Abstract
Despite their high success rates, peri-implantitis can affect the stability and function of dental implants. Various treatment modalities have been investigated for the treatment of peri-implantitis to achieve re-osseointegration. An electronic literature search was performed supplemented by a manual search to identify studies published until January 2022. Articles that evaluated re-osseointegration in peri-implantitis sites in animal models following laser therapy or antimicrobial photodynamic therapy (aPDT) were included. Case reports, case series, systematic reviews, and letters to the editor were excluded. Risk of bias and GRADE assessment were followed to evaluate the quality of the evidence. Six studies out of 26 articles identified on electronic search were included in this review. The studies included animal studies conducted on canine models. Four out of six studies reported a higher degree of re-osseointegration following treatment of implants with laser therapy. The findings suggest that laser decontamination shows potential in enhancing re-osseointegration, particularly with the Er: YAG laser, which effectively decontaminated implant surfaces. However, conflicting outcomes and limitations in the evidence quality warrant caution in drawing definitive conclusions. Based on the limited available evidence, laser therapy may show a higher degree of re-osseointegration of implants than mechanical debridement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dental care has significantly improved over the last decade with the technological advances in radiographic techniques, implant designs, guidance systems, etc. Implant-supported prosthesis represents a widely accepted predictable treatment of choice to replace missing teeth in partially and completely edentulous arches. The mechanical and prosthetic predictability of outcomes can be attributed to the enhancement in surgical and prosthetic implant protocols [1]. Prosthetic rehabilitation of patients with severe atrophy of the maxillary and mandibular arches poses a challenge to clinicians. Bone and soft tissue augmentation alone or in combination are often required to provide a fixed prosthesis that restores proper form, function, and esthetics [2].
More than 1 million dental implants are placed each year worldwide. However, more than 4.4% of patients and 1.4% of implants may experience early implant failure [3]. Complications in implant therapy may occur due to biological or prosthetic concerns. Peri-implant diseases can be classified as peri-implant mucositis, peri-implantitis, and peri-implant hard and soft tissue deficiencies. Peri-implantitis may be a result of microbial colonization of implant surfaces. Peri-implantitis is associated with progressive bone loss following inflammation of the peri-implant mucosa of an osseointegrated implant [4, 5]. The continued bone loss may jeopardize the stability and function of implants [6].
Albrektsson et al. defined osseointegration as the stable anchorage of an implant achieved by direct bone-to-implant contact. [7] Ihde et al. present a purely bone-based explanation for the beginning of the “bone loss” process around already “osseointegrated” implants. Fully healed bone indicates the development of the inner cortical (IC) layer around the implants, as well as mechanical coupling between the inner and the outer cortical layer. [8] Deeper understanding of the foreign body equilibrium suggests a role of macrophages and the importance of maintaining cellular balance for therapeutic reasons. [9] The foreign body reaction composed of macrophages and foreign body giant cells is the end-stage response of the inflammatory and wound healing responses following implantation of a medical device, prosthesis, or biomaterial. It takes more than 6 months (bone healing and remodeling) for the bone around the implant sites to fully heal [10].
Numerous studies have investigated and reported methods for the treatment of peri-implantitis. These can be classified broadly as mechanical debridement, surgical (open flap) debridement, chemical disinfection, laser therapy, and regenerative procedures [6, 11,12,13,14,15,16]. These therapies are primarily based on the principles and available evidence for the treatment of periodontitis. The ultimate goal is to achieve re-osseointegration of the implants [17].
Necessary modifications were made in the treatment approaches to overcome the disparities between implants and natural teeth, such as implant surface roughness. Laser decontamination of implants results from denaturation of proteins and cellular necrosis. Due to their excellent coagulation properties, diode lasers, CO2 lasers, Nd: YAG, and Nd: YAP lasers find tremendous applications in soft tissue surgeries. For hard tissue applications, Er: YAG and Er, Cr: YSGG are lasers of choice owing to their high absorption from hydroxyapatite [18]. Er: YAG laser is most commonly used to treat peri-implantitis due to its high bactericidal effect without substantial heat generation [19].
Antimicrobial photodynamic therapy (aPDT) is a contemporary intervention that comprises laser-induced inactivation of cells, microorganisms, or molecules. The process involves staining the bacteria with a photosensitizer dye followed by laser application [18]. It utilizes a laser beam of an appropriate wavelength to create an oxidative burst when interacting with the photosensitizer dye. The resultant cell wall lysis kills the pathogenic bacteria [20, 21]. aPDT as a supplementary treatment with mechanical debridement has substantially improved peri-implant pocket probing depth (PPD) and stabilized marginal bone levels [22]. Electron microscopic analysis of implant surfaces revealed osteoblast adherence and proliferation on the titanium surface of implants treated with CO2 and Er, Cr: YSGG lasers. Osteoblast adhesion and proliferation is a central feature in osseointegration. This offers a plausible mechanism for re-osseointegration of failing implants following treatment with aPDT [23]. The review aims to systematically analyze the efficacy of laser in treating dental implants with peri-implantitis and achieving re-osseointegration.
