Abstract
CoFe2O4 ferrites were synthesized by sol–gel method, having metal nitrates as precursors and PVA as surfactant, followed by a heat treatment at 960 °C for 2 h. The ultrafine ferrite powders obtained have been characterized by X-ray diffraction, thermal gravimetry, differential scanning calorimetry and room temperature magnetic measurement studies. The morphology of the powder was identified by high resolution-scanning electron microscopy. X-ray diffraction results indicate that the resultant CoFe2O4 crystallites consist of spinel phase. Significant differences in magnetic properties of CoFe2O4 samples synthesized with various concentrations of PVA were observed. The magnetisation measurements show that when the PVA concentration increased, coercivity initially decreased and then increased where as retentivity and magnetisation decreased. The optimum concentration of PVA for the synthesis of CoFe2O4 ferrites is obtained from this investigation. Obviously this material can be used as an efficient candidate for practical recording purpose.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Spinel ferrites have been widely investigated over the last two decades due to their specific magnetic and electrical properties. They have been used in high-density magnetic recording, micro wave devices and magnetic fluids [1, 2]. Among spinel ferrites, CoFe2O4 is an interesting magnetic material due to its high coercivity (5,400 Gauss) and moderate saturation magnetisation (about 80 Am2/kg) as well as remarkable chemical stability and mechanical hardness, which makes it a good candidate for the recording media [3, 4].
A large number of research papers have been reported in literature, concerning the preparation methods such as solid state reaction [5], micro emulsion [6], chemical co-precipitation [7], hydrothermal method [8], microwave synthesis [9] and sol–gel method [10]. The physical and chemical properties of spinel powders are affected not only by the synthesis route but also with the precursors and their concentrations. The sol–gel method is quite an efficient one, in producing high purity, crystalline and homogeneous particles of a particular size. The sol–gel process is a liquid phase synthesis involving hydrolysis and condensation reactions of metal precursors, leading to the formation of three-dimensional inorganic networks.
In this work, we attempt to synthesize CoFe2O4 particles having reasonable saturation magnetisation and high coercivity which can be used as practical recording materials, by simple sol–gel method using PVA as surfactant. Also we aim to investigate the effect of adding various PVA concentrations namely 3, 6, 9 and 15 wt% and to analyze the phase formation, densification, structure and magnetic properties of resultant CoFe2O4 powder.
2 Experimental techniques
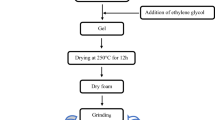
The chemicals used to synthesize CoFe2O4 powders were Co(NO3)2·6H2O, Fe (NO3)3·9 H2O, and Polyvinyl Alcohol (PVA). All chemicals were of analytical grade and used without purification. PVA solution was prepared by slowly sprinkling PVA powder in deionized water under continuous stirring to avoid clumping of the material in water. The sols were prepared by dissolving ferric nitrate and cobalt nitrate in deionized water in stoichiometric ratio of 2:1. After constant stirring for 1 h, PVA solution was added to sols. The subsequent mixture was then heated to 90 °C and subjected to constant stirring till a gel was obtained. The gel was then kept in hot air oven for 2 days to evaporate water. The precursor was then calcined at 960 °C for 2 h. Afterwards it was crushed for 30 min in a mortar to form powder. Four samples a,b,c,d were synthesized this way with various PVA concentrations 3, 6, 9 and 15 wt%, respectively, rest of the procedures being the same.
The precursor was subjected to TG/DSC analysis between 28 and 1,200 °C by NETZSCH STA 409 C/CD in static air atmosphere at a heating rate of 10 °C per minute. The phase structure analysis of calcined powder was identified using a X-ray diffractometer (PANalytical X’pert pro) of X-ray wave length (λ = 0.15406 nm) in a wide range of 2θ (20° < 2θ < 70°). The morphology and dispersibility of the products were measured by HR-SEM (FEI Quanta 200FEG). The magnetic saturation magnetization and coercivity of cobalt ferrite powder were measured by a Vibrating Sample Magnetometer (VSM)—Lakeshore 7304 with a maximum field of 20,000 Gauss (G) at room temperature.
3 Results and discussion
3.1 Thermal decomposition of the gel precursor
Figure 1 shows the TG/DSC curves for the gel precursor. Five stages of weight loss are observed in TG curve. The first stage in the temperature range of 25–190 °C, with the mass loss of 17.43%, is associated with the evaporation of water molecules from the gel precursor, which is characterised by an endothermic peak in DSC analysis. The second stage in the range of 190–230 °C, in TG curve, with a mass loss of 12.6%, is due to spontaneous combustion caused by interactions of nitrate ions in the gel. This is accompanied by an exothermic event in DSC analysis. The third stage in TG curve, in the temperature range of 230–290 °C, with the mass loss of 9.6%, is caused by the polymeric chain decomposition of PVA. The fourth stage in the temperature range of 290–330 °C, with the mass loss of 3.4% is due to loss of structural water. The final stage in the temperature range of 330–842 °C, accompanied by a mass loss of 2.2%, is due to the further thermal decomposition of residual compounds and the onset of crystallisation process.
