Abstract
Nearly monodispersed cobalt ferrite nanoparticles were synthesized by a low temperature sol–gel route using propylene oxide as a gelation agent. The nanoparticles were obtained by the reaction of FeCl2 and CoCl2 in ethanol solution with propylene oxide to form the sol, followed by the boiling of the sol solution. The unique chemistry of this procedure allows the formation of highly homogeneous gel intermediate, resulting in the great reducing of crystallization temperature of ferrites to less than 100 °C without postannealing step. This guarantees the preparation of well defined and non-aggregated ferrite nanoparticles on an ultra-large scale of about 75 g in a single reaction. This large scale synthesis strategy offers important advantages over other conventional routes for the preparation of CoFe2O4 nanoparticles, showing the promising application of this route in the industrial production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Spinel ferrites (MFe2O4, M=Fe, Co, Ni, Mn, etc.) are very promising magnetic compounds due to their high performance in applications of high density magnetic recording, microwave device, drug delivery, ferrofluid, magneto-optic, magnetocaloric refrigeration and magnetic resonance imaging [1–6]. Their fascinating properties are the nano-size effects, where their magnetic properties of blocking temperature, saturation, remanent magnetization and coercivity are greatly connected with particle size (size-dependent) [7–9], giving the nanomaterials excellent magnetic properties. When the particle size is reduced to a threshold value, small alternation of size can make their properties greatly change [10, 11], namely size-sensibility. Therefore, monodisperse or narrow size distribution is one of major factors for their magnetic performance, and one of primary objectives in the preparation of nano-magnetic materials.
Due to the asynchrony of the nucleation and phase formation, it is difficult to obtain ferrite nanoparticles with monodisperse state or narrow size distribution through the traditional coprecipitation route [12, 13]. A complicated seed-mediated method was advised to control the crystallization process during coprecipitation, followed by an exhaustive size selection procedure [14] (which is also frequently used by other routes). The crystallization process also can be carefully controlled by a soft reactor (reverse micelle [15], foam [16], etc.) or hard reactor (organic [17] or inorganic matrix [18]) to limit the growth of particles in order to keep their size in a narrow distribution. The non-hydrolytic [8] and decomposition process [19] of metal organic complex offer a much more successful way for the aim of size control due to the better control of nucleation and phase formation. However, their advantages are counteracted by the high reaction temperature in the organic solvent, complicated process and expensive precursors.
Herein, nearly monodispersed cobalt ferrite nanoparticles were successfully synthesized by a recently developed chemical strategy [20–23] on an ultra large, epoxide assisted sol–gel route using propylene oxide as gelation agent. Through the key choosing of iron salts as iron source and optimizing of reaction conditions, the unique chemistry of this route guarantees the lowering of cobalt ferrite crystallization temperature to less than 100 °C. The delicate control of the process without postannealing step results in the nearly monodispersed state and non-aggregation state of the ferrite nanoparticles.
2 Experimental
2.1 Materials
Iron (II) chloride tetrahydrate (FeCl2·4H2O), iron (III) chloride hexahydrate (FeCl3·6H2O), iron (III) nitrate nonahydrate (Fe(NO3)3·9H2O) cobalt (II) chloride hexahydrate (CoCl2·6H2O) and propylene oxide (PPO) were reagent grade and used as received.
2.2 Preparation of CoFe2O4 nanoparticles
All syntheses were performed under ambient atmosphere and same reaction parameters except for the concentration of metal ions and PPO. Stoichiometric FeCl2·4H2O and CoCl2·6H2O were dissolved in a certain amount of ethanol. After the addition of propylene oxide to the solution (molar ratio of propylene oxide and metal ions was kept at 10), the solution became brown with a few minutes. The obtained sol was stirred for 6 h and then boiled around 78 °C for another 15 min. During the boiling of the sol, it gradually became black coloration. The non-aggregated ferrite nanoparticles containing sol solution can be used directly for other applications such as transparent magnetic film and gel, or the boiling of the solution was continued until dry powder was obtained. 75 g CoFe2O4 can be produced in a single reaction, where the maximum capacity of production is only limited by the volume of the facilities used for the preparation. As a comparison, FeCl3·6H2O and Fe(NO3)3·9H2O were used as iron precursor salts to prepare the nanoparticles, following the same procedure.
