Abstract
Peri-implant diseases present in two forms: peri-implant mucositis and peri-implantitis. The prevalence of peri-implant complications is significantly rising. The aim of this study was to compare conventional treatment of inflamed peri-implant tissues with conventional treatment together with diode laser application. Twenty-seven patients (age 36 to 67, 15 women and 12 men, 12 smokers and 15 non-smokers) requiring treatment for mucositis or peri-implantitis were taken into account for this preliminary study. Plaque index (PI), pocket depth (PD), and bleeding on probing (BoP) were recorded at baseline evaluation. Patients in control group (CG) received conventional non-surgical periodontal treatment. Patients in test group received conventional non-surgical periodontal treatment together with diode laser application (810 nm, 30 s, 1 W, 50 Hz, t on = 100 ms, t off = 100 ms, energy density = 24.87 J/cm2). Paired t test was used to evaluate the difference in repeated measurements of considered indexes at T 0 and T 1 (1 year) in both groups. A total of 606 sites were taken into account in the test group (TG) and 144 in the CG. PD mean variation in the TG was 2.66 mm ± 1.07, while mean PD variation in the CG was 0.94 ± 1.13 mm. Paired t testing of the variation in PD in CG and TG revealed a statistically significant difference between the two groups (p < 0.0001). A reduction of pathological sites from 89 % (T 0) to 14.35 % (T 1) was achieved in the TG, while reduction obtained in the CG was from 75.69 % (T 0) to 50 % (T 1); BoP scores at time T 1 had fallen below 5 % in the TG and decreased to 59.7 %, in the CG. Within the limitations of this study, diode laser seems to be an additional valuable tool for peri-implant disease treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Peri-implant diseases present themselves in two forms: peri-implant mucositis and peri-implantitis. Peri-implant mucositis occurs in 80 % of the subjects that have an implant in the oral cavity and in 50 % of the implant sites. Peri-implantitis was identified, respectively, in 28–56 % of subjects and in 12–43 % of implant sites after 5 years from placement, depending on the different studies [1]. In the First European Workshop in Periodontology, the term peri-implantitis was defined as “an inflammatory process that affects the tissues around osseointegrated implants, subjected to normal functional load, and that causes a loss of alveolar bone support” [2, 3].
Recently, in the Seventh European Workshop on Periodontology [4], the definitions of peri-implant diseases have been revised: While peri-implant mucositis represents the host response of the peri-implant tissues to the bacterial challenge and does not fundamentally differ from gingivitis (considered as the host response to the bacterial challenge), peri-implantitis may differ from periodontitis, both in the extent and in the composition of cells in the lesion as well as in the progression rate [5].
Peri-implantitis is manifested by an inflammation of the mucosa and is associated with a pocket depth (PD) ≥4 mm, bleeding on probing (BoP), and presence/absence of suppuration; it is always associated with a radiographically visible bone loss; and it is clinically irreversible [4, 6]. In the past, the incidence of peri-implantitis ranged between 2 and 10 % in articles published before the year 2000 [7, 8]. In the most recent reviews of the literature, a prevalence rate inferior to 10 % is still retained only in patients who reported no history of periodontal disease, the so-called “healthy patient” [9]; on the contrary, the prevalence of peri-implantitis, in fact, has grown dramatically; currently, it oscillates between 30 and 70 % [8, 10]. Patients susceptible to periodontal disease result to be also vulnerable to peri-implant tissue inflammation. A previous history of periodontal disease, as evidence indicates [11], turns out to be one of the most significant risk factors for the onset of peri-implant diseases. There were, in 2008, an estimated 300,000 to 428,000 endosseous dental implants placed in the USA, and 1 million in Italy, and this statistic is projected to grow at ∼12 % annually [10]. As dental-implant-retained prostheses grow to be more widespread, the prevalence of peri-implant complications will as well enlarge, at least on “Perio” patient [9]. Although surviving, implants placed in patients with a history of chronic periodontitis may demonstrate a higher incidence of peri-implantitis, than implants placed in patients without a history of periodontitis [11].
These data should guide the clinician in the correct approach to the patient that will undergo implant treatment. The importance of treating periodontitis before implant placement in partially edentulous patients has been emphasized throughout the dental literature [12, 13].
Among the strategies adopted to treat peri-implant diseases, lasers have a noticeable role. Past studies have shown that peri-implant decontamination can decrease the relapse rate of the pathology, thus contributing to the success of the therapy [14].
The purpose of this retrospective controlled clinical trial was to investigate the effects of diode lasers as an additional tool to conventional treatment of inflamed peri-implant tissue in a follow-up period of 1 year. Seven hundred fifty sites with peri-implant diseases were evaluated before and after non-surgical periodontal therapy, with the adjunctive use of diode laser in the experimental group.