Above 842 °C no weight loss was observed and the crystallisation process was completed. Endothermic peak observed at 306 °C in DSC curve confirms the oxidation combustion of PVA main chain and a small another endothermic peak observed at 826 °C is due to further decomposition of nitrates. A broad endothermic event observed in DSC curve between 870 and 1,200 °C is due to densification of the powder. In TG curve no weight loss is observed above 842 °C, which means that pure ferrites could be observed above that temperature.
3.2 X-ray diffraction studies
The XRD patterns of the CoFe2O4 powder synthesized with 3, 6, 9 and 15 wt% of PVA concentration are shown in Fig. 2a–d, respectively. The prominent peaks observed at 2θ values of 30°, 35°, 43°, 57° and at 63° are assigned to (220) (311) (400) (511) (440) planes respectively. All these peaks confirm the cubic spinel type lattice of CoFe2O4 which matches well with the standard XRD pattern (JCPDS Card No: 22-1086). No additional peaks were observed in these XRD patterns, suggesting that no other phases besides cobalt ferrite structure are detected in all the samples. This is also in good agreement with the results deduced from TG/DSC curve that above 842 °C no sharp endothermic curve was observed signifying pure phase of CoFe2O4 has started above that temperature. Comparing Fig. 2a–d, we observe that the intensity of the peaks increases with increasing PVA concentration, which suggests that the addition of PVA significantly increased the crystalinity and composition of CoFe2O4 particles. The three dimensional network structure of PVA acts like a confined space that allows sufficient contact and reaction of the reactants and also limits the growth of the CoFe2O4 particles, leading to the formation of CoFe2O4 particles of good crystalinity and high dispersibility [5].
3.3 Morphology of CoFe2O4 ferrites
Figure 3 shows the HR-SEM images of the samples with various PVA concentrations. It can be seen from the micrographs that there are significant differences in the microstructures of the four samples. Figure 3a reveals that the CoFe2O4 particles prepared with 3 wt% of PVA shows the presence of very large lumps which are agglomerates of small spherical particles. The maximum agglomeration size even extends up to 1 μm. Within single agglomerate, the particles are in the limit of close-packing and they are supposed to interact strongly, yielding high magnetisation. For the sample with 6 wt% of PVA concentration, the CoFe2O4 particles are dispersed and crystallites of sizes in the range of 100–500 nm are present. As seen from Fig. 3c, it is clear that the particles are dispersed again and the individual particle size is further reduced. From Fig. 3d it is observed that the individual particles are more prominent with the crystallite sizes further more reduced in the ranges from 50 to 150 nm. In this case smaller crystallites are predominantly present in comparison with Fig. 3a–c. Considering all these facts, it is concluded that for the samples with increasing PVA concentration, the particle agglomeration tendencies decrease, and there are significant variations in grain size. The increase of PVA chains breaks up the network structure of agglomerated crystallites and results in the formation of dispersive CoFe2O4 particles of less grain size [5]. It is evident that the size, shape and agglomeration state of the as-prepared CoFe2O4 particles in Fig. 3d are better than that in Fig. 3a–c. The histograms of HR-SEM images shown in Fig. 3a–d are presented in Fig. 4a–d. From the histograms, it is confirmed that the ranges of crystallite sizes are getting reduced with the increasing PVA concentration.
3.4 Magnetic properties
To clarify the magnetic properties of CoFe2O4 powders, the hysteresis loops of the samples prepared by the sol–gel method were measured using VSM. The maximal magnetic field applied in the measurements is 20 kG and the detection was carried out at room temperature. Figure 5 shows the magnetisation loops of the samples with various PVA concentrations. Obviously, all the magnetic properties of CoFe2O4 powders show dependence upon the concentration of PVA.
As seen from Fig. 6a, the Ms value decreases from 106.6 to 50 Am2/kg as the PVA concentration increases from 3 to 15 wt%. Similar evolution trend is also found in Mr, where it reduced from 36.6 to 15.7 Am2/kg, with increasing PVA concentration. It is easy to deduce that the evolution behaviours of Ms and Mr are highly dependent on the dispersion of growth rates of CoFe2O4 crystallites. The decrease in Ms may be related with structural defects, or due to the presence of a relatively non-reactive surface layer that has low magnetisation. This could also be due to the evolution of the average residual strain effects [11].
