Abstract
Cobalt ferrite particles (CoFe 2 O 4 ) were synthetized using the proteic sol–gel method with pectin extracted from the albedo of orange peel as the gelling agent. The sol–gels were obtained by mixing pectin in different concentrations (4, 6 and 8 %) with salts of cobalt (II) nitrate hexahydrate and iron (III) nitrate nonahydrate. After gelling, the samples were calcined from 4 to 48 h at 1373 K. The formation of the spinel phase was investigated using X-ray diffraction, Fourier transform infrared and Mössbauer spectroscopy. Data analyses showed that 4 % pectin concentration is sufficient to produce cobalt ferrite particles. The grain size slightly increases with the thermal treatment time. The particles were successfully used to move a drop of sparged oil on water subjected to the action of a magnetic field.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Spinel-type ferrite oxides (AB 2 O 4 ), with high magnetic permeability and low magnetic losses, can be used for applications in the manufacturing of many electronic and magnetic devices. Their interesting electrical and magnetic properties allow the use of ferrites in ferrofluids, radar absorbing coatings, waveguides (in the gigahertz region), biomedical and clinical devices, repulsive suspension (for levitated systems), gas sensors and magnetic cores of reading/writing heads (for either high-speed digital tapes or disk recording) [1].
Cobalt ferrite is an important member of the spinel family. It has been extensively studied because of its interesting magnetic properties [2, 3]. Among other applications, cobalt ferrite (CoFe 2 O 4 ) is used in magnetic media [4] and as a removal agent of spilled crude oil on sea water [5–7].
Many methodologies have been proposed in the literature to obtain cobalt ferrite nanoparticles such as co-precipitation route [8], microemulsion [9], sol–gel process [10–16], organic acid precursor method [17], mechanochemical method [18], forced hydrolysis [19] and sonochemical [20].
Recently, there has been an increased interest in using sol–gel methods, including the proteic sol–gel (PSG) method to produce nanoparticles, with certain emphasis on the ferrite system [10, 12–15, 21–29].
Within the framework of the PSG method, gelatin [21, 30, 31] and coconut water [22, 32, 33] are the most commonly used precursors. The proteins in these precursors act as a catalyst because they bind with the metals and their convoluted geometry forces metallic atoms to approach one another and facilitates chemical reactions [21].
Orange peel residue is rich in pectin with a high degree of methyl esterification (ATM) and hydrophilic characteristics. Therefore, pectin from orange peel is an excellent colloid: In the presence of water, it forms a gel with similar characteristics to those formed from other precursors [34, 35].
There are significant advantages that justify the use of pectin as a precursor: (1) large polysaccharide chain, (2) simple material production process, (3) availability to be used at an industrial scale and (4) low cost.
Comparatively, the usage of pectin extracted from the waste of industrial juice has the advantage of promoting a solution with low cost even compared with the conventional sol–gel methodology. In addition to being an alternative, environmentally friendly method, it adds economic value to a type of industrial residue [32, 36].
This research aims to produce cobalt ferrite using a new gelling agent, pectin, extracted from orange albedo. The application of cobalt ferrite to move sparged oil on water is studied.
2 Experimental methodology
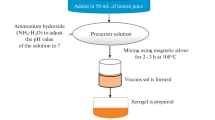
Figure 1 shows the process flowchart to obtain pectin from albedo orange. First, orange albedo was ground in a blender. The homogenized albedo was mixed with water in a 1:1 proportion. The solution was agitated in a tank with a thermostatic bath at 333 K for 30 min. Subsequently, the solution was filtered to obtain pectin. Then, acetone was added into the sample to speed up the water removal, which occurred in a natural convection oven at 323 K for 24 h.
