Abstract
Introduction
Peri-implantitis (PI) is one of the peri-implant diseases that causes destructive inflammatory process of the hard and soft tissues surrounding the implant. Recently, several types of lasers have been proven to be effective in PI. Despite the increase of scientific publications on laser treatment in PI, the best type of laser treatment is not evaluated until now. The primary aim of our systematic review is to provide a comprehensive review on the effect of different types of lasers that were used as a treatment modality for patients with peri-implantitis concerning the most effective type of laser in this field.
Material and methods
We used databases from scientific websites such as PubMed/Medline, Scopus, and Google Scholar to get related articles about this subject. The research process involved specific key words “peri-implantitis”-laser treatment”-peri-implantitis treatments”. We were more concerned about English human published studies including clinical trials, case-control, and case series of laser therapy in peri-implantitis.
Results
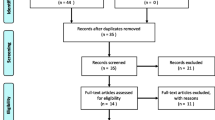
Our initial research yielded 174 articles. After scanning and screening the published articles, we excluded 152 articles; in total, 22 articles were included in this review.
Conclusion
We concluded that the determination of the optimal laser treatment for peri-implantitis is recondite due to the disharmony of results that have been documented. However, if lasers are not used according to proper protocols with proper temperatures, a damage can occur to the implant and peri-implant tissues.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Peri-implantitis (PI) is one of the peri-implant diseases that causes destructive inflammatory process of the hard and soft tissues surrounding the implant, which affects the osseointegration between the implant-bone connections [1]. PI is caused by pathogenic micro-organisims which accumulate and grow in the soft tissue surrounding an osseoingrated implant, triggering the host response, causing infectious, inflammatory processes limited to peri-implant mucosa; continuously, these reactions lead to pockets formation and supporting bone destruction, thereby, creating bacterial colonization on the implant surfaces which in turn prevent tissue re-attachment and bone re-generation [2]. The most common pathogenic microorganisms recognized with the implant failure are the gram-negative anaerobes, such as Prevotella intermedia, Porphyromonas gingivalis, Aggregatibacter actinomycetemcomitans, Bacterioides forsythus, Treponema denticola, Prevotella nigrescens, Peptostreptococcus micros, and Fusobacterium nucleatum [3, 4]. Furthermore, the combination of retrograde PI with implant failure could be due to micro-fractures of bone resulting from excessive mechanical loads [5]. Also, poor micro-design and macro-design of implant affects the osseointegration process. However, removal of plaque biofilm resolves inflammation and arrests the disease progression. Although implant surface modifications such as surface roughness are designed to promote the osseointegration and increase the long-term survival of implants, once its surface is contaminated by bacteria, it increases the risk of implant failure as it acts as retentive area of plaque that it is hard to be completely removed [6].
The diagnosis of PI is a challenge as it is often noticed during routine checkup dental visits, hereby, peri-implant tissue (PT) evaluation is needed by early observation for any changes of the hard and soft tissues to prevent its progression [7]. Presence of plaque and bleeding on probing (BOP) works as a positive predictor of PI. However, assessing the soft tissues can be achieved by inspection, palpation, and probing either by automated or manual probe, to evaluate signs of PI such as swelling, redness, BOP, and suppuration [5]. In addition, the radiographic examination considered as an important step in its diagnosis, which appears as a crater bone loss, although it is not reliable to see the facial and lingual/palatal bone levels. In this situation, gutta percha can be used to detect the facial/palatal/lingual defects and then estimate the crestal bone level in relation to implant length. Furthermore, the implant mobility is a diagnostic feature for a final stage of osseodisintegration in which implant becomes nonfunctional, called “failed implant”; low degrees of an implant mobility can be determined by Periotest or magnetic resonance frequency. However, histological examination shows infiltration of lymphocytes and plasma cells which are detected in PT [8]. There are several methods to predict high risk for PI including the use of DNA probes, bacterial cultures, polymerase chain reaction (PCR), monoclonal antibody, and enzyme assays to investigate the subgingival microflora [9].
Peri-implantitis is classified according to the pocket depth (PD) and bone loss, into three categories: early, moderate, and severe. Early PI is characterized by PD ≥ 4 mm with BOP and suppuration while bone loss ˂ 25% of the implant length, when PD ≥ 6 mm with BOP, suppuration and bone loss ˂ 25–50% of the implant length considered as moderate peri-implantitis, the sever form of PI, signified by increased PD ≥ 8 mm with BOP and suppuration, also severe bone loss ˃ 50% of the implant length [10]. Though, this classification provides clinical and radiographic information, but it gives insufficient details of the types of the bony defect.
However, there are more classifications of peri-implant defects that were mentioned to describe the bony defect. Schwarz et al. [11] classified peri-implant defect into two classes depending on the configuration of the bony defect as class I defect which is an intraosseous defect and class II defect which is a supra-alveolar defect in the crestal area of implant. While Nishimura et al. [12] classified amount of bone loss with the associated shape of bony defect of the PI into four classes; class 1 is light horizontal bone loss with minimal peri-implant defects; class 2 is moderate horizontal bone loss with isolated vertical defects; class 3 is moderate to advanced horizontal bone loss with broad, circular, bony defects, and class 4 is advanced horizontal bone loss with broad, circumferential, vertical defects, as well as loss of the oral and/or vestibular bony wall.
