Abstract
The purpose of this study was to systematically assess clinical studies on the effect of using a diode laser in the treatment of peri-implantitis. Study question was “In patients with peri-implantitis around functional dental implants, can treatment by a diode Laser (810 nm) versus conventional treatment be effective in reducing the probing depth?”. The study included only randomized controlled clinical trials that involved patients with peri-implantitis. Included articles evaluated a diode laser (810 nm) used as monotherapy or as adjuvant therapy in the non-surgical treatment while their control group received conventional methods of treatment for peri-implantitis. Studies that involved other types of laser treatment options, surgical therapy, photodynamic therapy, case series, or case reports were excluded. Three electronic databases were searched for published articles from 2010 to 2018: PubMed/Medline, Cochrane, and Web of Science. The references were manually hand searched for relevant articles. The search initially identified 44 studies, which were filtered to yield a total of 3 eligible studies. All included studies compared laser treatment by a diode laser (810 nm) to conventional therapy by mechanical debridement for a follow-up period ranging from 6 months to 1 year, and risk of bias was assessed for each of the three included studies. A qualitative analysis of the three studies was conducted. This systematic review could not support the usage of a diode laser in the treatment of peri-implantitis. To confirm this assumption, more clinical trials with long-term follow-up periods are recommended.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bacterial biofilms formed on implant surfaces cause inflammation of the surrounding tissues and lead to complications such as mucositis and peri-implantitis [1]. Mucositis is an inflammatory lesion in the surrounding mucosa of an implant, while peri-implantitis is an inflammatory condition that is accompanied by mucosal inflammation, pocket depth (PD) ≥ 4 mm and bleeding on probing (BOP). Sometimes, pus might be present [2], and bone loss can be seen radiographically [3]. This condition is irreversible [2]. Bacterial colonization begins at the abutment surface. The implant surfaces are covered by a pellicle due to adhesion of salivary components, especially proteins, that provide linking sites for bacterial adhesion [1]. A previous review showed that peri-implant mucositis occurs in 80% of patients, that 50% of implants are affected, and that peri-implantitis affects 12 to 40% of implants after 5 years of placement [2, 4]. However, peri-implantitis has been a perplexing and controversial unresolved problem for several years [5]. The characteristics of the implant surface include implant chemistry, surface-free energy, and roughness. All of these factors affect bacterial attachment and proliferation. Surface roughness specifically has been considered the main feature promoting biofilm development, although it improves osseointegration via several surface modifications [1]. In the oral cavity, the microorganism community is complex. Hundreds of species are present, each species with a specific micro-environment. Thus, the bacterial interaction with implant surfaces in the oral cavity is complex [1]. The management of peri-implantitis has been a clinical dilemma since the conventional methods have not shown any promising results [6, 7]. There has been a paradigm shift in the methods for managing peri-implantitis. In this regard, the application of lasers has been of great clinical significance [8,9,10,11,12,13,14,15]. Recently, new approaches have been introduced, including the application of lasers such as Er,Cr:YSGG and Er:YAG. These new methods have yielded better clinical outcomes [16,17,18]. Studies have revealed that the diode laser seems to be a valuable therapeutic tool for peri-implantitis [19, 20]. There are different treatment protocols, but there is still no certain modality for the treatment of peri-implant mucositis and peri-implantitis [4]. Different traditional therapies of implant surfaces and the surrounding tissues have been performed for peri-implant disease treatment, such as detoxification via mechanical curettage [21, 22]. Other methods include the application of citric acid, air flow, and laser application [4]. One treatment modality that involves dental lasers could be photodynamic therapy, which has a good prognosis for the treatment of different oral diseases. This therapy employs a laser light of a certain wavelength with a photosensitizer. The laser stimulates the photosensitizer dye molecules to form highly reactive and cytotoxic singlet oxygen, leading to bacterial death [21]. A laser is a device that emits light via amplification of photons by stimulated emission. Lasers are classified according to the active medium: solid, liquid, gas and semiconductors. The wavelength of diode lasers extends from the visible to the near infrared range. The most commonly used dental diode laser wavelengths range from 800 to 980 nm, and the new wavelength of 445 nm. The diode lasers have high transmission in hydroxyapatite and high absorption in melanin and haemoglobin [23]. Therefore, they are used for cutting, coagulation, bleaching, and disinfection [23]. Lasers can easily irradiate the whole surface, particularly in rough and irregular areas, which are difficult to reach with mechanical instruments. Lasers inactivate bacterial diffused toxins and kill bacteria [4]. A laser has a direct effect on gram-negative anaerobic bacterial rods, as it is pigmented in black and is thus absorbed by the bacteria [23]. In this regard, using a diode laser (810 nm) can be clinically valuable for