Materials and methods
Search criteria
The current systematic review was conducted with adherence to Preferred Reporting for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [24].
Inclusion criteria
-
Population (P): sites with peri-implantitis/experimentally induced peri-implantitis
-
Intervention (I): laser decontamination by direct application or aPDT
-
Control (C): mechanical debridement
-
Outcomes (O): re-osseointegration (measured by bone-implant contact/new bone formation/periotest values)
-
Study type (S): randomized control trials, comparative evaluations, clinical control trials, animal studies, and in vivo studies.
Exclusion criteria
-
Systematic reviews, case reports, letters to the editor, and case series were excluded.
-
Articles in languages other than English were excluded.
Search strategy
A comprehensive electronic literature search was performed in Scopus, PubMed, Embase, and Web of Science databases to identify studies published until January 2022. The keywords used to identify articles for the study are presented in Table 1.
Screening and selection of studies
The titles and abstracts of all the studies identified on electronic search were screened by two reviewers independently (S.G.P., L.T.). Duplicates were removed, following which the titles and abstracts were assessed for relevance. Full-text of the relevant articles were extracted for further review and evaluated for eligibility. The references from these articles were hand-searched. Any disagreements were rectified through discussion with a third reviewer (E.T.) until a consensus was reached. Studies that met the inclusion criteria were subjected to validity assessment and data extraction.
Extraction of data
Two reviewers independently carried out data extraction (S.B., M.M.A.). A third reviewer (K.J.A.) corroborated the data for accuracy. The year of publication, geographical details, author details, the participant demographics, type of interventions, outcome assessment method, time interval, and outcomes reported for each article included in the review were extracted onto a customized template (Microsoft Word, Microsoft Inc, Redwood, CA, USA).
Assessment of quality
The Cochrane Handbook for Systematic Reviews was used as a guideline to assess the quality of the selected studies [24]. Two reviewers (L.T., S.G.P.) independently assessed the studies included in this review using the Systematic Review Centre for Laboratory Animal Experimentation (SYRCLEs) risk of bias tool and Collaborative Approach to Meta-Analysis and Review of Animal Data from Experimental Studies (CAMARADES) checklist [24, 25]. The studies are assessed against ten specific domains to determine their validity. The domains included the randomization process, missing outcome data, measurement of outcomes, selective reporting, random housing, baseline characteristics, and compliance with regulatory requirements. Each response was evaluated as yes (Y) or no (N) [25].
Quality of evidence for outcomes in summary of findings table
We followed the GRADE recommendations mentioned in the Cochrane Handbook for Systematic Reviews of Interventions to assess each outcome in the summary of findings table [24, 26]. One review author (S.B.) applied the GRADE system, and the evidence ratings were applied after discussion with two other authors (E.T., L.T.). The final rating was decided after the three review team members reached a consensus. Evidence for each outcome was graded as “high quality” at the start in the case of randomized control trials (RCTs). The risk of bias, inconsistency of results, indirectness of evidence, imprecision of results, and publication bias were considered. Subsequently, the evidence rating was downgraded by one level for serious or two levels for very serious concerns regarding the study limitations, inconsistencies in the outcomes, indirectness of evidence, imprecision of effect estimates, or publication bias.
Results
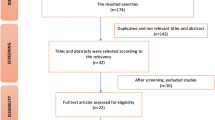
The electronic search identified a total of twenty-six studies from the four databases. Duplicates were removed. The remaining articles were screened for inclusion based on their titles and abstracts. Eleven articles that cleared the screening were subjected to full-text analysis to eliminate articles not relevant to the focus question. A total of six studies were included in this review that met the inclusion criteria. Figure 1 depicts the PRISMA flow diagram.
Risk of bias
All six studies showed a high risk of bias [27,28,29,30,31,32]. The “high” risk of bias assessment was mainly due to methodological insufficiencies in the studies. All the studies had two or more critical domains evaluated to be at a “high” risk of bias due to a lack of reporting or randomization process and blinding of outcome assessors [27,28,29,30,31,32]. A “high” attrition bias was also noted in two studies due to missing outcome data [27, 30]. Some studies lack vital information relevant to determining bias in specific domains, especially randomization, resulting in an “unclear” response. Figure 2 summarizes the risk of bias judgments for the included studies.
Characteristics of study settings
The present review comprised six animal studies, all of which focused on canine models. Among these, four studies utilized beagle dogs [29,30,31,32], one study employed mongrel dogs [27], and another study involved Jack Russel Terrier dogs [28]. The geographic distribution of the studies encompassed various regions, including Europe (Germany, Sweden, Switzerland), Asia (Japan, Iran), and South America (Brazil). A summary of the characteristics of the selected studies is shown in Table 2.