Materials and methods
Study design and participants
This study reports on the short-term follow-up of patients treated in a preliminary retrospective controlled clinical study, which investigated the effect of two different non-surgical treatment of peri-implant diseases. All the patients were properly informed on the treatment they would undergo and had to sign an informed consent before participation and before their clinical data could be used in the present study. Patients were recruited, treated, and subject to follow-up in the private office of the authors between March 2010 and July 2012. The study was carried out in accordance with the Declaration of Helsinki and its later amendments.
The inclusion criteria were as follows: Patients should be older than 18, they had to be in good health, with no systemic diseases, they should not have been on medication in the 6 previous months, they should have ≥1 site affected by mucositis or peri-implantitis, bleeding on probing, and pocket depth ≥4 mm. The exclusion criteria were as follows: age under 18, presence of systemic diseases, or being on daily medication. Inclusion and exclusion criteria are summarized in Table 1.
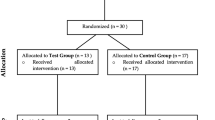
Twenty-one patients were taken into account in the test group (TG) and six in the control group (CG) The 27 patients selected, aged between 36 and 67, were affected by mucositis or peri-implantitis (15 women and 12 men, 12 smokers and 15 non-smokers). A total of 606 sites were taken into account in the TG and 144 in the CG.
Clinical assessment
At time T 0, before starting the treatment protocols, the first periodontal assessment was recorded, using a periodontal probe (Colorvue UNC12, HuFriedy Mfg, Rotterdam, Netherlands) and a pressure of ≤0.15 N. All the clinical measurements were performed by the same operator, at T 0 and at T 1. Clinical data scores, including plaque index (PI), bleeding on probing (BoP), and pocket depth (PD), were collected:
- PI :
-
Presence or absence of plaque in four points around the implant—mesial, vestibular, distal, and lingual
- BoP :
-
Presence or absence of bleeding on probing in six points around the implant—distal-vestibular, vestibular, mesial-vestibular, mesial-lingual, lingual, and distal-lingual
- PD :
-
The distance in millimeters from the mucosal margin to the bottom of the pocket was taken at six points around each implant
Oral hygiene protocol
The patients received individualized home oral hygiene instructions. All patients were taught the rolling brushing technique with manual or electric toothbrush, including tongue brushing. It was also recommended the use of gauze as follows: The gauze is wrapped around the finger of the dominant hand and used to cleanse gingival mucosa, teeth, and implants with rolling motion in an apical-coronal direction. Oral hygiene was completed by an appropriate device that could either be the interdental brush or floss, depending on proximal space viability.
Treatment protocol
Professional prophylaxis was performed for patients of both TG and CG using manual and power-driven instrumentation: ultrasonic piezoelectric unit (Piezon® Master 700, EMS, Nyon, Switzerland) with an implant dedicated tip (PI insert, EMS, Nyon, Switzerland), ultrasonic magnetostrictive unit (Cavitron® Plus, Dentsply Italia, Rome, Italy) with dedicated insert capped with a plastic disposable tip (Cavitron® SofTip, Dentsply Italia, Rome, Italy), and titanium curettes (Roncati Implant Care, KLS Martin, Tuttlingen, Germany).
The sites in TG were treated with a laser application as follows. Patients were asked to rinse with 0.2 % chlorhexidine mouthwash (Curasept ADS 0.20, Curaden Healthcare, Saronno, Italy), for 1 min. Laser optical fiber (diameter 320 μm) was inserted parallel to the longitudinal axis of the implant, up to 1 mm from the most apical portion of the pocket, and moved in an apical-coronal and mesial-distal direction for 30 s, two times for each site requiring treatment; the 810-nm diode laser (Stomatology 7, A2G, Rome, Italy) was used at 1 W in pulsating mode, 50 Hz, t on = 100 ms, t off = 100 ms, energy density = 24.87 J/cm2. Laser fiber movement can be estimated in 5 mm per second. Subsequently, each site was subjected to non-surgical periodontal instrumentation, using both manual and ultrasonic instruments, as needed, to remove all soft and calcified deposits. Laser application was repeated after mechanical instrumentation to complete the decontamination of the affected sites.
Finally, a 0.5 % chlorhexidine gel (Curasept ADS 0.5 %, Curaden Healthcare, Saronno, Italy) was applied into the site, using a disposable syringe with blunt needle.