The treatment of PI depends on plaque removal, detoxification of implant surface, debridement of PI, and maintenance of plaque control regimes [12, 13]. These could be achieved via mechanical debridement (MD) with or without raising a surgical flap, depending on the severity of the peri-implantitis lesion. The surgical considerations are required for moderate and severe peri-implantitis which include resective or regenerative surgeries in combination with non-surgical methods. The resective surgery is done by either ostectomy or osteoplasty in combination with implantoplasty that tends to eliminate the causative agents and maintain optimal conditions for cleaning the implant surfaces. While, regenerative surgery is used to establish bone regeneration (re-osseointegration) with either autogenous bone or bone replacement graft materials, with or without collagen membrane [1]. Although success rates have been documented, these procedures have limited efficacy [14]. However, membrane exposure is a frequent complication after such procedures, which leads to bacterial penetration and infection [15].
However, the non-surgical (conservative) therapy is done by the manual treatment such as curettes, ultrasonic devices, air polishing systems, lasers, and photodynamic therapy (PDT) methods. The aim of non-surgical options is to eliminate or decrease the amounts of pathogens present in the implant surfaces and the peri-implant pockets in order to re-establish the healthy conditions [16, 17]. However, using of manual and ultrasonic instruments in removing microbial biofilm and calcified deposits around the implant is still the main method of tissues debridement and surface decontamination [17]. However, Schwarz et al. [18] reported that residual biofilm areas were found less 30–40% by using ultrasonic methods than manual instruments, but may cause small metal chips and scratches on the implant surfaces even if carbon fiber tips are used [5, 19]. In 2005, Karring et al. [20] reported that sub-mucosal debridement alone, accomplished by utilizing either an ultrasonic device or carbon fiber curettes, is not sufficient for the implant decontamination if PD ≥ 5 mm and implant with exposed threads. Hereby, it seems that mechanical or ultrasonic debridement alone may not be satisfactory for PI management.
In addition, medications such as antiseptic rinses and application of systemic or locally delivered antibiotics are also considered as non-surgical treatment. It was reported in several studies that chemical antimicrobial agents such as tetracycline, doxycycline, amoxicillin, metronidazole, minocycline hydrochloride, ciprofloxacin, and sulfonamides with trimethoprim suppress periodontal pathogens more effectively compared to mechanical techniques, significantly reduce pocket depths, and improve the results of conventional mechanical anti-infective therapy [19,20,21,22,23,24]. On the contrast, the disadvantages of antimicrobial agents include the use of combined of different types of antibiotics due to the diversity of pathogens, bacterial resistant formation, and the incidence of unfavorable systemic reactions.
Mombelli [25] established the cumulative interceptive supportive therapy (CIST) protocol for maintenance of the dental implant and management of peri-implantitis regarding certain factors like infection, PD, BOP, suppuration, and radiographic radiolucency surrounding the implant. There is no therapy in case of PD ˂ 3 mm, no plaque or bleeding, while mechanical therapy and oral hygiene instructions are recommended if PD ˂ 3 mm but plaque and BOP are present; this is called protocol A. Protocol B is indicated when plaque, BOP, and pocket depth 4–5 mm are present without radiographic evidence of bone loss; in this stage, mechanical debridement, oral hygiene instructions, and local anti-infective therapy (e.g., CHX) are required. When PD exceeds 5 mm and bone loss ˂ 2 mm, protocol C is indicated which includes mechanical debridement, microbiological test, and local and systemic anti-infective therapy. The last protocol is D where PD exceeds 5 mm and bone loss is more than 2 mm; in this case, the respective or regenerative surgery is recommended [25]. Professional supportive care should be developed according to the individual needs of the patient (e.g., 3-, 6,- or 12-month recall intervals) and their compliance has to be confirmed.
Recently, several types of lasers have been proven to be effective in the dental field as they become cost effective, portable, lightweight, and reliable [13]. Despite the increase of scientific publications on laser treatment in PI, the best type of laser treatment is not evaluated until now. The primary aim of our systematic review was to provide a comprehensive review on the effect of different types of lasers that were used as a treatment modality for patients with peri-implantitis concerning the most effective type of laser in this field.
Material and methods
We used databases from scientific websites such as PubMed/Medline (National Library of Medicine, Bethesda, Maryland), Scopus, and Google Scholar to get related articles about this subject. The research process involved specific key words in the main query “peri-implantitis”- laser treatment”- peri-implantitis treatments” to find more articles about the subject. We were more concerned about English published articles only. Also, all prospective and retrospective human studies, as well as clinical trials, case-control, and case series of laser therapy in PI were evaluated; the selected studies had at least one failing rough-surface, screw-type implant that presented with all signs of PI including plaque index, BOP, pocket depth, and radiological examination, which were published from 2007 to August 2018. Articles of animal studies, in vitro, case reports, follow-up periods less than 6 months, and peri-implant mucositis cases were not assessed. Also, letters, editorials, reviews, and PhD thesis were excluded. All eligible studies were assessed in this article; the titles and abstracts of potential references were manually examined to exclude irrelevant publications, and the full texts of the relevant papers were then reviewed. Authors, years of publication, type of study, number of patients, number of implants, type of lasers, and results were extracted from the selected studies. The study targeted the use of laser treatments as monotherapy or as an adjunct modality, either with surgical or non-surgical interventions in the treatment of peri-implantitis. Several types of lasers were found and reviewed to identify the best option of using lasers in PI treatment. However, meta-analysis could not be performed due to the heterogeneity in the selected studies.
