g−1 of fresh tissue) (Table 4). The uninoculated control plants when plated similarly on NFb medium did not show presence of any diazotrophic endophytic bacteria. The plant growth promotion was analyzed by PCA analysis to determine the critical component of the study which influences the overall growth of the wheat plant. PCA analysis was performed using nine variables to extract the principal component (PC). The extracted PC1 - IAA (39% variance) and PC2- siderophore (22% variance) together gave the 61% (eigenvalue >1.5) variance. The loading score showed the PC1 correlation with the siderophore production, root length, wet and dry weight of root whereas PC2 showed the positive correlation with the shoot length, root length, the wet and dry weight of shoot, the wet and dry weight of root, and total chlorophyll content (Table 5). The scatter graph plot is divided into the four groups with correlation with PC1, PC2, both PC1 and PC2 and does not show any correlation with the principal component (Fig. 5).
Discussion
It is believed that the diversity of endophytic bacterial community varies according to different plants species, host specificity and geographical location (Zinniel et al. 2002) and successful colonization into the host plant depends on plant genotype and development stage (Andreote et al. 2010). Total endophytic communities have been studied from various plants such as rice (Rangjaroen et al. 2014), maize (Seghers et al. 2004), potato (Garbeva et al. 2001), citrus plants (Araujo et al. 2002) and grasses (Wemheuer et al. 2016). However, endophytic diazotrophic bacterial diversity has not been widely explored. Most reports on endophytic nitrogen fixing populations are available for rice plants (Prakamhang et al. 2009; Sessitsch et al. 2012; Rangjaroen et al. 2014; Ferrando and Scavino 2015). The present work is a systematic study of diazotrophic community residing within above-ground plant parts of five important cereal crops. The study analyzed diazotrophic endophyte diversity by DGGE as well as characterized several diazotrophic endophytic bacteria from the five plant species. In general, the DGGE analysis showed that diazotrophic communities were different as compared to the total communities of the same plant and also varied with the plant. However, in most cases the diazotrophic community appeared similar in different plant parts. DGGE band sequencing showed majority of endophytes affiliated to Proteobacteria. Bands matching with Pseudomonas were detected as part of the diazotrophic community in three plant species. It should however be pointed out that the identification based on short reads of the DGGE sequences as well as lower sequence identities might be of relevance only up to the phylum level. The best matching sequences have diverse isolation sources and only Agrobacterium tumefaciens strain ISSDS-369 among the matches is reported as diazotrophic. Earlier Bacillus sp., Serratia sp. have been reported from rice and wheat plants (Gyaneshwar et al. 2001; Larran et al. 2002; Mano et al. 2007; Liu et al. 2010), Pseudomonas aeruginosa from pearl millet (Gupta et al. 2013), Brevundimonas sp. from rice (Sun et al. 2008), Sphingomonas sp. from tomato and rice (Mano et al. 2007; Khan et al. 2014). Interestingly, our study found sequences affiliated to Rhizobium/Agrobacterium among the bands sequenced from the diazotrophic endophytic population.
The present work was also aimed at isolation and characterization of diazotrophic endophytic bacteria from Poaceae family plants. Our strategy used here for the isolation of diazotrophic endophytic bacteria was based on repeated subculturing on NFb medium. The success of this strategy is reflected by the detection of the nifH gene in each of the isolate. Pure cultures of 31 diazotrophic endophyte bacteria were characterized in the present work. They were found to be affiliated to α, β, γ- Proteobacteria, Actinobacteria, and Firmicutes. They belonged to 14 genera including Achromobacter, Arthrobacter, Streptomyces, and Rhizobium in decreasing order of abundance. Only a few bacteria were found in common between plants, such as Rhizobium spp. found in wheat, rice, and sorghum, Arthrobacter spp. found in maize and wheat, Bacillus spp. found in sorghum and pearl millet. Among the endophytes obtained, only Ralstonia spp. are known to cause disease in certain plants. However, there are reports of plant growth promoting Ralstonia spp. associated with banana and soybean (Kuklinsky-Sobral et al. 2004; Jimtha et al. 2014). In accordance with the DGGE results, several Rhizobium/Agrobacterium spp. were also isolated in as diazotrophs from different Poaceae plants. We have not come across any earlier reports showing the direct isolation of free living nitrogen fixing Rhizobium spp. growing on nitrogen-free medium. However, Yanni et al. (1997) showed that during crop rotation, the benefit to the cereal crop occurs because of the endophytic colonization of Rhizobium spp. on roots of rice plants. Interestingly, although the standard H. seropedicae Z67 showed efficient colonization and plant growth promotion of wheat plants, we did not obtain Herbaspirillum spp. in the sequences nor among the isolates.