Patients in the CG received mechanical instrumentation, after rinsing with 0.2 % chlorhexidine mouthwash (Curasept ADS 0.20, Curaden Healthcare, Saronno, Italy), for 1 min. They also received the final application of 0.5 % chlorhexidine gel (Curasept ADS 0.5 %, Curaden Healthcare, Saronno, Italy). Both non-surgical periodontal instrumentation and laser treatment were performed by the same operator.
Routine supportive periodontal therapy (SPT) was performed every 4 months, for both TG and CG; home care reinforcement was regularly provided. Adjunctive use of diode laser was associated to conventional SPT, after 4 months, during the follow-up period, only in the TG.
Periodontal probing was recorded 1 year after the treatment (T 1).
Statistical analysis
Statistical analysis was performed using SAS 9.2 (SAS, Cary, NC, USA) software.
Paired t test was used to evaluate the difference in repeated measurements of PD at T 0 and T 1 in both groups, and for matching the variations in PD in both groups. For BoP, only descriptive statistical analysis was applied.
Results
The study took into account 125 implants, for a total of 750 sites, monitored for a follow-up period of 1 year. A total of 606 sites were taken into account in the TG and 144 in the CG. The clinical variables such as PD and BoP were reduced more in the TG than in the CG.
Mean PD in CG was 4.465 ± 1.176 mm at baseline (T 0) (95 % confidence interval (CI) range 4.272–4.659), and 3.611 ± 1.123 mm was the mean ± the standard deviation (SD) at T 1 (95 % CI range 3.426–3.796), while mean PD and SD in TG were 5.218 ± 1.342 mm at T 0 (95 % CI range 5.111–5.325) and 2.543 ± 0.9811 mm at T 1 (95 % CI range 2.465–2.261). PD values are represented in Fig. 1. Paired t test of PD in CG at T 0 and T 1 and t test of PD in TG at T 0 and T 1 revealed statistically significant difference (p < 0.0001).
PD mean variation in the TG was 2.66 mm with a standard deviation (SD) of 1.07, while mean PD variation in the CG was 0.94 with a SD of 1.13 mm. Moreover, t testing of the variation in PD in CG and TG revealed a statistically significant difference between the two groups (p < 0.0001).
A reduction of pathological sites (PD >4 mm) from 89 % (T 0) to a 14.35 % (T 1) was achieved in TG and from 75.69 % (T 0) to a 50 % (T 1) in the CG.
The mean PD variation ranges from a maximum of 4.20 mm to a minimum of 1.80 mm.
For what concerns BoP, in TG at T 0, 546 sites (90.09 %) had a positive score (presence of bleeding), while at T 1, only 30 sites (4.95 %) had positive BoP; on the other hand, in CG, at T 0, sites with positive BoP were 126 (87.5 %) while at T 1 were 86 (59.72 %). The charts illustrate, respectively, the PD of 606 sites in TG at time T 0 (T 0-PD) and at time T 1 (T 1-PD), prior and after laser-assisted non-surgical periodontal treatment (Fig. 2), and the probing depth (PD) of 144 CG sites, prior and after non-surgical periodontal instrumentation (Fig. 3). In Fig. 4, BoP-positive sites are reported, both in TG and in CG. In Fig. 5, the sites are divided in groups depending on the PD <3 mm, 3 < PD < 5 mm, and PD >5 mm, at T 0 and at T 1.
TG (n = 606): at T 0, 65 sites with PD ≤3 mm, 341 sites with 3 < PD ≤ 5 mm, and 200 sites with PD value >5 mm. At T 1, 518 sites with PD ≤3 mm, 88 sites with 3 < PD ≤ 5 mm, and 0 sites with PPD >5 mm. CG (n = 144): at T 0, 32 sites with PD ≤3 mm, 82 sites with 3 < PD ≤ 5 mm, and 30 sites with PD >5 mm. At T 1, 72 sites with PD ≤3 mm, 60 sites with 3 < PD ≤ 5 mm, and 12 sites with PD value >5 mm
Discussion
Reasoning on the data collected, few issues deserve a debate.
Probing depths of 5 and 6 mm were the most prevalent pathological values recorded at T 0 (421 out of a total of 606 sites in the test group). Such pocket depth, 5–6 mm around single-rooted teeth, is generally treated by non-surgical periodontal treatment, at least as initial therapeutic approach. At T 1, residual scores of 5–6 mm (PD) were still present only on 20 sites (4.75 %), while 399 sites (94.77 %) of 5–6 mm (PD) at T 0 presented ≤3 mm (PD) at T 1, and negative BoP (Fig. 4), suggesting that the majority such pathological sites have been successfully treated. It is worth to comment that four of six patients in the control group were smokers, the most important risk factor, second only to plaque. The clinical results obtained from patients treated in this study, following the application of non-surgical periodontal therapy, associated with the use of lasers at high and low energy level, are considered satisfactory, compared to the control group.