However, as per Fig. 6b, coercivity (Hc) which is 72.1 kA/m for sample with PVA concentration (3 wt%) decreases to 59.2 kA/m for the sample with PVA concentration (6 wt%), then increases to 67.8 kA/m for sample with PVA concentration (9 wt%) and again decreases to 63 kA/m for sample with PVA concentration (15 wt%). Generally, coercivity Hc of a magnetic material is a measure of its magnetocrystalline anisotropy [12]. It seems to originate from exchange anisotropy due to spin disorder at the particle interface. This effect is expected to be larger for smaller particles due to the increase in the surface-to volume ratio [9]; however the dramatic decrease and increase in coercivity at 6 and 9 wt% of PVA concentration cannot be attributed only to magnetocrystalline anisotropy. As have been reported by other groups [13, 14], Hc is also closely related to the microstructure, particle/grain size, residual strain, domain structure, and many other complex factors.
According to Stoner Wohlfarth model [15], a theoretical value of Mr/Ms is 0.5 for non interacting uniaxial single domain particles with the easy axis being randomly oriented. The value of (Mr/Ms) ratio for all the samples is approximately 0.34 which suggests that all the samples exhibit uniaxial anisotropy. CoFe2O4 crystallites, when used as a recording material should possess reasonable Ms, high Hc and low media noise [16]. Taken altogether, these results implied that there existed an optimum PVA concentration (9 wt%), shown in the Fig. 5c. Bhame and Joy have reported [17] synthesis of CoFe2O4 particles of size 900 nm by ceramic method. The Ms and Hc values for the powder samples are 77 Am2/kg and 47.7 kA/m respectively. The Ms and Hc values reported by Gharagozlou [18] for CoFe2O4 synthesized by polymeric precursor method are 100.1 Am2/kg and 85.3 kA/m respectively. Compared to the reported values, the present investigation using sol–gel method with PVA 9 wt% concentration provides moderate magnetisation Ms and high coercivity Hc.
4 Conclusions
CoFe2O4 ferrites were synthesized by sol–gel method using PVA as surfactant in various concentrations. The XRD results confirm the cubic spinel structure of CoFe2O4 powder. Above 842 °C no endothermic peak was observed in TG/DSC curve, which corroborates well with the absence of other complex phases in XRD results. As revealed by the HR-SEM images, increasing the concentration of PVA breaks up the three dimensional network structure of the products and resulted in the formation of well dispersed CoFe2O4 particles. Significant differences in magnetic properties were also observed with respect to PVA concentrations. CoFe2O4 ferrites of high Hc and moderate magnetization Ms, which could be specifically used for practical recording materials, were achieved for an optimum PVA concentration of 9 wt% from the above method.
References
A. Goldman, Modern Ferrite Technology (Van Nostrand Reinhold, New York, 1990)
B.M. Berkovsky, V.F. Medvedev, M.S. Krakov, Magnetic Fluids: Engineering Applications (Oxford University Press, Oxford, 1993)
J.G. Lee, J.Y. Park, C.S. Kim, J. Mater. Sci. 33, 3965 (1998)
S.N. Okuno, S. Hashimoto, K. Inomata, J. Appl.Phys. 71, 5926 (1992)
R. Qin, F. Li, L. Liu, W. Jiang, J. Alloys Comps. 482, 508 (2009)
D.S. Mathew, R.S. Juang, Chem. Eng. J. 129, 51 (2007)
X.H. Yann, X. Wang, Z.D. Zhang, J. Crystal. Growth 277, 467 (2005)
L.J. Zhao, H.J. Zhang, Y. King, S.Y. Song, S.Y. Yu, W.D. Shi, J. Solid State Chem. 181, 245 (2008)
N. Kasapoglu, A. Baykal, Y. Koseoglu, M.S. Toprak, Scripta Mater. 57, 441 (2007)
F.X. Cheng, Z.Y. Peng, C.S. Liao, Z.G. Xu, S. Gao, C.H. Yan, D.J. Wang, J. Wang, Solid State Commun. 107, 471 (1996)
R.P. Moyet, Y. Cardona, P. Vargas, J. Silva, O.N.C. Uwakweh, Mater. Charact. 61, 1317 (2010)
P.A. Roy, S.K. Date, J. Magn. Magn. Mater 222, 33 (2000)
B.H. Liu, J. Ding, Appl. Phys. Lett 88, 042506 (2006)
Y.C. Wang, J. Ding, J.B. Yi, B.H. Liu, Z.X. Shen, Appl. Phys. Lett 84, 2596 (2004)
E.C. Stoner, E.P. Wohlfarth, Philos. Trans. R. Soc. A 240, 599 (1948)
S.H. Xiao, W.F. Jiang, L.Y. Li, X.J. Li, Mater. Chem. Phys. 106, 82 (2007)
S.D. Bhame, P.A. Joy, Sensor Actuator B 137, 256 (2007)
M. Gharagozlou, J. Alloys Comps 486, 660 (2009)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sivakumar, M., Kanagesan, S., Suresh Babu, R. et al. Synthesis of CoFe2O4 powder via PVA assisted sol–gel process. J Mater Sci: Mater Electron 23, 1045–1049 (2012). https://doi.org/10.1007/s10854-011-0545-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-011-0545-0