Results
Our initial research yielded 174 articles published in various journals. After scanning the title and abstract, we excluded 142 articles because they were not relevant to the subject and did not meet the included criteria of the study. Among the 32 studies, we excluded 10 articles because they were non-human studies, case studies, and with follow-up period less than 6 months. In total, 22 articles were included in this review. Original studying articles and case reports were investigated by the author. The results of this literature review are presented in Fig. 1. Also, we explore in our article, the peri-implantitis treatment strategies using different types of laser as monotherapy or adjunctive to non-surgical and surgical treatments.
However, the selected studies included eight studies on Er:YAG laser (Table 1); six randomized clinical trials (RCT), and two case series (CS). In addition, ten studies on diode laser were done (Table 2) where five of them were randomized clinical trials, two controlled clinical trials (CCT), one pilot study, and two case series. Two of these studies used diode laser as light source in photodynamic therapy of peri-implantitis. Also, there were two studies on CO2 laser (Table 3), which included one controlled clinical trial and one case series study. Furthermore, one study of case series evaluates the effect of Er, Cr:YSGG laser in the treatment of peri-implantitis as shown in Table 4. Moreover, only one randomized clinical trial was done on using Nd:YAG laser (Table 5) as adjunctive treatment compared to mechanical debridement alone in the treatment of peri-implantitis.
Although, all the included studies provide adequate oral hygiene care at the beginning of the treatment including instructions and peri-implant scaling before laser treatment, Deppe et al. [42] was the only one who commented on the maintenance of the oral hygiene during the follow up periods. In addition, all the studies were done on Ti implants except Schwarz et al. [28] who worked in Zirconia implants.
Only two studies used lasers as a monotherapy [2, 26] which limits the evidence of using lasers alone in the treatment of PI. While, most of the results of applying lasers were related to the use of lasers as an adjunct treatment to non-surgical therapies and surgical therapies. The selected studies included 11 non-surgical studies (using mechanical curettage by plastic, titanium (Ti) or carbon fiber curettes [28, 32,33,34,35, 40, 44], air abrasive [16, 38], and CHX rinses or local delivery agents [37, 39], while the surgical studies, were nine studies, including GBR either with resorbable or non-resorbable membranes [18, 27, 29, 36, 41,42,43, 45] as part of the surgical treatment in peri-implantitis cases.
The use of lasers in peri-implantitis treatment
Nowadays, treatment with lasers alone or adjunctive to conventional mechanical therapy shows positive results in the elimination of bacterial smear layer and in the treatment of peri-implantitis [38, 46]. Although, there are several types of laser used in the treatment and maintenance of PI, number of parameters should be considered before treating PI using laser such as type of laser, suitable power settings that effectively disinfect the implant while being safe for its surface, exposure time, and the distance from which the laser is applied [19]. Lasers can be used in either open flap or non-flap procedures depending on the severity of bone loss, type of laser used, and its wavelength. Laser types are grouped into hard-tissue lasers (Er,Cr:YSGG and Er:YAG wavelengths), which are useful in the improvement of early osseointegration after fixture placement, and soft-tissue lasers which are useful in improving hemostasis and wound healing processes [47]. All lasers can provide some degree of tissue healing and pain relief, according to their wavelength, especially the soft lasers with a wavelength range of 655–810 nm. The different types of lasers and their wavelengths are illustrated in Fig. 2.
Erbium laser
The hard-tissue erbium family lasers include Er:YAG and Er,Cr:YSGG. These lasers have two wavelengths—2780 nm for Er,Cr:YSGG laser and 2940 nm for Er:YAG. They are highly absorbed by water and hydroxyapatite, leading to the vaporization of the water within the mineral substrate. They are able to cut hard and soft tissue including tooth structure and gingiva [48]. Er:YAG laser is the most commonly used lasers for nonsurgical treatment of peri-implantitis [49]; it has shown significant effect of removing calculus, granulation tissues, and microbe-infiltrated oxide layer from the implant surfaces without damaging implant surfaces [50, 51].
Neodymium laser
The ND:YAG laser (neodymium-doped yttrium aluminum garnet) is the first laser that build for dentistry. This laser has a solid active medium with a wavelength of 1064 nm, located at the invisible near-infrared portion of the electromagnetic spectrum. It is more absorbed by hemoglobin, so it is desirable to be used for coagulation purpose. These lasers cause a rise in temperature, which may lead to melting of the titanium implant surfaces, physical change of the hydroxyapatite coating, cracks, and porosity loss which make it contraindicated in the treatment of peri-implantitis [5].
CO2 laser
In early 1990, the first soft tissue laser wavelengths; carbon dioxide (CO2) lasers were introduced in dentistry [48]. These lasers give positive outcomes in the treatment of peri-implantitis as it is very effective against anaerobic bacteria spectrum [52]. However, CO2 lasers have minimal depth penetration (less than 0.1 mm) and is absorbed by the watery tissues and hence vaporizes the bacterial cells in tissues and increases osteoblast attachment to implant surfaces [53]. Furthermore, it is minimally absorbed at the implant surface and has a reduced risk of causing temperature-induced tissue damage [54].
Diode laser
Diode lasers (DLs) have been grown and evolved as easy soft-tissue hand pieces due to their usefulness in being used around implants with minimum risk of creating iatrogenic damages [48]. For killing the bacterial micro-organisms, the laser is inserted towards the ulcerated pocket wall which causes a localized increase in temperature, targeting bacteria and their endotoxins which in turn leads to detoxification of implant surfaces [55]. DLs also stimulate fibroblast and osteoblast; this in turn causes RNA messenger production to increase, leading to a significant collagen formation during periodontal tissue healing [47]. DLs with wavelengths of 810 nm, 940 nm, and 980 nm have a less damaging effect on the implant when a good coolant is used [5]. However, diode laser (980 nm) does not damage Ti surfaces, even if it is used in high-power settings, while DLs with 810 nm wavelength at a high-power may cause damage to the implant surface, so such a laser must be used carefully [30].