Patients treated with the laser achieved a significant reduction in PD and BoP, which was reduced significantly to values ≤5 %.
This improvement is probably due to the laser associated with conventional therapy. The reduction of peri-implant PD is probably due to a healing process with the formation of an epithelium seal, similar to the long junctional epithelium.
BoP, an inflammatory marker with high prognostic value, was significantly reduced (≤5 %) in the TG from time T 0 to T 1, after laser-assisted periodontal therapy, while in the control group, bleeding score was still present in 59.7 % of sites at T 1.
Little data is available for what concerns clinical studies using diode laser in the treatment of peri-implantitis. Results from this work are comparable with those obtained with lasers and mechanical therapy in the treatment of chronic periodontitis. Lasers have demonstrated to be effective in the disinfection of root surfaces. Both hard tissue lasers (Er:YAG) and soft tissue lasers (potassium titanyl phosphate (KTP), diode lasers, Nd:YAG) have shown positive results for what concerns adjunctive periodontal treatment [15–17]. Schwarz et al. suggested that from a clinical point of view, the Er:YAG may be as an alternative treatment option to conventional periodontal therapy [18]. Kreisler et al. obtained similar results using an 809-nm diode laser in a split mouth design study. Teeth treated with the laser revealed a significantly higher reduction in tooth mobility, pocket depth, and clinical attachment loss. The laser was used as an adjunctive treatment after conventional scaling and root planning, with power of 1 W in continuous mode, for 10 s per each site, with 30-s cooldown period [19].
Thermal increase
One of the major concerns about the use of laser in the treatment of peri-implant pathologies is the thermal increase. Kreisler et al. studied thermal changes induced during simulated surface decontamination of the implant bone interface. Different power settings have been tested, showing that the threshold of 47 °C was exceeded only after 30 s, when using the laser at 1 W in continuous mode; this happened both on sand-blasted/acid-etched surface and on hydroxyapatite-coated surface [20]. Therefore, power settings employed in this study have to be considered as safe for the peri-implant tissues.
Bacterial elimination and surface modification
For what concerns efficacy of the treatment, several studies and different wavelengths have been reviewed recently in a work by Romanos et al. [21]. From this review of the literature, diode laser seems to have the possibility to effectively eliminate the bacteria from the implant surface, without changing the morphology of the implant surface [21]. Long-term evaluation of laser-assisted therapy of peri-implantitis revealed a decrease of relapses after 5 years, when compared with the values of literature where decontamination was not included in the therapy [14].
In this article, implant sites, and not the individual subjects, were evaluated: This allows to draw conclusions not influenced by the clinical status of the residual teeth, in the case of mixed dentition. The laser therapy has attracted considerable attention in periodontology. In its larger context, it includes the use of laser light at low or high level of energy. The use of diode laser with both high or low power mode may represent a useful protocol for the maintenance of serious complications post-implant rehabilitation.
Recent evidence suggests that the application of light energy is promising and has many potential uses in the treatment of periodontal disease and implant in tissue regeneration, including the ablation of tissue, killing bacteria, and inflammation control [22]. The high-level laser therapy can help to detoxify thoroughly diseased peri-implant tissues and can thus promote improved healing and regeneration [23, 24].
Several limitations have to be considered in this work. First of all, this is not a randomized blinded clinical trial, and the results obtained must be considered as a preliminary work for such a kind of clinical study. The absence of a placebo control group, together with the effects of having a large percentage (four on six patients) of smokers in the control group, may have also influenced the outcome of the study. The small number of subjects in the groups is a common weak point in the studies regarding peri-implantitis, due to difficulty in finding a large number of subjects with comparable clinical features. A larger, blinded placebo controlled study has to be carried out in order to confirm the observations derived from our work.
Conclusions
Within the limitations of this study, diode laser seems to be a valuable tool in mucositis and peri-implantitis treatment, in combination to conventional, non-surgical periodontal instrumentation. Randomized longitudinal clinical trials are still needed, to confirm the conclusions expressed in the present article.