Figure 2 depicts the schematic sketch of the apparatus to produce the sol–gel synthesis. This apparatus consists of a stir tank reactor with controlled temperature: 100 g of water and 4, 6 or 8 g of orange albedo pectin (which correspond to our 4, 6 and 8 % pectin in solution) were mixed in the tank. The resulting solutions were heated at 323 K for 30 min. Then, 15 g of cobalt (II) nitrate hexahydrate (Co(NO 3 ) 2 .6H 2 O) and 45 g of iron(III) nitrate nonahydrate (Fe(NO 3 ) 3 .9H 2 O) were added to the solution to obtain the precursor. The resulting brown and slightly viscous liquid was dried in a natural convection dryer for 24 h at 393 K. This process was followed by calcination at 1373 K in a muffle furnace at different dwell times (4, 8, 12, 24 and 48 h).
After the isothermal treatment, the calcinated samples were cooled in air, ground in an agar mortar, homogenized, and stored at 277 K.
The as-produced and treated samples were studied with X-ray diffraction (XRD). XRD data were collected using a Rigaku Ultima IV diffractometer with Cu-Kα (λ = 1.5409 Å) radiations in the 2θ range from 20° to 100°.
The Mössbauer spectra were measured in the transmission mode with 57Co diffused into a Rh matrix as the source, moving with constant acceleration. The spectrometer was calibrated using a standard α-Fe foil, and the isomer shift was expressed with respect to this standard at 293 K. The spectra were fitted using the NORMOS program [37].
Fourier transform infrared (FTIR) spectra were measured at room temperature in a Nicolet 6700 spectrometer using 500 scans and a resolution of 2 cm−1 in the transmission geometry.
After characterization, an experiment was performed to test the ability of the cobalt ferrite particles to move a drop of oil (apparent viscosity, η = 46 cP, density, d = 0.9171 at 323 K) spilled on water. A simple system was assembled using a Petri plate filled with distilled water on a sheet of millimeter paper. Two straight lines were used to mark the distance that the oil drop traveled, and magnets were placed to attract the cobalt ferrite (Fig. 3).
Cobalt ferrite (CoFe 2 O 4 ) was deposited onto the spilled oil in the water. The required time to traverse the distance between the lines was measured in triplicate, and the averaged values were analyzed.
3 Results and discussion
3.1 X-Ray diffraction
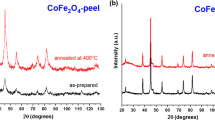
Figure 4 shows XRD of cobalt ferrite nanoparticles synthetized using 4 % pectin concentration and isothermally treated at 1373 K for a period of 4–48 h. The diffractograms show the peaks characteristic of CoFe 2 O 4 with small (<5 %) amounts of α-Fe 2 O 3 . The sample treated for 48 h, though, does not have any detectable impurities in its diffractogram.
Figures 5 and 6 show the X-ray diffractograms of the samples produced with 6 and 8 % pectin, respectively. Although the peaks due to cobalt ferrite are the strongest in all diffractograms, it is clear that there is an increase in the amount of α-Fe 2 O 3 with pectin concentration.
Figure 7 shows the Rietveld plot of the sample that was synthetized using 4 % pectin and calcined at 1173 K/48 h. The data were fitted using the MAUD program [38] and the Cobalt Ferrite CIF data file from the Crystallography Open Database Web site [39]. Based on the data analysis, the cobalt ferrite lattice parameter a = 8.336 Å was obtained, which is consistent with the values in the literature.
Figures 8 and 9 show the change in grain size as a function of the treatment time. Grain size remains constant for samples made with 4 % pectin (Fig. 8) and has a tendency to grow with time for the sample produced with 6 % pectin (Fig. 9).
3.2 Infrared spectra
An infrared spectrum of the sample produced with 4 % pectin is shown in Fig. 10. The two main bands at 400 and 595 cm−1 are typical of cobalt ferrite [40, 41] and are assigned to the M–O stretching in octahedral sites (M being either iron or cobalt) and Fe–O stretching in tetrahedral sites, respectively. The ratio between these peaks with the peak due to iron in tetrahedral sites (FeTh) being bigger than the peak due to metal in octahedral sites indicates that the sample has an inverse spinel structure [42]. There is a small peak at 470 cm−1 and a shoulder at 540 cm−1 due to the presence of a small amount of α-Fe 2 O 3 [41, 43]. No shoulder at or near 635 cm−1 was found, which means that this sample has no significant amount of vacancies [40, 44].
