Abstract
Starch from the stem of sago palm (Metroxylon sagu) was demonstrated as a chelating agent in the sol-gel synthesis of cobalt ferrite (CoFe2O4) nanoparticles. Variations in the weight ratio of metal nitrates to sago starch from 1:8 to 1:12 led to the particle size of 30–500 nm. However, further increase in sago starch to 1:16 resulted in ferrimagnetic hysteresis loops with a reduced coercive field and magnetization due to the second phase of hematite (α-Fe2O3). The synthesis conditions and products were comparable to those of the polyvinyl alcohol (PVA) sol-gel synthesis. Thus, sago starch can be implemented in the green synthesis of nanosized ferrites whose magnetic properties are tuned by varying of sol-gel compositions for each application.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sago palm (Metroxylon sagu) is a tropical plant, commonly found in swampy environments. Substantial sago starch can be cultivated from its stem and used in traditional cooking. Since the commercial application of sago was realized in 1970s [1], a variety of food products has been derived from the sago starch including noodle, biscuit, jelly, and sago pearl. Furthermore, sago starch is useful in non-food manufacturing of biodegradable plastics, adhesives, and other chemicals. Because of its biopolymeric compatibility and degradability, the starch network is employed to control chemical elements in the reaction process. Recently, sago starch has been used as capping agent for silver nanoparticles [2, 3]. Nanoparticles are stabilized, and the agglomeration is dependent on the concentration of sago starch in the sol-gel synthesis.
Cobalt ferrite (CoFe2O4) is under research and development for its magnetic, catalytic, and antibacterial properties [4–10]. In the sol-gel reaction, CoFe2O4 as well as other ferrites (e.g., NiFe2O4, MnFe2O4, ZnFe2O4, CuFe2O4) can be synthesized from the controlled decomposition of precursors while the chelating gel is dried during the heat treatment at a relatively low temperature. The common chelating agents are polyacrylamide [4], propylene oxide [5], ethylene glycol [6], pectin [7], citric acid [8], and polyvinyl alcohol (PVA) [8–10]. Magnetic properties of CoFe2O4 obtained in these works were influenced by the amount of chelating agent.
Since sago starch is abundant natural biopolymer with encapsulation properties, it is tested in this work as a biological chelating agent for sol-gel derived CoFe2O4. In the characterization stage, the phase and magnetic properties of the ferrites of three varying sol-gel compositions are compared in order to find the optimum synthetic conditions for nanosized CoFe2O4.
Experimental
Native sago starch, purchased from a store in Nakhon Si Thammarat, was dissolved in distilled water (35% w/v) under constant stirring at a room temperature. Cobalt nitrate (Sigma-Aldrich) and iron nitrate (Sigma-Aldrich) powders were then mixed with the sago starch solution with varying weight ratios of metal nitrates to sago starch as 1:8, 1:12, and 1:16, respectively referred to as Samples A, B, and C. The reaction mixture was initially stirred for 6 h, and the temperature was then increased to 80 °C until the gel was dry.
The sintering temperature was determined by thermogravimetric analysis (TGA) (Perkin Elmer TGA7), and crystalline structures of cobalt ferrite product were characterized by powder X-ray diffraction (XRD) (Bruker D8 Advance). The wavelength of 1.54058 Å was obtained from Kα radiation with 40 kV between the cathode and the copper target. The measurement was performed in the range of 2θ angles from 10° to 80° with a rotating step of 0.02°. The morphology of the ferrite products was examined by field emission scanning electron microscopy (FESEM) (Zeiss Merlin compact). The elemental compositions were obtained by the energy dispersive x-ray spectroscopy (EDS) attached to the FESEM. To study the effect of the sol-gel composition on the magnetic properties, the samples were characterized by vibrating sample magnetometry (VSM).
Results and Discussion
The thermal effect on sol-gel products is demonstrated in the TGA curve in Fig. 1. The weight loss can be divided into three regimes based on the slope and hence DTG. The initial increase in temperature up to 300 °C brings about 26% decreases in weight. Then, the sharp decrease of 36% is observed between 300 and 600 °C. By further increases in temperature, the weight loss is minimal. The calcination temperature was then selected as 800 °C. The product after the calcination, as exemplified in an FESEM micrograph in Fig. 2, exhibits a variation in morphology. The diameter of particles ranges from 30 to 500 nm, and the edges tend to be smooth like those synthesized by using PVA gel [10]. Large clusters indicate the sintering effect at the temperature up to 800 °C. The EDS spectrum shows, in the inset of Fig. 2, elemental compositions of 11.4% Co and 20.9% Fe which are comparable to the stoichiometric ratio of CoFe2O4. Excessive O (51.7%) and C (15.7%) suggest the contributions from residual chemicals.
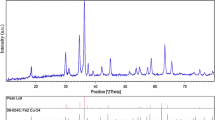
From the XRD patterns in Fig. 3, the cubic spinel CoFe2O4 phase [JCPDS 22–1086] can be identified in each sample by reflections from the 111, 220, 311, 400, 422, 511, and 440 planes at 18.2o, 30.0o, 35.4o, 43.0o, 53.4o, 56.9o, and 62.5o, respectively. In addition, sample C prepared by using the highest amount of sago gel (the ratio of metal nitrate to sago starch is 1:16) also contains hematite (α-Fe2O3 ) [JCPDS 33-0664] as indicated by the minor peaks in Fig. 2. For samples A (the ratio is 1:8) and B (the ratio is 1:12), these Fe2O3 peaks are absent, and the CoFe2O4 peaks are particularly sharp. Likewise, the single phase CoFe2O4 was only obtained from some sol-gel compositions, and α-Fe2O3 may exist as a second phase when PVA was used as a chelating agent [10].