References
Albrektsson T, Isidor F (1994) Consensus report of session IV. In: Lang NP, Karring T (eds) Proceedings of the First European Workshop on Periodontology. Quintessence, London, pp 365–369
Aoki A, Mizutani K, Takasaki AA et al (2008) Current status of clinical laser applications in periodontal therapy. Gen Dent 56:674–687
Brägger U (1994) Radiographic parameters for the evaluation of peri-implant tissues. Periodontol 2000(4):87–97
Esposito M, Hirsch JM, Lekholm U, Thomsen P (1998) Biological factors contributing to failures of osseointegrated oral implants. (II). Etiopathogenesis. Eur J Oral Sci 106:721–764
Ishikawa I, Aoki A, Takasaki AA, Mizutani K, Sasaki KM, Izumi Y (2009) Application of lasers in periodontics: true innovation or myth? Periodontol 200050:90–126
Izumi Y, Aoki A, Yamada Y et al (2011) Current and future periodontal tissue engineering. Periodontol 2000(56):166–187
Karoussis IK, Kotsovilis S, Fourmousis I (2007) A comprehensive and critical review of dental implant prognosis in periodontally compromised partially edentulous patients. Clin Oral Implants Res 18:669–679
Lang NP, Berglundh T (2011) Working group 4 of seventh european workshop on periodontology. Periimplant diseases: where are we now?--consensus of the seventh european workshop on periodontology. J Clin Periodontol 38(Suppl 11):178–181
Lang NP, Bosshardt DD, Lulic M (2011) Do mucositis lesions around implants differ from gingivitis lesions around teeth? J Clin Periodontol 38(Suppl 11):182–187
Lindhe J, Meyle J, Group D of European Workshop on Periodontology (2008) Peri-implant diseases: consensus report of the sixth european workshop on periodontology. J Clin Periodontol 35(Suppl 8):282–285
Meissner G, Kocher T (2011) Calculus-detection technologies and their clinical application. Periodontol 2000(55):189–204
Misch CE, Perel ML, Wang HL et al (2008) Implant success, survival, and failure: the International Congress of Oral Implantologists (ICOI) pisa consensus conference. Implant Dent 17:5–15
Mombelli A, Lang NP (1998) The diagnosis and treatment of peri-implantitis. Periodontol 2000(17):63–76
Bach G, Neckel C, Mall C, Krekeler G (2000) Conventional versus laser-assisted therapy of periimplantitis: a five-year comparative study. Implant Dent 9:247–251
Castro GL, Gallas M, Nunez IR, Borrajo JLL, Garciavarela L (2006) Histological evaluation of the use of diode laser as an adjunct to traditional periodontal treatment. Photomed Laser Surg 24:64–68
Schwarz F, Sculean A, Rothamel D, Schwenzer K, Georg T, Becker J (2005) Clinical evaluation of an Er: YAG laser for non-surgical treatment of periimplantitis: a pilot study. Clin Oral Implants Res 16:44–52
Romeo U, Palaia G, Botti R, Leone V, Rocca JP, Polimeni A (2010) Non-surgical periodontal therapy assisted by potassium-titanyl-phosphate laser: a pilot study. Lasers Med Sci 25:891–899
Schwarz F, Sculean A, Berakdar M, Szathmari L, Georg T, Becker J (2003) In vivo and in vitro effects of an Er:YAG laser a GaAIAs diode laser, and scaling and root planing on periodontally diseased root surfaces: a comparative histologic study. Lasers Surg Med 32:359–366
Kreisler M, Al Haj H, d’Hoedt B (2005) Clinical efficacy of semiconductor laser application as an adjunct to conventional scaling and root planing. Lasers Surg Med 37:350–355
Kreisler M, Al Haj H, D’Hoedt B (2003) Temperature changes induced by 809-nm GaAlAs laser at the implant-bone interface during simulated surface decontamination. Clin Oral Implants Res 14:91–96
Romanos GE, Gutknecht N, Dieter S, Schwarz F, Crespi R, Sculean A (2009) Laser wavelengths and oral implantology. Lasers Med Sci 24:961–970
Sculean A, Schwarz F, Becker J (2005) Anti-infective therapy with an Er:YAG laser: influence on peri-implant healing. Expert Rev Med Devices 2:267–276
Soukos NS, Goodson JM (2011) Photodynamic therapy in the control of oral biofilms. Periodontol 2000(55):143–166
Takasaki AA, Aoki A, Mizutani K et al (2009) Application of antimicrobial photodynamic therapy in periodontal and peri-implant diseases. Periodontol 2000(51):109–140
Compliance with ethical standards
The study was carried out in accordance with the existing local ethical requirements.
Conflicts of interests
The authors report no conflicts of interest related to this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lerario, F., Roncati, M., Gariffo, A. et al. Non-surgical periodontal treatment of peri-implant diseases with the adjunctive use of diode laser: preliminary clinical study. Lasers Med Sci 31, 1–6 (2016). https://doi.org/10.1007/s10103-015-1785-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-015-1785-7