3.3 Mössbauer spectra
Mössbauer spectra of the samples were measured at 300 and 77 K and are shown in Fig. 11.
The room temperature spectra of the as-prepared sample (Fig. 11a) were fitted using two components:
-
(1)
Doublet characteristic of a non-cubic symmetry for Fe-atoms.
-
(2)
Hyperfine field distribution.
The parameters of the doublet are isomer shift (δ) = 0.320 mm/s and quadrupolar split (Δ) of 0.72 mm/s; values typical of the Fe 3+ state [45].
The hyperfine field distribution extends from 20 up to 50 T, reflecting the presence of distinct Fe environments. This behavior suggests that the dried material is in a chemically disordered state or is composed of small particles in the super-paramagnetic state.
The 77 K Mössbauer spectra of the as-prepared sample are displayed in Fig. 11b and were fitted with three components:
-
(1)
The doublet measured for the as-prepared sample.
-
(2)
Two sextets, one with: B HF = 52 T, δ = 0.335 mm/s, Δ = −0.190 mm/s and the other with: B HF = 50 T, δ = 0.332 mm/s and Δ = −0.206 mm/s. These sextets are due to Fe ions in A sites and in B sites, respectively [45].
The 77 K spectrum of the sample annealed isothermally at 1373 K for 48 h (Fig. 11c) is better fitted with two well-defined sextets. One sextet has B HF = 54 T, δ = 0.331 mm/s, Δ = 0.059 mm/s, and the other has B HF = 51 T, δ = 0.236 mm/s, Δ = 0.001 mm/s. These sextets correspond to the A and B sites, respectively.
These results are in agreement with results reported in literature for samples of cobalt ferrite prepared by different methods [45].
The relative intensity between B and A sites is I B/A = 1.6. There is no evidence of the α-Fe 2 O 3 phase in Mossbauer spectra for the sample isothermally treated at 1373 K for 48 h.
3.4 Use of cobalt Ferrite nanoparticles to move spilled oil on water
Figure 12 shows the displacement of a drop of oil (1 g) spilled on water and later covered with cobalt ferrite nanoparticles; the displacement was induced by a magnetic field. It is noticeable that the set of spilled oil with cobalt ferrite nanoparticles moves on water in the direction of the magnetic field without a significant deformation. This result contributes a promising engineering application in separating spilled oil on water.
Figure 13 shows a sketch of the forces that act on the spilled oil drop on water. In the beginning, in Fig. 13a, it can be noted that the spilled drop is maintained static on the water because the forces—i.e., gravity (F g ) and buoyancy (F b )—are balanced. Fig. 13b shows that when a magnetic field is applied, the magnetic force (F m ) makes the spilled oil flow on water, and this movement of the spilled oil creates a viscous force (F v ) in the opposite direction of the flow. This reasoning may explain the spilled oil behavior in Fig. 12.
4 Conclusion
Cobalt ferrite particles were successfully produced using the proteic sol–gel method with pectin from orange albedo as the precursor.
Based on the XRD data analysis, Mossbauer and FTIR, it was proved that this methodology produced particles with small concentrations of impurities and defects.
Cobalt ferrite was produced from 4 to 8 % pectin concentration. For the set of operating conditions used, it was found that crystalline grain size was slightly dependent on pectin concentration and time (4 to 48 h) of isothermal treatment.
When a magnetic field is applied to spilled oil mixed with cobalt ferrite particles that float on water, the mixture is moved in the direction of the magnets. Thus, it may be possible to use cobalt ferrite and a magnetic field to separate spilled oil on water.