In five of the included studies, peri-implantitis was induced experimentally by placing ligatures around the implants [27, 29,30,31,32]. However, one study adopted a distinct approach by assessing peri-implantitis on previously failed implants that underwent decontamination and re-implantation in healthy Jack Russel Terrier dogs [28].
Characteristics of interventions
In all of the included studies, full-thickness mucoperiosteal flaps were raised at the implant sites [27,28,29,30,31,32]. Thorough debridement of granulation tissue was accomplished using plastic curettes. Subsequently, the sites underwent various treatments, including laser therapy (in the test group/s), mechanical debridement alone, or a combination of both [27,28,29,30,31,32]. Two studies included subgroups that received different implant surface treatments, including turned surface, sand-blasted large grit acid-etched (SLA) surface, titanium plasma-sprayed surface, commercially pure titanium surface, hybrid surface, and sand-blasted with titanium oxide surface [27, 32].
Among the interventions employed, three studies utilized CO2 lasers [28, 31, 32], two studies utilized Er:YAG lasers [29, 30], and one study utilized diode lasers [27]. In one study, photosensitization or aPDT was utilized as a delivery method for laser therapy [27].
Laser parameters
Various laser types and wavelengths were employed in the included studies. Direct laser application was employed in four studies [28, 29, 31, 32], while one study compared both direct laser application and aPDT [30]. Three studies employed CO2 lasers with a wavelength of 10.6 μm [28, 31, 32], while two studies utilized Er:YAG lasers with a wavelength of 2.940 nm [29, 30]. In two studies, aPDT was utilized, involving the use of a GaAlAs 830 nm diode laser and toluidine blue O (TBO) at a concentration of 100 μg/ml for photosensitization [27, 30].
Techniques for delivery
Various techniques were employed to enhance the effectiveness of CO2 laser therapy [32], including combining it with continuous irrigation using hydrogen peroxide solution and utilizing the Swiftlase scanner system to reduce tissue carbonization. In one study, CO2 irradiation was combined with continuous irrigation using a 10 mM water solution of hydrogen peroxide [32]. Another study utilized a continuous wave CO2 laser along with the Swiftlase scanner system to minimize tissue carbonization [31]. This system involved sweeping a focused beam over a 3.0-mm diameter area for 0.1 s, resulting in a dwell time of less than 1 ms per point [31]. Kasraei et al. employed a special jig where the implant was placed and irradiated using a CO2 laser with a wavelength of 10.6 μm. The laser was applied for 60 s at a rate of 2 mm/s from a distance of 20 mm perpendicular to the implant surface [28].
Er:YAG laser irradiation was utilized in two studies, both of which emphasized the importance of copious water irrigation during the procedure [29, 30]. Both studies employed an ERL device emitting pulsed infrared radiation, which was guided onto the implant surfaces using a cone-shaped glass fiber tip emitting a radial and axial laser beam [29, 30].
aPDT delivery
aPDT was employed as a delivery method for laser therapy in certain studies, involving the careful application of TBO and subsequent irradiation using a GaAIAs diode laser. The scanning method and specific surfaces targeted during the laser application were described in detail in the studies.
For studies employing aPDT as a delivery method for laser therapy, TBO at a concentration of 100 μg/ml was carefully applied to the implant surface and peri-implant defect for 5 min. Subsequently, the area was irradiated with a GaAIAs 830 nm diode laser at a wavelength of 2.940 nm. The laser was applied to four surfaces of the implant (mesial, buccal, distal, and lingual) for 20 s on each surface using a scanning method [30]. Shibli et al. injected TBO into the peri-implant defect for 1 min using a thin needle. The area was then irradiated with a GaAlAs diode laser using a scanning method on the mesial, distal, buccal, and lingual surfaces for 20 s on each surface [27].
Characteristics of outcome measures
All studies measured re-osseointegration as the primary outcome [27,28,29,30,31,32]. Five out of six studies assessed re-osseointegration based on the new bone to implant contact (BIC) 3–6 months post-operatively [27, 29,30,31,32]. Block biopsies were obtained for each implant site, and histological analysis was done by fluorescence microscopy. New bone to implant contact was measured as the linear distance from the bottom of the defect to the most coronal part of new bone formation in intimate contact with the implant on histologic examination [27, 29,30,31,32]. One study assessed re-osseointegration based on periotest values (PTV) [28]. Periotest is an electronic device initially developed to determine the mobility of teeth. Their use was extended to assess the stability of implants, but their reliability and reproducibility remain uncertain [33]. Periotest values were assessed on the day of the surgery and 1, 3, and 6 months postoperatively [28].