Photodynamic therapy (PDT)
Furthermore, there is another method to get rid of bacteria in periodontal sulcus of implant, which is called photodynamic therapy. PDT has high-target specificity with healthy human cells and low probability of microbial resistance and low risk of chemical and thermal side effects respectively [56]. This type of treatment combines two components which are photosensitizer (like toluidine or methylene blue) and a light source of a specific wavelength. It takes place when the visible light irradiation forms biochemical interactions in the presence of the photosensitizer which undergoes excitation and produces toxic oxygen species such as singlet oxygen and free radical that generate bactericide effects against both aerobic and anaerobic bacteria by specific cellular destruction, membrane lysis, and protein inactivation [57]. Some studies [58, 59] had reported that the microbial load significantly reduced after PDT in peri-implantitis cases. While, another study concluded that PDT showed no significant differences in the reduction of pocket depth or reduction of the bacterial load in the periodontal pockets in comparison with local antibiotic therapy [60]. On the other hand, PDT found to be effective on moderate and severe PI; this study suggested that conventional debridement with or without PDT depends on the severity of PI [61].
Laser-assisted peri-implantitis protocol
It is an emerging experimental technique where an implant-specific modification of the laser-assisted new attachment protocol (LAPIP) [62, 63]. Researchers used Nd:YAG laser-ablation to remove inflamed sulcular tissues and decontaminate the implant surface, followed by nonsurgical periodontal therapy. The LAPIP technique is designed to create a blood clot that allows the defect area to heal apico-coronally by preventing downgrowth of the gingival epithelium. However, no randomized controlled trials to date have evaluated its efficacy for the treatment of PI [56].
Discussion
Although, the presence of long term sucess rate of implants, failures sometimes occur. Regardless the type of peri-implantitis treatment, most of the studies approved that the mainstay of treatment protocol of peri-implantitis must include decontamination of the implant surface [61] either with or without surgical intervention. Both soft and hard lasers can be used in such decontamination procedures [30]. Lasers are more efficient than mechanical methods, as they can irradiate small areas of the implant surface in which mechanical methods are unable to reach [13]. In addition, Leonhard et al. [64] conducted that 42% of PI cases treated with the conventional treatment have shown failure as well as the study that was done by Karring et al. [20] who revealed that debridement alone is not sufficient for the treatment of peri-implantitis. However, some clinical investigations [2, 29, 33, 34, 42, 65] presented positive clinical outcomes and stated that lasers are promising treatment modalities in the decontamination of the different implant surfaces, which showed bacterial reduction, improvement of clinical parameters of PI, and healing acceleration but beneficial long-term have been to be demonstrated. While, some of the current published data [18, 26, 28, 36] concluded that the use of lasers in combination with surgical/non-surgical therapy gave minimal benefits in improvements of tissues around the implants. The explanation for the contradiction and inconsistency of results may be due to the wide variability of clinical parameters of peri-implantitis, types, and wavelengths of laser among the clinical studies.
However, in comparison with the included studies, the results revealed that since 2007 until August 2018, DLs are the most commonly used lasers in the treatment of PI; this was in disagreement with what was documented by Charalampakis and Belibasakis [49] who reported that the most used laser in PI management was the Er:YAG laser, although it was reported by Tosun et al. [52] Er:YAG laser has the ability to remove 100% of bacteria on Ti surfaces while diode laser can only eliminate 97% of the bacteria. One explanation for this result is that diode laser was known to be used in the dental field after Er:YAG laser with its lightweight and safety.
Numerous benefits were stated in using DLs such as the ability to decontaminate the implant’s surface, bacterial removal, and ease of accessing. Also, it has an biostimulative result which supports regeneration process [60]. Therefore, diode laser is useful not only in treating PI but also in the healing process [37, 39]. Several investigations [32,33,34, 38] conducted that the use of diode laser with both high or low power modes represented a good modality when used as adjunctive treatment to MD or abrasive devices without surgical intervention, and it could be used as a protocol for the maintenance of the post-implant rehabilitation complications. While in 2013, Thierbach and Eger [40] worked on 40 implants with and without pus discharge and reported that peri-implantitis with pus discharge could be treated much better by diode laser with surgical intervention.
However, Bombeccari et al. [41] and Papodopoulos [36] et al. made randomized clinical trials on diode lasers in surgical intervention treatments, and they revealed that there were no additional benefits to surgical treatments after follow-up period of 6 months. Though Arisan [35] in 2015, made a clinical trial on 48 implants of 20 patients, comparing the effect of diode laser 810 nm with the mechanical debridement to the mechanical debridement alone, after 6 months of follow-up, he reported that there was no added value of using a diode laser in non-surgical intervention.
In addition, two randomized clinical trials of PDT were made by Schaar et al. [39] and Bassetti et al. [37] in 2013 and 2014 using diode laser with a phenothiazine chloride dye without surgical intervention. They used same clinical procedures and same number of implants, but different follow-up periods 6 and 12 months respectively. Both studies applied PDT twice a week to a group of 20 implants and compared them to a single application of local delivery minocycline hydrochloride microspheres in a group of 20 implants’ sulci. In addition, both groups were irrigated with 3% hydrogen peroxide. Both studies reported that there was a significant reduction in both treatment modalities in reduction of BOP and PD but complete resolution of the disease was not observed. Also, there was no statistically significant difference between the two groups either after 6 months or 12 months of follow-up periods. However, diode laser seems to be efficient in the treatment of peri-implantitis in short periods of time only.