Our results thus suggest that nitrogen fixation in cereal plants tissues is dominated by phylum Proteobacteria. Many of the bacterial genera reported here such as Pantoea, Staphylococcus, Brevundimonas, Methylobacterium, Pseudomonas, Ralstonia and Bacillus have been previously found as endophytes from banana plants (Thomas et al. 2008) indicating similarity in distribution in cereal and non-cereal plants. Our results suggest that they are diazotrophic and have potential to increase N availability to plants. In contrast to endophytes recovered from cereal plants grown in normal agriculture fields, endophytes as well as rhizospheric populations recovered from plants grown in heavy metal contaminated soils showed the predominance of phyla Firmicutes and Actinobacteria with very few members of Proteobacteria (Sharaff and Archana 2015; Román-Ponce et al. 2016). These findings, along with the results presented here, indicate that metal stress might lead to loss of nitrogen fixing endophytic population.
In this work endophytic bacterial entry was confirmed in the wheat plants via gfp tagging. Endophytic bacterial isolates were able to colonize intracellular spaces passing through the epidermis, endodermis and then entered into the central cylinder of the plant. Many bacterial isolates showed the hydrolytic enzyme production which may assist their entry into the host plant. Due to the problem of high auto-fluorescence in the stem and leaves part of the plant we are not able to detect all gfp tagged endophytic bacterial occurrence in this part of the plant. For addressing this problem we performed the plate counts to confirmed presence of endophytes in these plant parts and found them to be present in significant numbers. Interestingly, no matter from which plant the isolate was obtained, all were able to colonize wheat plants indicating low plant specificity.
Phytohormones play an important role in plant growth promotion (Glick 2014). Our findings suggest high IAA production by the endophytic isolates ranging from 33 to 890 μg ml−1 with the highest IAA production by Arthrobacter sp. M6. Among all the isolates, phosphate solubilization was highest with Acinetobacter sp. M5 which solubilizes the phosphate even in buffered conditions. Acinetobacter spp. are considered as effective producers of pyrroloquinoline quinone (PQQ) which is important for gluconic acid production and P-solubilization (Ogut et al. 2010). All isolates obtained in this study showed siderophore production which is not surprising since many plant associated α, β, γ-Proteobacteria, bacilli, actinobacteria demonstrate the ability of siderophore production under low iron availability (Tian et al. 2009). Rhizobium spp. isolated in this study showed the hydroxamate, catecholate and mixed type of siderophore production. This is interesting since earlier similar observation has been made with legume nodulating rhizobia (Khan et al. 2006). Among all endophytic bacterial isolates only Streptomyces spp. showed the biocontrol activity against the fungal pathogens. These Streptomyces spp. do not produce chitinase and protease enzyme, suggesting synthesis of antifungal metabolites production. Different combinations of PGP traits in these diazotrophic endophytic bacterial isolates were observed (Fig. 4b).
All the isolates obtained in this study promoted the growth of wheat plants. Higher nitrogen content was found in aerial part of the wheat as compared to the uninoculated control plants indirectly supporting the in planta nitrogen fixation ability of the isolates. The importance of nitrogen fixation ability of bacteria associated with cereal plants is sometimes the main factor promoting the plant growth, as shown for a nifH defective mutant of Klebsiella pneumoniae which when inoculated on the wheat plant showed stunted growth and yellowing of plant (Iniguez et al. 2004). This may explain the observation that isolates showing lower amount of IAA production were also effective in enhancing plant growth. Interestingly, different endophytic bacterial isolates in this study supported the plant growth by different ways. Some endophytic bacteria showed an increase in the wet and dry weight of shoot and root, while others improved shoot and root length. All the diazotrophs showed an increase in chlorophyll content as compared to control plants. In the present study, we found that all the isolates showed cross-colonization of wheat plants regardless of their source of isolation. This capacity of endophytes makes them a potential candidate for the broad host range biofertilizer development.
To summarize, the diversity study of total bacterial community and nitrogen fixer community residing in plant parts showed the presence of only few nitrogen fixing bacteria inside the plants of Gujarat region, India. This study also indicates the culture dependent and independent methods both very useful for exploring the bacterial diversity inside the plants. Future studies will be focused on detailed characterization of some of these isolates for their plant protection and growth promotion ability in various plants pathogen systems.