Characteristics of outcomes
Four out of six studies reported a higher degree of re-osseointegration following treatment with laser therapy [27,28,29, 31]. Of the two studies that compared mechanical debridement with aPDT, one study [32] stated that regardless of the implant surface, aPDT combined with guided bone regeneration allowed for better re-osseointegration around the peri-implant sites (p = 0.05, n =24). Another study [30] reported better re-osseointegration with mechanical debridement combined with chemical decontamination (p < 0.05, n =30). All three studies that employed CO2 laser showed significantly higher bone formation and greater implant stability (p < 0.05) [28, 31, 32]. Two studies used Er: YAG laser decontamination and reported conflicting results. [29, 30] Greater re-osseointegration was seen with Er: YAG lasers (p = 0.05, n = 30) [29], while the other study reported better re-osseointegration with combined chemical and mechanical debridement therapy (p < 0.05, n = 30) [30]. The implant surfaces were also believed to affect re-osseointegration with greater osseointegration associated with rough surface implants than turned surface implants [32]. One study did not find the implant re-osseointegration to be significant and reported similar results irrespective of the implant surface characteristics (p = 0.05, n = 40) [27].
Quality of the evidence
Our review included six studies involving 200 participants. Based on GRADE, the overall quality of evidence was low. The quality of evidence was downgraded by one level to reflect the high risk of bias due to methodological insufficiencies and attrition bias in the included studies. An inconsistency in results across the small number of studies included in the present review was noted. The potential impact of heterogeneity in size or direction of two studies is significant since only six studies were included in the review, leading us to downgrade the evidence by one level. Table 3 depicts the quality of evidence using the GRADE system.
Discussion
This review included six studies that examined 200 implants and explored the efficacy of laser decontamination in the re-osseointegration of failed implants compared to mechanical debridement in dogs.
The principal findings of this review was that surface decontamination with lasers appears to have some potential to promote re-osseointegration based on findings from four out of six studies, regardless of the implant surface characteristics. Er: YAG laser was effective in decontaminating implant surfaces, promoting bone regeneration, and rendering the surfaces biocompatible for implant success [34,35,36]. However, these findings were not unanimous. Persson et al. reported comparable re-osseointegration between implants treated with laser therapy and those subjected to mechanical debridement [32]. Conversely, another study indicated that a combination of mechanical and chemical treatment outperformed laser therapy in terms of implant re-osseointegration [31].
These findings broadly align findings from various studies investigating the treatment of peri-implantitis using different lasers [35, 36]. Romanos et al. [18] proposed that lasers might enhance the adhesion of blood cells and stabilize blood clots, potentially leading to accelerated wound healing. This mechanism could offer a plausible explanation for the observed improvements in wound healing and re-osseointegration following laser therapy [18]. Schou et al., Schwarz et al., and Romeo et al. conducted a series of studies on surface decontamination of dental implants using mechanical debridement [15, 25, 32, 33]. They observed that combined treatment of flap surgery with citric acid, air powder abrasive, and saline irrigation resulted in the highest re-osseointegration of implants [37,38,39,40]. Schwarz et al., in their experimental peri-implantitis model, performed implantoplasty on implant surfaces with Arkansas stones and diamond burs and suggested that implantoplasty is an adequate substitute for the treatment of peri-implantitis [41].
The type of laser and its application method varied among the studies included in this review. There is a difference in the mechanism of action of lasers when used directly or as aPDT. On direct application, there is a disparity in the properties of lasers depending upon their wavelength [42]. aPDT involves using a low-level laser application and a photosensitizer dye. The decontamination occurs due to irreversible damage to the cytoplasmic membrane of the bacteria by the free radicals generated as a result of energy transfer from a photon of light to the photosensitizer agent [43]. The effects of direct laser application depend on the laser-tissue interactions: photomechanical, photochemical, and photothermal. These differences could explain the disparity in the outcomes of the studies.
Four out of six studies reported a higher degree of re-osseointegration following laser decontamination. The results can be attributed to the difference in surfaces of natural teeth and implants, including the variations among the different implant surfaces. The rough surface implants tend to accumulate more plaque, and initial bacterial adhesion is more significant in areas of high wettability [44]. Mechanical debridement alone may not be sufficient to eliminate bacterial plaque from these niches, which dictates the need for adjunctive or alternate treatment modalities. Similar observations were reported by Renvert et al. and Valero et al [13, 43].
The review conducted by Renvert et al. on re-osseointegration of contaminated implants evaluated all modes of decontamination, including mechanical debridement, surgical (open flap) debridement, chemical disinfection, laser therapy, and regenerative procedures. In their systematic review, the authors stated that surface decontamination alone is not sufficient to promote re-osseointegration and that no method showed predictable results in the treatment of peri-implantitis [17]. Valero et al., in their review on different methods of implant surface decontamination, suggest that mechanical removal of biofilm in contaminated implants should be accompanied by chemical decontamination for long term success [44]. These studies are consistent with the findings of our systematic review proposing that mechanical debridement alone may not be adequate in decontamination of implant surfaces and subsequent re-osseointegration of the implants.
The present review included two studies that reported a higher degree of re-osseointegration associated with rough surface implants [27, 32]. Marwa et al. reported similar results with regenerative approaches such as guided bone regeneration (GBR) in treating peri-implantitis. The authors suggested that rough surface implants showed better re-osseointegration than smooth surface implants [6].