In 2017, John et al. [26] conducted that Er:YAG laser is an effective modality treatment when used as monotherapy, but it failed to achieve a complete resolution of peri-implantitis cases after 36 months of follow-up periods. This was in agreement with Schwarz et al. [28] who estimated in 2015 that treatment of peri-implantitis using Er:YAG lasers contributed significant short-term clinical improvements without the complete resolution of the disease after 6 months of follow-up periods.
In addition, a randomized clinical study made by Renvert et al. [16] in 2011, evaluated the effectiveness of Er:YAG lasers compared to the abrasive air polishing system without surgical intervention. The study revealed a significant reduction in clinical parameters and bacteria strains in both groups after 1 month, but after 6 months, there were no effect in reducing bacterial account and reduction of PD measurements, only decrease in BOP in both groups. Furthermore, Schwarz et al. [27, 29, 31] studies revealed that there were no significance differences in BOP, pocket depth reduction, or improvement of clinical attachment level or bone regeneration, when using Er:YAG in comparison with mechanical treatments in surgical intervention up to 7 years of follow-up periods. In 2013, Schwarz et al. [18] reported negative outcomes of Er:YAG laser in a 48-month follow-up clinical study. The effect of surface denomination by laser was evaluated in comparison to the mechanical treatment (using plastic curettes and sterile saline) after resection and regenerative surgeries. Their results showed a significant reduction in clinical parameters such as BOP, plaque index, and attachment loss in the mechanically treated group more than the Er:YAG laser-treated group.
Yet, results showed that there is a possibility of clinical parameters’ relapse with the longer follow-up periods as the single use of Er:YAG gives short-term progresses and no complete resolution of the disease. However, the Er:YAG laser has been shown to be safe for use on implant surfaces when used at 100 mJ/pulse and 10 pulses/s for 60 s and no microscopic changes occurred in the implant surface [66]. But Kim et al. in 2011 [67] stated that there were alterations in implant surface characteristics when using lasers with energies exceeding 140–180 mJ/pulse. Hereby, considerations should be taken when using Er:YAG lasers, otherwise damage of implant surface could occur.
Deppe et al. [42] performed a prospective clinical study on using CO2 laser and conventional debridement with surgical intervention in 54 implants. They investigated 29 implants in the test group by using CO2 laser in the decontamination of implants’ surfaces, while 25 implants in the control group were treated with conventional MD. Then, each group was further divided into two subgroups receiving either adjunctive soft tissue resection or guided bone regeneration that were followed for at least 6 months to 5 years. They concluded that both groups gave successful results in gaining CAL when combined with bone augmentation, but CO2 laser was more significant than the conventional decontamination when only combined with soft tissue resection.
In 2008, Romanos and Netwing [43] applied CO2 laser with GBR to 19 implants with peri-implantitis and reported that CO2 laser was effective in decontamination of implant surface because of the physical properties of its energy, its absorption, and interaction with the tissues; also, BOP and PD were significantly reduced, and bone formation was achieved due to the increase of osteoblast attachment to implant surfaces [53]. In addition, other studies [14, 42] conducted that using of CO2 lasers accelerate the healing, increase the liability of bone formation in peri-implant defect sites, and treat the disease. There is no significant increase neither in temperature of the implant nor alteration of the implant surface [30]. But it was reported in an early study that using of CO2 lasers at continuous and pulsed settings causes increase in temperature of implants’ surfaces 9.5 to 12.2 °C respectively [68], which in turn will cause bone necrosis. Also, Romanos et al. [14] stated that carefulness of time exposure should be taken otherwise temperature may increase affecting the osseointegration process.
In 2014, Al-Falaki [2] conducted a study on 28 implants using Er,Cr:YSGG laser with 6-month follow-up period. The results showed that Er,Cr:YSGG laser has a significant effect on decreasing the peri-implant inflammation, BOP, and PD.
Abduljabbar et al. [44] made a randomized clinical trial without surgical intervention on 63 patients with 74 implants where they assessed the effect of Nd:YAG with MD in comparison with MD alone in the treatment of peri-implantitis. They concluded that using of this laser with MD showed improvement in the clinical parameters of PT more than using MD alone in short term but not in long term.
Through the relevant evidences, a wide heterogeneity was found in the studies regarding the types, wavelengths, and methods of laser applications in the management of PI. Also, implant materials, clinical parameters, and indices were different in some cases; thus, a clear and reliable inference could not be made. In addition, some studies used a combination of laser therapy with other treatments, and insufficient studies supported the use of lasers as monotherapy. Also, the lack of longitudinal data on the implant survival in most of the included studies, CAL and PD were used as pertinent substitution. Moreover, some researches presented a small number of patients, which is relevant to low incidence of PI. Although, systemic diseases and smoking are known to be risk factors with adverse effect on PI treatment, some researchers did not even mention the health or the smoking situation of their patients.
Subsequently, the relative influence of the laser application could not be assessed accurately, but it was found in most of clinical trials that lasers can sterile implant surface, remove the granulation tissues, and accelerate healing. However, the most prevalent lasers in peri-implantitis treatment are diode, Er:YAG, and CO2 lasers, while Nd:YAG laser was effective, but least in use as it has the potential to damage tissues as well as the implant surface due to its ability to penetrate tissues and increase the temperature.