the treatment of peri-implantitis [24]. Several studies have claimed its potential in bio-stimulation and disinfection, which are crucial characteristics for achieving a successful line of treatment [24, 25]. Previous studies have shown that the treatment may yield good results if curettage and laser therapy are combined together in the treatment of peri-implant diseases [21]. Since diode lasers (810 nm) can decontaminate the implant surface without any side effects on the implants and the surrounding tissues [23], they can be considered an important tool in the treatment of mucositis and peri-implantitis if used with conventional mechanical debridement [2]. Some systematic reviews have evaluated the effect of various treatments on peri-implantitis, including different laser types [19, 20, 26,27,28]. However, there are no studies regarding the usage of a diode laser in this condition or its effectiveness compared with that of conventional therapy. However, because a diode laser (810 nm) is a high-power laser, especially highly absorbed by the melanin present in big amounts in bacteria that are pigmented, it shows high potentiality for bacteria eradication. This feature is in addition to its bio-modulatory effects in stimulating fibroblast growth factor and reducing the number of inflammatory mediators, thus promoting wound healing and repair. Also Silva TSO et al. concluded in their study in 2014 that low level laser therapy (LLLT) seems to speed up the process of bone repair at implant sites. This is the role of the heat (secondary effect) of irradiating high-power laser on implant and bone surface if used in correct settings. This laser was found to be the most widely used type of diode laser in clinical trials [8,9,10,11, 29]. Since no other systematic reviews that evaluated the use of a diode laser (810 nm) compared with conventional therapy were found, the present study systematically reviewed the relevant literature to investigate the effect of a diode laser (810 nm) in combination with conventional mechanical debridement in the treatment of peri-implantitis.
Review
Rationale and focus question
Since there is no conclusive answer to the best of our knowledge regarding the treatment of peri-implantitis, this study systematically assessed clinical investigations on the effect of using a diode laser in the treatment of peri-implantitis. The addressed, focused question was as follows: In patients with peri-implantitis around functional dental implants, can treatment by a diode laser (810 nm) versus conventional treatment be effective in reducing the probing depth?
Material and methods
Search strategy
The search was performed in the following electronic bibliographic databases (PubMed/MEDLINE, Cochrane, and Web of Science) from 2010 to 2018, as this date limit represents the beginning of the evidence-based literature for the laser-assisted treatment of peri-implantitis. There were no language limitations. The search strategy in PubMed/MEDLINE included the following terms: Implant*OR Implant decontamination OR Periimplantitis OR Peri-implantitis or Periimplant*OR Periimplantitis [Mesh Terms] AND Diode laser OR Dental diode laser OR Diode dental lasers OR Diode 810 laser OR Diode 810 [Mesh Terms] OR Diode laser in dentistry OR Diode laser therapy OR Low level laser therapy AND Mechanical debridement OR Scaling* OR Curettage OR Conventional periodontal treatment* OR Conventional periodontal therapy* OR Non-surgical periodontal treatment OR Non-surgical periodontal therapy OR Periodontal therapy* OR Traditional treatment of periimplantitis (Table 1). A PubMed alert was placed for the search.
The references of eligible articles found were hand searched for further relevant articles.
Protocol and registration
The protocol was developed according to PRISMA-P reporting guidelines and was registered in PROSPERO (http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42018112972).
Focus question
The following focus question was developed according to the population, intervention, comparison, and outcome (PICO) study design: In patients with peri-implantitis around functional dental implants, can treatment by a diode laser (810 nm) versus conventional treatment be effective in reducing the probing depth?
Information sources
Search
Table 1 presents the full electronic search strategy for one major database: PubMed/MEDLINE. Keywords are as mentioned in the table.
Selection of studies
After running searches through three databases, the articles found were further filtered to remove the duplicates; the rest were screened by title and abstract, and then what remained was followed by a further assessment for eligibility. The review process then proceeded to further exclude other articles for not meeting the prerequisites for inclusion.
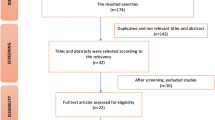
Based on titles and abstract searches, 44 studies were initially identified. After the removal of duplicates, 35 publications were screened by title and abstract, and 14 full text articles were further assessed for eligibility after the exclusion of 21 non-eligible articles. The review process then proceeded to further exclude 11 articles for not meeting the prerequisites for inclusion (Table 2 and Fig. 1).
Types of publications
Published articles conducted from randomized controlled clinical trials.
Types of studies
Randomized controlled clinical trials in published articles from 2010 to 2018.
Types of participants/population
Patients whose natural dentition had been replaced by functioning dental implants.
Disease definition
Peri-implantitis is defined as probing depth around a functioning dental implant of more than 4 mm due to bone loss and inflammation.
Inclusion and exclusion criteria
-
1.