References
Paiva JAC et al (2009) Spectroscopy studies of NiFe2O4 nanosized powders obtained using coconut water. J Alloy Compd 485(1–2):637–641
Singhal S et al (2005) Preparation and characterization of nanosize nickel-substituted cobalt ferrites (Co1 − xNixFe2O4). J Solid State Chem 178(10):3183–3189
Šepelák V et al (2007) Nanocrystalline nickel ferrite, NiFe2O4: mechanosynthesis, nonequilibrium cation distribution, canted spin arrangement, and magnetic behavior. J Phys Chem C 111(13):5026–5033
Somaiah N et al (2012) Magnetic and magnetoelastic properties of Zn-doped cobalt-ferrites-CoFe2-xZnxO4 (x = 0, 0.1, 0.2, and 0.3). J Magn Magn Mater 324(14):2286–2291
Salamanca-Buentello F et al (2005) Nanotechnology and the developing world. PLoS Med 2(5):383–386
Vejpravova J et al (2005) Magnetism of sol-gel fabricated CoFe2O4/SiO2 nanocomposites. J Appl Phys 97(12):124304
Vejpravova J et al (2005) Sol-gel fabricated CoFe2O4/SiO2 nanocomposites: synthesis and magnetic properties. IEEE Trans Magn 41(10):3469–3471
Gul IH et al (2010) Optical, magnetic and electrical investigation of cobalt ferrite nanoparticles synthesized by co-precipitation route. J Alloy Compd 507(1):201–206
Pillai V, Shah DO (1996) Synthesis of high-coercivity cobalt ferrite particles using water-in-oil microemulsions. J Magn Magn Mater 163(1–2):243–248
Zhang CY et al (2007) Preparation of spinel ferrite NiFe2O4 fibres by organic gel-thermal decomposition process. J Sol-Gel Sci Technol 42(1):95–100
Cannas C et al (2011) Simple and fast preparation of pure maghemite nanopowders through sol–gel self-combustion. J Sol-Gel Sci Technol 60(3):266–274
Cui HT et al (2010) Facile and ultra large scale synthesis of nearly monodispersed CoFe2O4 nanoparticles by a low temperature sol-gel route. J Sol-Gel Sci Technol 55(1):36–40
Holec P et al (2009) Preparation of MgFe2O4 nanoparticles by microemulsion method and their characterization. J Sol-Gel Sci Technol 51(3):301–305
Montemayor SM et al (2007) Comparative study of the synthesis of CoFe2O4 and NiFe2O4 in silica through the polymerized complex route of the sol-gel method. J Sol-Gel Sci Technol 42(2):181–186
Wang ZY et al (2012) Structure and magnetic properties of CoFe2O4 ferrites synthesized by sol-gel and microwave calcination. J Sol-Gel Sci Technol 61(2):289–295
Silva J et al (2005) Characterization of Porous Nanocomposites Formed by Cobalt Ferrites Dispersed in Sol-Gel Silica Matrix. J Sol-Gel Sci Technol 35(2):115–122
Mohamed RM et al (2010) Structure and magnetic properties of nanocrystalline cobalt ferrite powders synthesized using organic acid precursor method. J Magn Magn Mater 322(14):2058–2064
Shi Y, Ding J, Yin H (2000) CoFe2O4 nanoparticles prepared by the mechanochemical method. J Alloy Compd 308(1–2):290–295
Hamdeh HH et al (2005) Mossbauer evaluation of cobalt ferrite nanoparticles synthesized by forced hydrolysis. J Appl Phys 97(6):064310
Shafi KVPM, Gedanken A, Prozorov R (1998) Sonochemical preparation and characterization of nanosized amorphous Co-Ni alloy powders. J Mater Chem 8(3):769–773
Menezes AS et al (2007) Sintering of nanoparticles of α-Fe2O3 using gelatin. J Non Cryst Solids 353(11–12):1091–1094
Muniz EP et al (2013) Influence of heat-treatment environment on Ni-ferrite nanoparticle formation from coconut water precursor. J Mater Sci 48(4):1543–1554
Reddy MP et al (2014) Microwave sintering of nickel ferrite nanoparticles processed via sol-gel method. J Sol-Gel Sci Technol 70(3):400–404
Brown P et al (2012) Controlling the morphology of a zinc ferrite-based aerogel by choice of solvent. J Sol-Gel Sci Technol 61(1):104–111