References
Ahmad F, Ahmad I, Khan MS (2008) Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiol Res 163:173–181
Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Andreote FD, da Rocha UN, Araújo WL, Azevedo JL, van Overbeek LS (2010) Effect of bacterial inoculation, plant genotype and developmental stage on root-associated and endophytic bacterial communities in potato (Solanum tuberosum). Antonie Van Leeuwenhoek 97:389–399
Araujo WL, Marcon J, Maccheroni W, van Elsas JD, van Vuurde JW, Azevedo JL (2002) Diversity of endophytic bacterial populations and their interaction with Xylella fastidiosa in citrus plants. Appl Environ Microbiol 68:4906–4914
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1–15
Chanway CP, Shishido M, Nairn J, Jungwirth S, Markham J, Xiao G, Holl FB (2000) Endophytic colonization and field responses of hybrid spruce seedlings after inoculation with plant growth-promoting rhizobacteria. For Ecol Manag 133:81–88
Chaturvedi R, Archana G (2012) Novel 16S rRNA based PCR method targeting Deinococcus spp. and its application to assess the diversity of deinococcal populations in environmental samples. J Microbiol Methods 90:197–205
Cheng HP, Walker GC (1998) Succinoglycan is required for initiation and elongation of infection threads during nodulation of alfalfa by Rhizobium meliloti. J Bacteriol 180:5183–5191
Compant S, Clément C, Sessitsch A (2010) Plant growth-promoting bacteria in the rhizo- and endosphere of plants: their role, colonization, mechanisms involved and prospects for utilization. Soil Biol Biochem 42:669–678
Döbereiner J, Baldani VLD, Reis VM (1995) Endophytic occurrence of diazotrophic bacteria in non-leguminous crops. In: Fendrik I, del Gallo M, Vanderleyden J, de Zamaroczy M (eds) Azospirillum VI and related microorganisms. Springer, Berlin, pp 3–14
Ferrando L, Scavino AF (2015) Strong shift in the diazotrophic endophytic bacterial community inhabiting rice (Oryza sativa) plants after flooding. FEMS Microbiol Ecol 91:fiv104
Garbeva P, van Overbeek LS, van Vuurde JWL, van Elsas JD (2001) Analysis of endophytic bacterial communities of potato by plating and denaturing gradient gel electrophoresis (DGGE) of 16S rRNA based PCR fragments. Microb Ecol 41:369–383
Glick BR (2014) Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol Res 169:30–39
Gupta G, Panwar J, Jha PN (2013) Natural occurrence of Pseudomonas aeruginosa, a dominant cultivable diazotrophic endophytic bacterium colonizing Pennisetum glaucum (L.) R. Br. Appl Soil Ecol 64:252–261
Gyaneshwar P, James EK, Mathan N, Reddy PM, Reinhold-Hurek B, Ladha JK (2001) Endophytic colonization of rice by a diazotrophic strain of Serratia marcescens. J Bacteriol 183:2634–2645
Gyaneshwar P, Parekh LJ, Archana G, Poole PS, Collins MD, Hutson RA, Kumar GN (1999) Involvement of a phosphate starvation inducible glucose dehydrogenase in soil phosphate solubilization by Enterobacter asburiae. FEMS Microbiol Lett 171:223–229
Hammer Ø, Harper DAT, Ryan PD (2001) Past: paleontological statistics software package for education and data analysis. Palaeontol Electron 4:1–9
Holt JG, Krieg NR, Sneath PHA, Staley JT, Williams ST (1994) Bergey’s Manual of determinate bacteriology, 9th edn. Williams and Wilkins, Baltimore
Iniguez AL, Dong Y, Triplett EW (2004) Nitrogen fixation in wheat provided by Klebsiella pneumoniae 342. Mol Plant-Microbe Interact 17:1078–1085
Jimtha JC, Smitha PV, Anisha C, Deepthi T, Meekha G, Radhakrishnan EK, Remakanthan A (2014) Isolation of endophytic bacteria from embryogenic suspension culture of banana and assessment of their plant growth promoting properties. Plant Cell Tissue Organ Cult 118:57–66
Kang SH, Cho HS, Cheong H, Ryu CM, Kim JF, Park SH (2007) Two bacterial entophytes eliciting both plant growth promotion and plant defense on pepper (Capsicum annuum L.) J Microbiol Biotechnol 17:96–103