The utilization of aPDT as a localized treatment presents a potential alternative to antibiotics for addressing local infections. The interaction between laser light and microbial cells is multifaceted, involving various photophysical and photochemical processes. Er:YAG and CO2 lasers, operating in the infrared range, exhibit strong absorption by water, leading to rapid vaporization and mechanical disruption of microbial cells [45]. In contrast, GaAlAs lasers, typically in the visible and near-infrared spectrum, rely on photochemical reactions to promote bactericidal effects through the generation of reactive oxygen species (ROS) and nitric oxide (NO) [46, 47]. In the context of peri-implantitis therapy, CO2 laser demonstrated superior efficacy compared to Nd:YAG and HO:YAG laser systems [48]. However, it is generally considered as a secondary or tertiary option when compared to GaAlAs lasers due to the limited impact of diode lasers on implant surfaces. Additionally, the CO2 laser has certain drawbacks, such as its rigid optical delivery system for intra-oral applications, which can be challenging and expensive in comparison to Er:YAG and GaAlAs lasers. Despite these limitations, it is important to acknowledge that CO2 laser, as a powerful laser source, may still possess decontamination effects on dental implants based on the findings of this in vivo animal assessment [28].
One notable advantage of employing CO2 laser irradiation on implant surfaces is its ability to mitigate the risk of overheating, which distinguishes it from other laser wavelengths such as diode, Nd:YAG, and Er:YAG lasers [49,50,51]. In vitro studies have indicated a significant increase in implant surface temperature when subjected to diode laser irradiation for more than 10 s [50,51,52]. It is plausible that the unfavorable and unpredictable clinical outcomes reported by some authors in their studies could be attributed to overheating resulting from inconsistent power settings [53].
Re-osseointegration in the studies varied depending on the implant surface characteristics, with sand-blasted large-grit acid-etched implants showing a high degree of re-osseointegration, while turned surface implants exhibited minimal re-osseointegration [32]. Laser therapy’s “decontamination” effect appeared to have less impact on re-osseointegration compared to surface characteristics [32]. Implants with a commercially pure titanium surface demonstrated higher re-osseointegration percentages, while titanium plasma-sprayed surfaces and coated surfaces showed lower levels [27]. Additionally, anodized surface implants were associated with increased biofilm accumulation on the exposed implant surface [30].
There are challenges and variations in assessing and comparing osseointegration between animal models and humans. The literature acknowledges that early osseointegration in animal models has demonstrated twice the effectiveness compared to humans [54]. However, there is a lack of consensus regarding the standardized methodology for assessing osseointegration and facilitating comparison across studies. Consequently, establishing a direct parallel between the biological process of osseointegration becomes challenging [54]. Additionally, it is evident that the species model employed has a significant impact on osseointegration, with the dog model exhibiting a faster rate compared to the human model [55].
Comparison of laser types for decontaminating implant surfaces reveals varying suitability and potential risks associated with different lasers. Both the Nd:YAG and Ho:YAG lasers were unsuitable for decontaminating implant surfaces, regardless of their power output. The use of Er:YAG and CO2 lasers, on the other hand, requires careful regulation of the power output to prevent any potential surface damage. In contrast, the GaAIAs laser appears to be a safer option with minimal surface alterations observed [48].
Implant surface characteristics significantly impact treatment outcomes and success in peri-implantitis. Implants with pure titanium surface and titanium plasma-sprayed coating demonstrated the most favorable outcomes in terms of treatment for peri-implantitis [27, 56] suggesting that the surface characteristics of implants may play a crucial role in determining treatment success. Specifically, there was radiographic bone gain in implants with turned, TiOblast, and SLA surfaces, while additional bone loss was observed in TiUnite implants following surgical treatment. Furthermore, implant surface characteristics influenced the treatment outcome in an experimental model of peri-implantitis. While further bone loss was prevented in implant types A, B, and C, the resolution of peri-implantitis lesions was achieved only in sites associated with implant types A and B. In contrast, no signs of resolution were observed in sections representing TiUnite implants [57].
This systematic review provides evidence suggesting that successful re-osseointegration is possible through proper decontamination of implant surfaces. The review lists available treatment modalities with their merits and limitations to assist clinicians in making informed choices. However, due to limited evidence, a definitive conclusion on the efficacy of laser therapy for contaminated implant re-osseointegration could not be reached.
Applicability of evidence
All the studies included in this review examined the effectiveness of lasers in implant re-osseointegration following peri-implantitis. The evidence primarily consisted of animal models with experimental peri-implantitis. It is important to note that experimental peri-implantitis differs from its clinical counterpart in several aspects. Experimental peri-implantitis introduces an additional foreign body (ligatures) onto an existing foreign body (implants), potentially resulting in a tissue response that encompasses both bacterial biofilm-induced inflammation and a foreign body component. Consequently, the extent to which experimental peri-implantitis faithfully reproduces clinical peri-implantitis remains uncertain and reducing generalizability [58]. Heterogeneity in the mode and type of laser application precluded performance of a meta-analysis.