2.3 Characterization
The XRD patterns of the samples were measured in a Siemens D8 diffractometer using CuKα radiation. The morphology of the particles was observed using a JEOL 2000 transmission electron microscope working at 200 kV.
3 Results and discussion

During the preparation, the aquo complexes [M(H2O)6]n+ (M=Co, Fe) react with propylene oxide (PPO) to form M–OH bonds as shown in equation (1). The hydrolysed ions will undergo condensation, releasing water and forming M–O–M bonds. The PPO acts as a proton scavenger, reacting with the protons which come from the hydrolysis of the aquo complexes. The protonated propylene oxide is then irreversibly ring-opened.
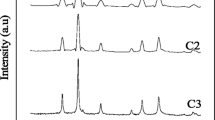
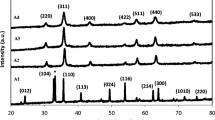
The XRD patterns of samples prepared with Fe(NO3)3·9H2O, FeCl3·6H2O and FeCl2·4H2O are shown in Fig. 1. The samples synthesized with Fe(NO3)3·9H2O and FeCl3·6H2O present amorphous structure, however, it is very interesting to notice that the samples prepared with FeCl2·4H2O exhibit a structure of cobalt ferrite (JCPDS 22-1086). This means that the different structures of the final products depend on the reaction kinetic of the three iron salts. Fe3+ ions react with propylene oxide to form iron (III) hydroxide which cannot be crystallized to iron oxide just by the boiling of the ethanol solution. While, the precursor intermediate, which was obtained from the drying of the sol solution (prepared from FeCl2·4H2O without adding of CoCl2·6H2O) at room temperature without heat treatment, presents a crystallized phase of iron oxide hydroxide (JCPDS 89-3850) as shown in Fig. 2a. The sample, which was prepared by the boiling and vacuum drying of the same sol solution at room temperature, presents a spinel structure of Fe3O4 (Fig. 2b, JCPDS 19-0629). It can be concluded that part of Fe2+ ions was oxidized by the oxygen in the solution and crystallized to form mixed valence iron oxide hydroxide during the sol formation process, which was then converted to Fe3O4 by the following boiling. This Fe3O4 sample can be oxidized easily to γ-Fe2O3 (Fig. 2c, JCPDS 39-1346) in the air atmosphere within a few hours. Therefore, it is reasonable to infer that Co2+ doped Fe3O4 was formed during the boiling process of the sol, which was transformed into spinel CoFe2O4 by the oxidization of the Fe2+ ions of the Fe3O4 to Fe3+ in air atmosphere.
The unique chemistry and the low synthesis temperature of this sol–gel route allows the preparation of nanoparticles with very small particle size and narrow size distribution as observed in their TEM micrographs (Fig. 3). The sample, which was prepared with metal ions concentration of 0.3 mol l−1, shows the well defined and non-aggregated CoFe2O4 nanoparticles with diameter of about 9 nm (Fig. 3a). When the concentration is increased to 0.6 mol l−1, its TEM micrograph reveals the particle size of about 15 nm (Fig. 3b). The particle size distributions (Fig. 3c) of the two samples were easily calculated from their TEM micrographs due to the well dispersed state of the nanoparticles. The size distribution demonstrates that the nanoparticles prepared at lower concentration (0.3 mol l−1) are in very narrow size distribution around 9 nm, nearly monodispersed. On the other hand, the higher metal ions concentration of 0.6 mol l−1 not only results in the increase of particle size to about 15 nm but also makes the size distribution a little wider than the former.
As a comparison, γ-Fe2O3 nanoparticles was prepared by the boiling of FeCl2 resulted sol solution with the same synthesis procedure of CoFe2O4, which is described in our previous work [23]. The sample produced with metal ions concentration of 0.3 mol l−1 presents 2.3 nm of particle median diameter (Fig. 4a) and a very narrow size distribution (Fig. 4c). With the increase of metal ions concentration to 0.6 mol l−1, the median diameter of particles also increases to 5.1 nm (Fig. 4b) with slightly wider size distribution (Fig. 4c) than that of former. This indicates that Co2+ ions promote the increase of particle size even in the exactly same reaction conditions with synthesis of γ-Fe2O3.