Kembhavi AA, Kulkarni A, Pant A (1993) Salt-tolerant and thermostable alkaline protease from Bacillus subtilis NCIM no.64. Appl Biochem Biotechnol 38:83–92
Khan A, Geetha R, Akolkar A, Pandya A, Archana G, Desai AJ (2006) Differential cross-utilization of heterologous siderophores by nodule bacteria of Cajanus cajan and its possible role in growth under iron-limited conditions. Appl Soil Ecol 34:19–26
Khan AL, Waqas M, Kang SM, Al-Harrasi A, Hussain J, Al-Rawahi A, Lee IJ (2014) Bacterial endophyte Sphingomonas sp. LK11 produces gibberellins and IAA and promotes tomato plant growth. J Microbiol 52:689–695
Khatri BP, Bhattarai T, Shrestha S, Maharjan J (2015) Alkaline thermostable pectinase enzyme from Aspergillus niger strain MCAS2 isolated from Manaslu conservation area, Gorkha, Nepal. SpringerPlus 4:488
Kuklinsky-Sobral J, Araujo WL, Mendes R, Geraldi IO, Pizzirani-Kleiner AA, Azevedo JL (2004) Isolation and characterization of soybean-associated bacteria and their potential for plant growth promotion. Environ Microbiol 6:1244–1251
Larran S, Perello A, Simon MR, Moreno V (2002) Isolation and analysis of endophytic microorganisms in wheat (Triticum aestivum L.) leaves. World J Microbiol Biotechnol 18:683–686
Liu X, Jia J, Atkinson S, Cámara M, Gao K, Li H, Cao J (2010) Biocontrol potential of an endophytic Serratia sp. G3 and its mode of action. World J Microbiol Biotechnol 26:1465–1471
Lorck H (1948) Production of hydrocyanic acid by bacteria. Physiol Plant 1:142–146
Mano H, Tanaka F, Nakamura C, Kaga H, Morisaki H (2007) Culturable endophytic bacterial Flora of the maturing leaves and roots of rice plants (Oryza sativa) cultivated in a paddy field. Microbes Environ 22:175–185
Nagpure A, Gupta RK (2013) Purification and characterization of an extracellular chitinase from antagonistic Streptomyces violaceusniger. J Basic Microbiol 53:429–439
Ogut M, Er F, Kandemir N (2010) Phosphate solubilization potentials of soil Acinetobacter strains. Biol Fertil Soils 46:707–715
Pillai P, Archana G (2008) Hide depilation and feather disintegration studies with keratinolytic serine protease from a novel Bacillus subtilis isolate. Appl Microbiol Biotechnol 78:643–650
Poly F, Monrozier L, Bally R (2001) Improvement in the RFLP procedure for studying the diversity of nifH genes in communities of nitrogen fixers in soil. Res Microbiol 152:95–103
Prakamhang J, Minamisawa K, Teamtaisong K, Boonkerd N, Teaumroong N (2009) The communities of endophytic diazotrophic bacteria in cultivated rice (Oryza sativa L.) App soil ecol 42:141–149
Rangjaroen C, Rerkasem B, Teaumroong N, Sungthong R, Lumyong S (2014) Comparative study of endophytic and endophytic diazotrophic bacterial communities across rice landraces grown in the highlands of northern Thailand. Arch Microbiol 196:35–49
Román-Ponce B, Ramos-Garza J, Vásquez-Murrieta MS, Rivera-Orduña FN, Chen WF, Yan J, Wang ET (2016) Cultivable endophytic bacteria from heavy metal(loid)-tolerant plants. Arch Microbiol 198:941–956
Saha S, Roy RN, Sen SK, Ray AK (2006) Characterization of cellulase-producing bacteria from the digestive tract of tilapia, Oreochromis mossambica (Peters) and grass carp, Ctenopharyngodon idella (Valenciennes). Aquac Res 37:380–388
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory Manual, 3rd edn. Cold Springs Harbor, Cold Springs Harbor Laboratory Press
Seghers D, Wittebolle L, Top EM, Verstraete W, Siciliano SD (2004) Impact of agricultural practices on the Zea mays L. endophytic community. Appl Environ Microbiol 70:1475–1482
Sessitsch A, Hardoim P, Döring J, Weilharter A, Krause A, Woyke T, Hurek T (2012) Functional characteristics of an endophyte community colonizing rice roots as revealed by metagenomic analysis. Mol Plant-Microbe Interact 25:28–36
Sharaff M, Archana G (2015) Assessment of microbial communities in mung bean (Vigna radiata) rhizosphere upon exposure to phytotoxic levels of copper. J Basic Microbiol 55:1299–1307