Quality of the evidence
Definitive conclusions regarding the efficacy of laser in enhancing successful osseointegration cannot be drawn due to the limitations in the quality of the available evidence. The primary limitations observed in the included studies were inadequate reporting of study methods, the presence of attrition bias, and the potential for performance bias. Our assessment of the evidence quality for the reported outcomes indicates that it is generally low or very low.
The strengths of our review include a comprehensive search of four distinct databases supplemented with a manual search of the references to identify all relevant articles with multiple reviewers independently participating at every stage of the review process to minimize bias. However, this review is not without limitations as we only considered studies published in the English language, as translated articles may lack veracity. The articles included are animal studies conducted on canine models. Extrapolating these results into humans should be done with caution. Further research focusing on human clinical trials with well-matched subjects with homogeneity in the type and method of laser applications will derive conclusive results on the efficacy of lasers in the re-osseointegration of implants.
Conclusion
The present systematic review assessed the efficacy of laser in the treatment of peri-implantitis and their role in achieving re-osseointegration in dental implants. Based on limited evidence, there appears to be low certainty evidence indicating that laser surface treatment may enhance the re-osseointegration of implants. However, it is important to note the disparities observed in the study settings, treatment methods, laser application, and outcome measurement parameters, which contribute to the overall uncertainty of the findings. Additional clinical and histological investigations are warranted to deepen our understanding of the effects of laser on re-osseointegration. Furthermore, well-designed randomized controlled trials should focus on exploring the influence of implant surface characteristics and the potential benefits of adjuvant therapies, such as bone grafts combined with laser decontamination, in the treatment of peri-implantitis.
Data availability
Not applicable.
References
Siu G (2015) Full mouth rehabilitation with dental implants and fixed dental prostheses. Oral Health, pp 26–32. https://issuu.com > p_26-32_siu.r1
Bencharit S, Schardt-Sacco D, Border MB, Barbaro CP (2010) Full mouth rehabilitation with implant-supported prostheses for severe periodontitis: a case report. Open Dent J 4:165–171. https://doi.org/10.2174/1874210601004010165
Takamoli J, Pascual A, Martinez-Amargant J et al (2021) Implant failure and associated risk indicators: a retrospective study. Clin Oral Implants Res 32:619–628. https://doi.org/10.1111/clr.13732
Berglundh T, Armitage G, Araujo MG et al (2018) Peri-implant diseases and conditions: consensus report of workgroup 4 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J Periodontol 89:S313–S318
Caton JG, Armitage G, Berglundh T et al (2018) A new classification scheme for periodontal and peri-implant diseases and conditions – introduction and key changes from the 1999 classification. J Clin Periodontol 45:S1–S8. https://doi.org/10.1111/jcpe.12935
Madi M, Htet M, Zakaria O et al (2018) Re-osseointegration of dental implants after periimplantitis treatments: a systematic review. Implant Dent 27:101–110
Albrektsson T, Johansson C (2001) Osteoinduction, osteoconduction and osseointegration. Eur Spine J 10:S96–S101
Ihde S, Ihde A, Sipic O, Pałka Ł (2022) Peri-implantitis: a new definition proposal based on unnatural spatial arrangement and late mechanical coupling between two cortical bone layers during osseointegration phase part II. Appl Sci 12:5589
Amengual-Peñafiel L, Brañes-Aroca M, Marchesani-Carrasco F et al (2019) Coupling between osseointegration and mechanotransduction to maintain foreign body equilibrium in the long-term: a comprehensive overview. J Clin Med 8:139
Anderson JM, Rodriguez A, Chang DT (2008) Foreign body reaction to biomaterials. In: Seminars in immunology. Elsevier, pp 86–100
Parham PL Jr, Cobb CM, French AA et al (1989) Effects of an air-powder abrasive system on plasma-sprayed titanium implant surfaces: an in vitro evaluation. J Oral Implantol 15:78–86
Rühling A, Kocher T, Kreusch J, Plagmann H (1994) Treatment of subgingival implant surfaces with Teflon®-coated sonic and ultrasonic scaler tips and various implant curettes. An in vitro study. Clin Oral Implants Res 5:19–29
Heitz-Mayfield LJA, Salvi GE, Mombelli A et al (2012) Anti-infective surgical therapy of peri-implantitis. A 12-month prospective clinical study. Clin Oral Implants Res 23:205–210