Conclusion
The main key of treatment success is that the implant surface should be surrounded by a healthy PT after surface decontamination to achieve desirable successful treatment. Failure in the plaque control, patient motivation, and implant maintenance could be a serious impenetrable factor that may affect the final outcomes, so as to say that prevention is the best form of treatment. We concluded that the determination of the optimal laser treatment for PI is recondite due to the disharmony of results that have been documented. However, if lasers are not used according to proper protocols with proper temperatures, a damage can occur to the implant and peri-implant tissues. To produce more conclusive results, clinical protocols must be simplified and standardized and evaluated in controlled clinical trials. More clinical studies, larger numbers of patients, and longer follow-up periods are recommended to achieve definitive verdict on the efficacy of lasers in the treatment of peri-implantitis.
Data availability
A meta-analysis was not conducted for this systematic review.
Abbreviations
- BOP:
-
bleeding on probing
- CS:
-
case series
- CAL:
-
clinical attachment level
- CCT:
-
controlled clinical trial
- DLs:
-
diode lasers
- LAPIP:
-
Laser-Assisted Peri-Implantitis Protocol
- MD:
-
mechanical debridement
- PT:
-
peri-implant tissues
- PI:
-
peri-implantitis
- PDT:
-
photodynamic therapy
- PD:
-
pocket depth
- PCR:
-
polymerase chain reaction
- RCT:
-
randomized clinical trials
- TI:
-
titanium
References
Smeets R, Henningsen A, Jung O, Heiland M, Hammächer C, Stein JM (2014) Definition, etiology, prevention and treatment of peri-implantitis – a review. Head Face Med 10:34
Al-Falaki R, Cronshaw M, Hughes FJ (2014) Treatment outcome following use of the erbium, chromium:yttrium, scandium, gallium, garnet laser in the non-surgical management of peri-implantitis: a case series. Br Dent J 217(8):453–457
Heydenrijk K, Meijer JA, Van der Reijden WA, Raghoebar GM, Vissink A, Stegenga B (2002) Microbiota around root-form endosseous implants: a review of the literature. Int J Oral Maxillofac Implants 17:829–838
Shibli JA, Compagnoni MM, Moreira RF, Marcantonio E (2003) Microbiologic and radiographic análisis of ligature-induced peri-implantitis with different dental implant surfaces. Int J Oral Maxillofac Implants 18:383–390
Gupta S, Mahapatra NA (2015) Review of perimplantitis and lasers. IOSR J Dent Med Sci 14(3):01–04
Schwarz F, Bieling K, Nuesry E, Sculean A, Becker J (2006) Clinical and histological healing pattern of peri-implantitis lesions following non-surgical treatment with an Er:YAG laser. Lasers Surg Med 38:663–671
Mahato N, Wu X, Wang L (2016) Management of peri-implantitis: a systematic review, 2010– 2015. SpringerPlus 5:105
Algraffee H, Borumandi F, Cascarini L (2012) Peri-implantitis. Br J Oral Maxillofac Surg 50:689–694
Gupta HK, Garg A, Bedi NK (2011) Peri-implantitis: a risk factor in implant failure. J Clin Diagn Res 5(1):138–141
Froum SJ, Rosen PS (2012) A proposed classification for peri-implantitis. Int J Periodontics Restorative Dent 32(5):533–540
Schwarz F, Sahm N, Becker J (2008) Aktuelle Aspekte zur Therapie periimplantärer Entzündungen. Quintessenz 59:00
Nishimura K, Itoh T, Takaki K, Hosokawa R, Natio T, Yokota M (1997) Periodontal parameters of osseointegrated dental implants. A 4-year controlled follow-up study. Clin Oral Implants Res 8:272–278
Ashnagar S, Nowzari H, Nokhbatolfoghahaei H, Zadeh BY, Chiniforush N et al (2014) Laser treatment of peri-Implantitis: a literature review. J Lasers Med Sci 5(4):153–162
Romanos G, Ko HH, Froum S, Tarnow D (2009 Jun) The use of CO2 laser in the treatment of peri-implantitis. Photomed Laser Surg 27(3):381–386
Nowzari H, Slots J (1995) Microbiologic and clinical study of polytetrafluoroethylene membranes for guided bone regeneration around implants. Int J Oral Maxillofac Implants 10:67–73
Renvert S, Lindahl C, Roos Jansa°ker AM, Persson GR (2011) Treatment of peri-implantitis using an Er:YAG laser or an air abrasive device: a randomized clinical trial. J Clin Periodontol 38(1):65–73
Roncati M, Adriaens LM (2013) Treatment of peri-implantitis: nonsurgical therapeutic approaches. Annal Oral Maxillofac Surg 1(3):21
Schwarz F, Hegewald A, John G, Sahm N, Becker J (2013) Four-year follow-up of combined surgical therapy of advanced peri-implantitis evaluating two methods of surface decontamination. J Clin Periodontol 40:962–967
Peters N, Tawse-Smith A, Leichter J, Tompkins G (2012) Laser therapy: the future of peri-implantitis management? Braz j periodontol 22(1):26–33
Karring ES, Stavropoulos A, Ellegaard B, Karring T (2005) Treatment of periimplantitis by the vectors system. A pilot study. Clin Oral Implants Res 16:288–293
Novaes AB Jr, Schwartz-Filho HO, de Oliveira RR, Feres M, Sato S, Figueiredo LC (2012) Antimicrobial photodynamic therapy in the non-surgical treatment of aggressive periodontitis: microbiological profile. Lasers Med Sci 27(2):389–395