4 Conclusions
In this work, cobalt ferrite nanoparticles were prepared on an ultra-large scale of about 75 g in a single reaction by a low temperature sol–gel route using simple procedure and cheap precursors. The unique chemistry of this route results in the crystallization of CoFe2O4 at low temperature, leading to the preparation of small and nearly monodispersed nanoparticles without aggregation. The phase formation of CoFe2O4 was achieved by the boiling of the sol solution for some minutes and the obtained particles containing solution can be used directly for some applications such as ferrofluid or the preparation of transparent film and gel.
As compared with the well known synthetic methods such as decomposition of organometallic and metal complexes in high boiling point solvent [8, 19], this epoxide assisted sol–gel route provides important advantages for the large scale production of ferries nanoparticles. First, the maximum production capacity is only limited by the volume of the facilities used for the preparation. Second, inexpensive reagents and solvent are used with the characteristic of non-toxity, and there is almost no waste released during the process. Third, the procedure that is carried under mild reaction conditions is quite facile and economical. These guarantee the promising application of this route in the industrial preparation.
References
Pardavi-Horvath M (2000) J Magn Magn Mater 215:216171
Häfeli U, Schütt W, Teller J, Zborowski M (1997) Scientific and clinical applications of magnetic carriers. Kluwer, Amsterdam
Arulmurugan R, Vaidyanathan G, Sendhilnathan S, Jeyadevan B (2005) Physica B: Condensed Mater 363:225
Zhou B, Zhang Y, Liao C, Yan C, Chen L, Wang S (2003) Solid State Commun 126:593
Poddar P, Gass J, Rebar DJ, Srinath S, Srikanth H, Morrison SA, Carpenter EE (2006) J Magn Magn Mater 307:227
Hogemann D, Josephson L, Weissleder R, Basilion JP (2000) Bioconj Chem 11:941
Kumar V, Rana A, Yadav MS, Pant RP (2008) J Magn Magn Mater 320:1729
Song Q, Zhang Z (2004) J Am Chem Soc 126:6164
Vestal CR, Zhang Z (2002) Chem Mater 14:3817
Liu C, Rondinone AJ, Zhang ZJ (2000) Pure Appl Chem 72:37
Manova E, Kunev B, Paneva D, Mitov I, Petrov L, Estournès C, D’Orléans C, Rehspringer JL, Kurmoo M (2004) Chem Mater 16:5689
Olsson RT, Salazar-Alvarez G, Hedenqvist MS, Gedde UW, Lindberg F, Savage SJ (2005) Chem Mater 17:5109
Salavati-Niasari M, Davar F, Mahmoudi T (2009) Polyhedron 28:1455
Perales-Perez O, Sasaki H, Kasuya A, Jeyadevan B, Tohji K, Hihara T, Sumiyama K (2002) J Appl Phys 91:6958
Liu C, Zou B, Rondinone AJ, Zhang ZJ (2000) J Am Chem Soc 122:6263
Bala T, Sankar CR, Baidakova M, Osipov V, Enoki T, Joy PA, Prasad BLV, Sastry M (2005) Langmuir 21:10638
Ahmed SR, Kofinas P (2002) Macromolecules 35:3338
Vejpravováa J, Sechovský V, Plocek J, Nižòanský D, HutlováA Rehspringer JL (2005) J Appl Phys 97:124304
Bao N, Shen L, Wang YA, Ma J, Mazumdar D, Gupta A (2009) J Am Chem Soc 131:12900
Cui H, Zayat M, Levy D (2005) J Non-Crys Solids 351:2102
Cui H, Zayat M, Levy D (2005) J Sol- Gel Sci Techn 35:175
Cui H, Zayat M, Levy D (2005) Chem Mater 17:5562
Cui H, Ren W (2008) J Sol-Gel Sci Techn 47:81
Acknowledgments
This work is supported by National Natural Science Foundation of China (20971107) and Shandong Provincial Science and Technology Project (2009ZRB0199M and 2009ZRB019AP).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cui, H., Jia, Y., Ren, W. et al. Facile and ultra large scale synthesis of nearly monodispersed CoFe2O4 nanoparticles by a low temperature sol–gel route. J Sol-Gel Sci Technol 55, 36–40 (2010). https://doi.org/10.1007/s10971-010-2210-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-010-2210-0