Sharaff M, Archana G (2016) Copper-induced modifications in early symbiotic signaling factors of Ensifer (Sinorhizobium)–Medicago interactions. Arch Microbiol 198:701–709
Sharma V, Kumar V, Archana G, Kumar GN (2005) Substrate specificity of glucose dehydrogenase (GDH) of Enterobacter asburiae PSI3 and rock phosphate solubilization with GDH substrates as C sources. Can J Microbiol 51:477–482
Simon R, Priefer U, Pühler A (1983) A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Nature Biotechnol 1:784–791
Subrahmanyam G, Archana G, Chamyal LS (2011) Microbial activity and diversity in the late Pleistocene paleosols of alluvial Mahi River basin, Gujarat, western India. Curr Sci 101:202–209
Sun L, Qiu F, Zhang X, Dai X, Dong X, Song W (2008) Endophytic bacterial diversity in rice (Oryza sativa L.) roots estimated by 16S rDNA sequence analysis. Microbial ecol 55:415–424
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Tanaka K, Shimizu T, Zakria M, Njoloma J, Saeki Y, Sakai M, Akao S (2006) Incorporation of a DNA sequence encoding green fluorescent protein (GFP) into endophytic diazotroph from sugarcane and sweet potato and the colonizing ability of these bacteria in Brassica oleracea. Microbes Environ 21:122–128
Teather RM, Wood PJ (1982) Use of Congo red-polysaccharide interactions in enumeration and characterization of cellulolytic bacteria from the bovine rumen. Appl Environ Microbiol 43:777–780
Thomas P, Swarna GK, Roy PK, Patil P (2008) Identification of culturable and originally non-culturable endophytic bacteria isolated from shoot tip cultures of banana cv. Grand Naine. Plant Cell Tissue Organ Cult 93:55–63
Tian F, Ding Y, Zhu H, Yao L, Du B (2009) Genetic diversity of siderophore-producing bacteria of tobacco rhizosphere. Braz J Microbiol 40:276–284
Verma SC, Ladha JK, Tripathi AK (2001) Evaluation of plant growth promoting and colonization ability of endophytic diazotrophs from deep water rice. J Biotechnol 91:127–141
Villegas E, Ortega Martinez EI, Bauer R (1984) Chemical methods used at CIMMYT for determining protein quality in cereal grains. CIMMYT, Mexico
Wang Q, Garrity GM, Tiedje JM, Cole JR (2007) Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73:5261–5267
Wemheuer F, Wemheuer B, Kretzschmar D, Pfeiffer B, Herzog S, Daniel R, Vidal S (2016) Impact of grassland management regimes on bacterial endophyte diversity differs with grass species. Lett Appl Microbiol 62:323–329
Yanni YG, Rizk RY, Corich V, Squartini A, Ninke K, Philip-Hollingsworth S, Dazzo FB (1997) Natural endophytic association between Rhizobium leguminosarum bv. trifolii and rice roots and assessment of its potential to promote rice growth. Plant Soil 194:99–114
Zinniel DK, Lambrecht P, Harris NB, Feng Z, Kuczmarski D, Higley P, Vidaver AK (2002) Isolation and characterization of endophytic colonizing bacteria from agronomic crops and prairie plants. Appl Environ Microbiol 68:2198–2208
Acknowledgements
JKP is grateful to Department of Biotechnology, Government of India, for fellowship under DBT-MSUB-ILSPARE project. Authors acknowledge the support from Dr. Vikram Sarabhai Central Instrument Facility, The M.S. University of Baroda for confocal microscopy. We are grateful to Department of Biochemistry, Anand Agricultural University for providing their facility for estimating plant nitrogen content.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Stéphane Compant.
Electronic supplementary material
ESM 1
(DOCX 39 kb)
Rights and permissions
About this article
Cite this article
Patel, J.K., Archana, G. Diverse culturable diazotrophic endophytic bacteria from Poaceae plants show cross-colonization and plant growth promotion in wheat. Plant Soil 417, 99–116 (2017). https://doi.org/10.1007/s11104-017-3244-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-017-3244-7