Feres M, Bernal M, Matarazzo F, Faveri M, Duarte PM, Figueiredo LC (2015) Subgingival bacterial recolonization after scaling and root planing in smokers with chronic periodontitis. Aust Dent J 60(2):225–232
Zhu C, Yang J, Sun J, Shi J, Gou J, Li A (2013) Induction of immune response and prevention of alveolar bone loss with recombinant Porphyromonas gingivalis peptidylarginine deiminase. Arch Oral Biol 58(12):1777–1783
Feres M, Soares GMS, Mendes JAV, Silva MP, Faveri M, Teles R, Socransky SS, Figueiredo LC (2012) Metronidazole alone or with amoxicillin as adjuncts to non-surgical treatment of chronic periodontitis: a 1-year double- blinded, placebo-controlled, randomized clinical trial. J Clin Periodontol 39(12):1149–1158
Mombelli A, Lang NP (1998) The diagnosis and treatment of periimplantitis. Periodontology 17:63–76
John G, Becker J, Schmucker A, Schwarz F (2017) Non-surgical treatment of peri-implant mucositis and peri-implantitis at two-piece zirconium implants: a clinical follow-up observation after up to 3 years. J Clin Periodontol 44(7):756–761
Schwarz F, John G, Schmucker A, Sahm N, Becker J (2017) Combined surgical therapy of advanced peri-implantitis evaluating two methods of surface decontamination: a 7-year follow-up observation. J Clin Periodontol 44(3):337–342
Schwarz F, John G, Hegewald A, Becker J (2015) Non-surgical treatment of peri-implant mucositis and peri-implantitis at zirconia implants: a prospective case series. J Clin Periodontol 42(8):783–788
Schwarz F, Sahm N, Becker J (2014) Combined surgical therapy of advanced peri-implantitis lesions with concomitant soft tissue volume augmentation. A case series. Clin Oral Implants Res 25:132–136
Romanos G (2005) Point of care. J Can Dent Assoc 71(2):117–122
Schwarz F, John G, Mainusch S, Sahm N, Becker J (2012) Combined surgical therapy of peri-implantitis evaluating two methods of surface debridement and decontamination. A two-year clinical follow up report. J Clin Periodontol 39(8):789–797
Lerario F, Roncati M, Gariffo A, Attorresi E, Lucchese AAG, Palai G, Romeo U (2016) Non-surgical periodontal treatment of peri-implant diseases with the adjunctive use of diode laser. preliminary clin. study. Lasers Med Sci 31:1–6
Al Amri MD, Kellesarian SV, Ahmed A, Al-Kheraif AA, Romanos GE, Javed F (2016) Efficacy of periimplant mechanical debridement with and without adjunct antimicrobial photodynamic therapy in patients with type 2 diabetes mellitus. Photodiagn Photodyn Ther 14:166–169
Mettraux GR, Sculean A, Bürgin WB, Salvi GE (2016) Two-year clinical outcomes following non-surgical mechanical therapy of peri-implantitis with adjunctive diode laser application. Clin Oral Implants Res 27:845–849
Arısan V, Karabuda ZC, Arıcı SV, Topçuoğlu N, Külekçi G (2015) A randomized clinical trial of an adjunct diode laser application for the nonsurgical treatment of peri-implantitis. Photomed Laser Surg 33(11):547–554
Papadopoulos CA, Vouros I, Menexes G, Konstantinidis A (2015) The utilization of a diode laser in the surgical treatment of peri-implantitis. A randomized clinical trial. Clin Oral Investig 19(8):1851–1860
Bassetti M, Schär D, Wicki B, Eick S, Ramseier CA, Arweiler NB, Sculean A, Salvi GE (2014) Anti-infective therapy of peri-implantitis with adjunctive local drug delivery or photodynamic therapy: 12-month outcomes of a randomized controlled clinical trial. Clin Oral Implants Res 25:279–287
Deppe H, Mücke T, Wagenpfeil S, Kesting M, Sculean A (2013) Nonsurgical antimicrobial photodynamic therapy in moderate vs severe peri-implant defects: a clinical pilot study. Quintessence Int 44:609–618
Schär D, Ramseier CA, Eick S, Arweiler NB, Sculean A, Salvi GE (2013) Anti-infective therapy of peri-implantitis with adjunctive local drug delivery or photodynamic therapy: six-month outcomes of a prospective randomized clinical trial. Clin Oral Implants Res 24(1):104–110
Thierbach R, Eger T (2013) Clinical outcome of a nonsurgical and surgi- cal treatment protocol in different types of peri-implantitis: a case series. Quintessence Int 44:137–148
Bombeccari GP, Guzzi G, Gualini F, Gualini S, Santoro F, Spadari F (2013) Photodynamic therapy to treat periimplantitis. Implant Dent 22:631–638
Deppe H, Horch HH, Neff A (2007) Conventional versus CO2 laser-assisted treatment of peri-implant defects with the concomitant use of pure-phase beta-tricalcium phosphate: a 5-year clinical report. Int J Oral Maxillofac Implants 22(1):79–86
Romanos GE, Nentwig GH (2008) Regenerative therapy of deep peri-implant infrabony defects after CO2 laser implant surface decontamination. International Journal of Periodontics & Restorative Dentistry 28(3):245–255
Abduljabbar T, Javed F, Kellesarian SV, Vohra F, Romanos GF (2017) Effect of Nd:YAG laser-assisted non-surgical mechanical debridement on clinical and radiographic peri-implant inflammatory parameters in patients with peri-implant disease. J Photochem Photobiol B 168:16–19