Hysteresis loops of ferrite products are shown in Fig. 4, and magnetic parameters from these loops are compared in Table 1. All samples are ferrimagnetic. Nevertheless, the saturation is not completely obtained even in 8 kOe field, which is likely attributed to smaller particles. The approximate saturation magnetizations are 50–70 emu/g, comparable to the value recently reported for nanosized CoFe2O4 synthesized by the sol-gel technique using other chelating agents [4–10]. The coercive field and magnetizations are substantially reduced in the case of the highest amount of sago gel, coinciding with occurrence of the second phase α-Fe2O3. This marked difference in magnetic behavior is then linked to the purity of CoFe2O4 product via the amount of sago starch in the synthesis.
Conclusions
CoFe2O4 nanoparticles are synthesized by the sol-gel reaction with sago starch as a biological chelating agent. Variations in a weight ratio of metal nitrates to sago starch as 1:8, 1:12, and 1:16 influence the phase of ferrite products, which in turn control their magnetic properties. All samples are ferrimagnetic with the saturation magnetization as high as 70 emu/g which is consistent with those of CoFe2O4 derived using PVA gel. However, the magnetization and coercive field are substantially reduced in the case of the highest amount of sago gel due to the coexistence of α-Fe2O3 phase with CoFe2O4.
References
Karim, A.A., Pei-Lang Tie, A., Manan, D.M.A., Zaidul, I.S.M.: Starch from the sago (Metroxylon sagu) palmtree—properties, prospects, and challenges as a new industrial source for food and other uses. Compr Rev Food Sci Food Saf. 7(3), 215–228 (2008)
Mandal, A., Sekar, S., Chandrasekaran, N., Mukherjee, A., Sastry, T.P.: Vibrational spectroscopic investigation on interaction of sago starch capped silver nanoparticles with collagen: a comparative physicochemical study using FT-IR and FT-Raman techniques. RSC Adv. 5(21), 15763–15771 (2015)
Djokovic, V., Krsmanovic, R., Bozanic, D.K., Mcpherson, M., Tendeloo, G.V., Nair, S.P., Georges, M.K., Radhakrishnan, T.: Adsorption of sulfur onto a surface of silver nanoparticles stabilized with sago starch biopolymer. Colloids Surf B Biointerfaces. 73(1), 30–35 (2009)
Wang, W.P., Yang, H., Xian, T., Wei, Z.Q., Li, R.S., Feng, W.J.: Polyacrylamide gel synthesis, characterization and magnetic properties of CoFe2O4 nanopowders. Chinese J Inorg Chem. 27(6), 1071–1076 (2011)
Cui, H., Jia, Y., Ren, W., Wang, W.: Facile and ultra large scale synthesis of nearly monodispersed CoFe2O4 nanoparticles by a low temperature sol-gel route. J Sol-Gel Sci Technol. 55(1), 36–40 (2010)
Khajeh, M.A.Z., Shokrollahi, H., Avazpour, L., Toroghinejad, M.R.: Study on the effect of sol–gel parameters using the Taguchi technique to achieve the optimal crystallite size and magnetic properties of cobalt ferrite powders. J Sol-Gel Sci Technol. 76(2), 271–278 (2015)
Proveti, J.R.C., Porto, P.S.S., Muniz, E.P., Pereira, R.D., Araujo, D.R., Silveira, M.B.: Sol-gel proteic method using orange albedo pectin for obtaining cobalt ferrite particles. J Sol-Gel Sci Technol. 75(1), 31–37 (2015)
Sanpo, N., Wang, J., Berndt, C.C.: Influence of chelating agents on the microstructure and antibacterial property of cobalt ferrite nanopowders. J Aust Ceram Soc. 49(1), 84–91 (2013)
Sivakumar, M., Kanagesan, S., Babu, R.S., Jesurani, S., Velmurugan, R., Thirupathi, C., Kalaivani, T.: Synthesis of CoFe2O4 powder via PVA assisted sol-gel process. J Mater Sci Mater Electron. 23(5), 1045–1049 (2012)
Hunyek, A., Sirisathitkul, C., Harding, P., Harding, D.J.: Structural and magnetic properties of cobalt ferrites synthesized using sol-gel techniques. Mater Sci Poland. 30(3), 278–281 (2012)
Acknowledgements
This work is financially supported by Rajamangala University of Technology Rattanakosin (Grant no. A44/2560). The authors would like to thank the Center of Scientific and Technological Equipment, Walailak University for the access to FESEM/EDS. VSM was characterized with the assistance of Asst Prof Dr. Pongsakorn Jantaratana at Kasetsart University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hunyek, A., Sirisathitkul, C., Mahaphap, C. et al. Sago starch: chelating agent in Sol-gel synthesis of cobalt ferrite nanoparticles. J Aust Ceram Soc 53, 173–176 (2017). https://doi.org/10.1007/s41779-017-0022-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41779-017-0022-1