Dörtbudak O, Haas R, Bernhart T, Mailath-Pokorny G (2001) Lethal photosensitization for decontamination of implant surfaces in the treatment of peri-implantitis. Clin Oral Implants Res 12:104–108
Hayek RRA, Araújo NS, Gioso MA et al (2005) Comparative study between the effects of photodynamic therapy and conventional therapy on microbial reduction in ligature-induced peri-implantitis in dogs. J Periodontol 76:1275–1281
Aoki A, Mizutani K, Schwarz F et al (2015) Periodontal and peri-implant wound healing following laser therapy. Periodontology 2000 68:217–269
Renvert S, Polyzois I, Maguire R (2009) Re-osseointegration on previously contaminated surfaces: a systematic review. Clin Oral Implants Res 20:216–227
Romanos GE, Gupta B, Yunker M et al (2013) Lasers use in dental implantology. Implant Dent 22:282–288
Schwarz F, Aoki A, Sculean A, Becker J (2009) The impact of laser application on periodontal and peri-implant wound healing. Periodontology 2000 51:79–108
Ding H, Yu H, Dong Y et al (2011) Photoactivation switch from type II to type I reactions by electron-rich micelles for improved photodynamic therapy of cancer cells under hypoxia. J Control Release 156:276–280
Huang L, Xuan Y, Koide Y et al (2012) Type I and type II mechanisms of antimicrobial photodynamic therapy: an in vitro study on gram-negative and gram-positive bacteria. Lasers Surg Med 44:490–499
Vohra F, Al-Rifaiy MQ, Lillywhite G et al (2014) Efficacy of mechanical debridement with adjunct antimicrobial photodynamic therapy for the management of peri-implant diseases: a systematic review. Photochem Photobiol Sci 13:1160–1168
Romanos G, Crespi R, Barone A, Covani U (2006) Osteoblast attachment on titanium disks after laser irradiation. Int J Oral Maxillofac Implants 21:232–236
Higgins JPT, Thomas J, Chandler J et al (2019) Cochrane handbook for systematic reviews of interventions. Cochrane Handb Syst Rev Interv:1–694. https://doi.org/10.1002/9781119536604
Hooijmans CR, Rovers MM, De Vries RBM et al (2014) SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol 14:1–9. https://doi.org/10.1186/1471-2288-14-43
Schünemann, Brożek J, Guyatt G, Oxman A (2013) Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. https://gradepro.orghandbook
Shibli JA, Martins MC, Ribeiro FS et al (2006) Lethal photosensitization and guided bone regeneration in treatment of peri-implantitis: an experimental study in dogs. Clin Oral Implants Res 17:273–281
Kasraei S, Torkzaban P, Shams B et al (2016) CO2 laser surface treatment of failed dental implants for re-implantation: an animal study. Lasers Med Sci 31:985–989
Schwarz F, Jepsen S, Herten M et al (2006) Influence of different treatment approaches on non-submerged and submerged healing of ligature induced peri-implantitis lesions: an experimental study in dogs. J Clin Periodontol 33:584–595
Htet M, Madi M, Zakaria O et al (2016) Decontamination of anodized implant surface with different modalities for peri-implantitis treatment: lasers and mechanical debridement with citric acid. J Periodontol 87:953–961
Stübinger S, Henke J, Donath K, Deppe H (2005) Bone regeneration after pen-implant care with the CO2 laser: a fluorescence microscopy study. Int J Oral Maxillofac Implants 20:203–210
Persson LG, Mouhyi J, Berglundh T et al (2004) Carbon dioxide laser and hydrogen peroxide conditioning in the treatment of periimplantitis: an experimental study in the dog. Clin Implant Dent Relat Res 6:230–238
Bilhan H, Cilingir A, Bural C et al (2015) The evaluation of the reliability of periotest for implant stability measurements: an in vitro study. J Oral Implantol 41:e90–e95. https://doi.org/10.1563/AAID-JOI-D-13-00303
Schwarz F, Rothamel D, Becker J (2003) Influence of an Er: YAG laser on the surface structure of titanium implants. Schweizer Monatsschrift fur Zahnmedizin= Rev Mens suisse d’odonto-stomatologie= Riv Mens Svizz di Odontol e Stomatol 113:660–671
Schwarz F, Sculean A, Romanos G et al (2005) Influence of different treatment approaches on the removal of early plaque biofilms and the viability of SAOS2 osteoblasts grown on titanium implants. Clin Oral Investig 9:111–117
Matsuyama T, Aoki A, Oda S et al (2003) Effects of the Er: YAG laser irradiation on titanium implant materials and contaminated implant abutment surfaces. J Clin Laser Med Surg 21:7–17
Schou S, Holmstrup P, Jørgensen T et al (2003) Autogenous bone graft and ePTFE membrane in the treatment of peri-implantitis. I. Clinical and radiographic observations in cynomolgus monkeys. Clin Oral Implants Res 14:391–403