Guo LP et al (2011) Characterization and magnetic exchange observation for CoFe2O4-CoFe2 nanocomposite microfibers. J Sol-Gel Sci Technol 58(2):524–529
Li L (2011) Glycol-assisted autocombustion synthesis of spinel ferrite CoFe2O4 nanoparticles: magnetic and electrochemical performances. J Sol-Gel Sci Technol 58(3):677–681
Prasad AS et al (2013) Sol-gel synthesized high anisotropy magnetic nanoparticles of NiCr (x) Fe2-x O-4. J Sol-Gel Sci Technol 66(3):372–377
Yuan T et al (2011) The microstructure and magnetic properties of Ni0.4Zn0.6Fe2O4 films prepared by spin-coating method. J Sol-Gel Sci Technol 58(2):501–506
Zaki T et al (2013) Synthesis and characterization of MFe2O4 sulfur nanoadsorbents. J Sol-Gel Sci Technol 65(2):269–276
Yonezawa T et al (2008) Easy preparation of stable iron oxide nanoparticles using gelatin as stabilizing molecules. Jpn J Appl Phys 47:1389–1392
Medeiros AML et al (2006) Obtenção de Cr2O3 Nanoparticulado através do Método Sol-Gel Protéico, in 17° CBECIMat—Congresso Brasileiro de Engenharia e Ciência dos Materiais, Foz do Iguaçu
Santos JVA (2002) Filmes Finos de NiFe2O4 obtidos a partir de Água de Coco Utilizando o Processo Sol–Gel Protéico, in Núcleo de Pós Graduação em Física, Universidade Federal de Sergipe, São Cristovão, p 94
da Silva SW et al (2007) The influence of cobalt population on the structural properties of Co(x)Fe(3-x)O(4). J Appl Phys 101(9):09M514
Ueno H et al (2008) Extraction of valuable compounds from the flavedo of Citrus junos using subcritical water. Sep Purif Technol 62(3):513–516
Mccready RM, Mccomb EA (1952) Extraction and determination of total pectic materials in fruits. Anal Chem 24(12):1986–1988
Suresh P, Srinath S (2014) A comparative study of sol-gel and solid-state prepared La3 + doped multiferroic BiFeO3. Adv Mater Lett 5(3):127–130
Brand RA (1992) Normos programs. Laboratorium für Angwandte Physik, Universität Duisburg, Duisburg
Lutterotti L, Matthies S, Wenk HR (199) MAUD: a friendly Java program for material analysis using diffraction. IUCr Newsl CPD 21:14–15
Grazulis S et al (2009) Crystallography open database—an open-access collection of crystal structures. J Appl Crystallogr 42(4):726–729
Manova E et al (2004) Mechano-synthesis, characterization, and magnetic properties of nanoparticles of cobalt ferrite, CoFe2O4. Chem Mater 16(26):5689–5696
Gillot B, Jemmali F (1983) Dependence of electrical-properties in iron cobalt, iron zinc ferrites near stoichiometry on firing temperature and atmosphere. Phys Status Solidi a-Appl Res 76(2):601–608
Jacob J, Khadar MA (2010) Investigation of mixed spinel structure of nanostructured nickel ferrite. J Appl Phys 107:114310
Gabal MA (2003) Non-isothermal decomposition of NiC2O4-FeC2O4 mixture aiming at the production of NiFe2O4. J Phys Chem Solids 64(8):1375–1385
Gillot B, Jemmali F, Rousset A (1983) Infrared studies on the behavior in oxygen of cobalt-substituted magnetites: comparison with zinc-substituted magnetites. J Solid State Chem 50(2):138–145
Grigorova M et al (1998) Magnetic properties and Mössbauer spectra of nanosized CoFe2O4 powders. J Magn Magn Mater 183(1–2):163–172
Acknowledgments
The authors acknowledge UFES (Universidade Federal do Espírito Santo), FAPES (Fundação de Amparo à Pesquisa e Inovação do Espírito Santo), CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) and CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Proveti, J.R.C., Porto, P.S.S., Muniz, E.P. et al. Sol–gel proteic method using orange albedo pectin for obtaining cobalt ferrite particles. J Sol-Gel Sci Technol 75, 31–37 (2015). https://doi.org/10.1007/s10971-015-3671-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-015-3671-y