Schwarz F, Sahm N, Iglhaut G, Becker J (2011) Impact of the method of surface debridement and decontamination on the clinical outcome following combined surgical therapy of peri-implantitis: a randomized controlled clinical study. J Clin Periodontol 38(3):276–284
Geisinger ML, Holmes CM, Vassilopoulos PJ, Geurs NC, Reddy MS (2014) Is laser disinfection an effective adjunctive treatment to bone augmentation for peri-implantitis? A review of current evidence. Clinical Advances in Periodontics 4(4):274–279
Roncati M, Lauritano D, Tagliabue A, Tettamanti L (2015) Nonsurgical periodontal management of iatrogenic peri-implantitis: a clinical report. J Biol Regul Homeost Agents 29(3):164–169
Van As GA (2015) Lasers in implant dentistry, Part 1. Dent Today 34(7):134
Charalampakis G, Belibasakis GN (2015) Microbiome of peri-implant infections: lessons from conventional, molecular and metagenomic analyses. Clin 6:183–187
Schwarz F, Sculean A, Romanos G, Herten M, Horn N, Scherbaum W, Becker J (2005) Influence of different treatment approaches on the removal of early plaque biofilms and the viability of SAOS2 osteoblasts grown on titanium implants. Clin Oral Investig 9(2):111–117
Matsuyama T, Aoki A, Oda S, Yoneyama T, Ishikawa I (2003) Effects of the Er:YAG laser irradiation on titanium implant materials and contaminated implant abutment surfaces. J Clin Laser Med Surg 21(1):7–17
Tosun E, Tasar F, Strauss R, Kıvanc DG, Ungor C (2012) Comparative evaluation of antimicrobial effects of Er:YAG, diode, and CO2 lasers on titanium discs: an experimental study. J Oral Maxillofac Surg 70(5):1064–1069
Romanos G, Crespi R, Barone A, Covani U (2006) Osteoblast attachment on titanium disks after laser irradiation. Int J Oral Maxillofac Implants 21:232–236
Romanos GE, Weitz D (2012) Therapy of peri-implant diseases. Where is the evidence? J Evid Based Dent Pract 12:204–208
Cobb CM, Low SB, Coluzzi DJ (2010) Lasers and the treatment of chronic periodontitis. Dent Clin N Am 54(1):35–53
Alshehri FA (2016) The role of lasers in the treatment of peri-implant diseases: a review. The 108–Saudi Dent J 28:103
Mostafa D, Tarakji B (2015) Photodynamic therapy in treatment of oral lichen planus. J Clin Med Res 7(6):393–399
Eick S, Markauskaite G, Nietzsche S, Laugisch O, Salvi GE, Sculean A (2013) Effect of photoactivated disinfection with a light-emitting diode on bacterial species and biofilms associated with periodontitis and peri-implantitis. Photodiagn Photodyn Ther 10:156–167
Marotti J, Tortamano P, Cai S, Ribeiro MS, Franco JE, de Campos TT (2013) Decontamination of dental implant surfaces by means of photodynamic therapy. Lasers Med Sci 28:303–309
Koldsland OC, Scheie AA, Aass AM (2011) The association between selected risk indicators and severity of peri-implantitis using mixed model analyses. J Clin Periodontol 38:285–292
Mombelli A, van Oosten MA, Schurch E Jr, Land NP (1987) The microbiota associated with successful or failing osseointegrated titanium implants. Oral Microbiol Immunol 2(4):145–151
Aoki A, Mizutani K, Schwarz F, Sculean A, Yukna RA, Takasaki AA, Romanos GE, Taniguchi Y, Sasaki KM, Zeredo JL, Koshy G, Coluzzi DJ, White JM, Abiko Y, Ishikawa I, Izumi Y (2015) Periodontal and peri-implant wound healing following laser therapy 2000. Periodontology 68:217–269
Nevins M, Nevins ML, Yamamoto A, Yoshino T, Ono Y, Wang CW, Kim DM (2014) Use of Er:YAG laser to decontaminate infected dental implant surface in preparation for reestablishment of bone-to-implant contact. Int J Periodontics Restorative Dent 34:461–466
Leonhard A, Dahlén G, Renvert S (2003) Five-year clinical, microbiological, and radiological outcome following treatment of peri-implantitis in man. J Periodontol 74(10):1415–1422
Badran Z, Bories C, Struillou X, Saffarzadeh A, Verner C, Soueidan A (2011) Er: YAG laser in the clinical management of severe peri-implantitis: a case report. Journal of Oral Implantology 37(sp1):212–217
Monzavi A, Shahabi S, Fekrazad R, Behruzi R, Chiniforush N (2014) Implant surface temperature changes during Er: YAG laser treatment irradiation with different cooling systems. J Dent (Tehran) 11:210–215
Kim JH, Herr Y, Chung JH, Shin SI, Kwon YH (2011) The effect of erbium-doped: yttrium, aluminium and garnet laser irradiation on the surface microstructure and roughness of double acid-etched implants. J Periodontal Implant Sci 41:234–241
Oyster DK, Parker WB, Gher ME (1995) CO2 lasers and temperature changes of titanium implants. J Periodontol 66:1017–1024
Author information
Authors and Affiliations
Contributions
DM analyzed and wrote all data related to subject. The author read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The author declares that there is no competing interests
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mostafa, D. Different laser approaches in treatment of peri-implantitis: a review. Laser Dent Sci 3, 71–82 (2019). https://doi.org/10.1007/s41547-019-00063-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41547-019-00063-w