Schou S, Holmstrup P, Jørgensen T et al (2003) Implant surface preparation in the surgical treatment of experimental peri-implantitis with autogenous bone graft and ePTFE membrane in cynomolgus monkeys. Clin Oral Implants Res 14:412–422
Schou S, Holmstrup P, Jørgensen T et al (2003) Anorganic porous bovine-derived bone mineral (Bio-Oss®) and ePTFE membrane in the treatment of peri-implantitis in cynomolgus monkeys. Clin Oral Implants Res 14:535–547
Schou S, Holmstrup P, Skovgaard LT et al (2003) Autogenous bone graft and ePTFE membrane in the treatment of peri-implantitis. II. Stereologic and histologic observations in cynomolgus monkeys. Clin Oral Implants Res 14:404–411
Schwarz F, Sahm N, Mihatovic I et al (2011) Surgical therapy of advanced ligature-induced peri-implantitis defects: cone-beam computed tomographic and histological analysis. J Clin Periodontol 38:939–949
Elavarasu S, Naveen D, Thangavelu A (2012) Lasers in periodontics. J Pharm Bioallied Sci 4:S260–S263. https://doi.org/10.4103/0975-7406.100245
AlAhmari F, Shaikh L, AlDhubaiban D (2020) Photodynamic therapy in the treatment of periodontal diseases: a review. J Int Oral Heal 12:102
Mellado-Valero A, Buitrago-Vera P, Solá-Ruiz M-F, Ferrer-García J-C (2013) Decontamination of dental implant surface in peri-implantitis treatment: a literature review. Med Oral Patol Oral Cir Bucal 18:e869–e876. https://doi.org/10.4317/medoral.19420
Parker S, Anagnostaki E, Mylona V et al (2020) Systematic review of post-surgical laser-assisted oral soft tissue outcomes using surgical wavelengths outside the 650-1350 nm optical window. Photobiomodul Photomed Laser Surg 38:591–606. https://doi.org/10.1089/photob.2020.4847
Dougherty TJ, Gomer CJ, Henderson BW et al (1998) Photodynamic therapy. JNCI J Natl cancer Inst 90:889–905
Kömerik N, Wilson M (2002) Factors influencing the susceptibility of Gram-negative bacteria to toluidine blue O-mediated lethal photosensitization. J Appl Microbiol 92:618–623
Kreisler M, Götz H, Duschner H, d’Hoedt B (2002) Effect of Nd: YAG, Ho: YAG, Er: YAG, CO2, and GaAlAs laser irradiation on surface properties of endosseous dental implants. Int J Oral Maxillofac Implants 17:202–211
Oyster DK, Parker WB, Gher ME (1995) CO2 lasers and temperature changes of titanium implants. J Periodontol 66:1017–1024
Geminiani A, Caton JG, Romanos GE (2011) Temperature increase during CO2 and Er: YAG irradiation on implant surfaces. Implant Dent 20:379–382
Geminiani A, Caton JG, Romanos GE (2012) Temperature change during non-contact diode laser irradiation of implant surfaces. Lasers Med Sci 27:339–342
Leja C, Geminiani A, Caton J, Romanos GE (2013) Thermodynamic effects of laser irradiation of implants placed in bone: an in vitro study. Lasers Med Sci 28:1435–1440
Schwarz F, Sahm N, Iglhaut G, Becker J (2011) Impact of the method of surface debridement and decontamination on the clinical outcome following combined surgical therapy of peri-implantitis: a randomized controlled clinical study. J Clin Periodontol 38:276–284
Lang NP, Salvi GE, Huynh-Ba G et al (2011) Early osseointegration to hydrophilic and hydrophobic implant surfaces in humans. Clin Oral Implants Res 22:349–356
Botticelli D, Lang NP (2017) Dynamics of osseointegration in various human and animal models-a comparative analysis. Clin Oral Implants Res 28:742–748
Shibli JA, Martins MC, Nociti FH Jr et al (2003) Treatment of ligature-induced peri-implantitis by lethal photosensitization and guided bone regeneration: a preliminary histologic study in dogs. J Periodontol 74:338–345
Albouy J, Abrahamsson I, Persson LG, Berglundh T (2011) Implant surface characteristics influence the outcome of treatment of peri-implantitis: an experimental study in dogs. J Clin Periodontol 38:58–64
Reinedahl D, Chrcanovic B, Albrektsson T et al (2018) Ligature-induced experimental peri-implantitis-a systematic review. J Clin Med 7:492. https://doi.org/10.3390/jcm7120492
Author information
Authors and Affiliations
Contributions
Conceptualization: S.G.P., F.L., and E.T.; methodology: M.M.A.; software: L.T.; validation: S.B., B.S., and K.J.A; formal analysis: S.G.P.; investigation: S.B.; resources: K.J.A.; data curation: E.T.; writing—original draft preparation: S.G.P., K.A., E.T., and S.B.; writing—review and editing: K.J.A., B.S., M.M.A., F.L., and L.T.; visualization: L.T. and F.L.; supervision: M.M.A. and E.T.; project administration: S.B. and B.S.; and funding acquisition: K.J.A. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Patil, S., Bhandi, S., Alzahrani, K.J. et al. Efficacy of laser in re-osseointegration of dental implants—a systematic review. Lasers Med Sci 38, 199 (2023). https://doi.org/10.1007/s10103-023-03860-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10103-023-03860-9