Inclusion Criteria
-
Randomized clinical trials
-
Diode laser (810 nm)
-
Patients with peri-implantitis
-
Comparison with conventional treatment by mechanical debridement
-
Follow-up: a minimum of 6 months
-
Studies that evaluated the following outcomes:
-
Bleeding on probing
-
Probing depth
-
Clinical attachment level
-
-
-
2.
Exclusion Criteria
-
In vitro studies
-
Case reports
-
Literature reviews
-
Single arm studies
-
Erbium lasers
-
Nd: YAG laser
-
Diode lasers at wavelengths 940 nm, 980 nm, 660 nm, or 445 nm
-
Carbon dioxide lasers
-
Photodynamic therapy
-
Surgical treatment
-
Studies performed on natural teeth and not on implants
-
Micro-biological studies
-
Biofilms
-
Sequential search strategy
The results identified by PubMed alerts were evaluated until 2/7/2019, with no additional articles fitting the inclusion. Three eligible studies were selected for final review.
Data extraction
The following summarizes the information on studies that were obtained and extracted by 3 reviewers and then included in a standardized table: first study ID, i.e., author and year of publication, gender, age, demographic data, sample size, type of study (study design), diagnostic criteria, intervention (treatment protocol), follow-up period, treatment frequency, different parameters of lasers used, and clinical parameters (probing depth, BOP, and clinical attachment loss). These pieces of information are listed in Tables 3, 4, 5:
Data items
Characteristics of the included studies
Among the three included studies, two were categorized as RCTs [4, 21], and one was a retrospective controlled clinical trial [2]. The trials were originated from Istanbul [2], and the other two studies were unclear [4, 21]. In all studies, the number of patients ranged from 10 to 30 with a mean age of 55 years. The peri-implantitis diagnostic criteria differed across studies. One study included patients with PD ˃ 4 mm [4], another study included subjects with PD from 4 to 6 mm [2], and a third study recruited patients with PD ˃ 5 mm [21]. The follow-up period ranged from 6 to 60 months in all studies.
The risk of bias is presented in Fig. 2; one study is considered to be of low risk [4], while the other two studies were considered to be of high risk [2, 21].
Laser-related parameters
All studies included in the review used a diode laser with a wavelength of 810 nm. The power output was 1 W in all studies, with a range of irradiation times of 20 to 30 s in all studies, Lerario et al. [2] used 30 s cw, Arisan et al. [4] used 60 s Chopped mode, while Bach et al. [21] used 20 s. The optical fibre diameter was 400 μm, and one study used different optical fibre diameters: 200, 400, and 600 μm (140).
The emission mode in the included studies was pulsed mode in the two studies by Lerario et al. [2] and Arisan et al. [4], yet it was continuous mode in the third study by Bach et al. [21].
Main outcomes of the studies
Two studies showed a significant improvement in the reduction in PD for the diode laser group compared with the group treated with the conventional method [4, 22]. However, one study reported no statistically significant difference between the laser group and the conventional non-surgical group in the reduction in PD [2].
Risk of Bias within studies
The risk of bias assessment for the Randomized Control Trials was performed independently by three reviewers and was based on the Cochrane Handbook for Systematic Reviews of Interventions, categorized as (1) low risk of bias, (2) high risk of bias, or (3) unclear risk of bias. The following characteristics were evaluated: random sequence generation, treatment allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective outcome reporting. Disagreements about the risk of bias in any study were discussed between the three reviewers to reach a consensus. Authors were contacted for unclear risk of bias points to be clarified. However, only one author replied with a clarification as to the method of random sequence generation and allocation concealment.
Risk of Bias across studies
-
1.
Non-surgical periodontal treatment of peri-implant diseases with the adjunctive use of a diode laser: preliminary clinical study [2].
-
A.
Selection bias:
-
A.
-
1.
Randomization sequence generation: unclear
-
2.
Allocation concealment: unclear
-
B.
Performance bias:
-
B.
-
3.
Blinding of participants: unclear
-
4.
Blinding of personnel: not applicable (low risk)
-
C.
Detection bias:
-
C.
-
5.
Blinding of outcome assessors: unclear
-
D.
Attrition bias:
-
D.
-
6.
Outcome data: all participants that the study started with continued until the end of the study (low risk)
-
E.
Reporting bias:
-
E.
-
7.
Selective reporting: PI measurements were said to be recorded but were not (high risk)
-
8.
Other bias: none
-
2.
A randomized clinical trial of an adjunct diode laser application for the non-surgical treatment of peri-implantitis [4]
-
2.
Critical Appraisal:
-
A.
Selection bias:
-
1.
Randomization sequence generation: simple randomization (coin toss) (low risk)
-
2.
Allocation concealment: unclear
-
B.
Performance bias:
-
B.
-
3.
Blinding of participants: unclear
-
4.
Blinding of personnel: not applicable (low risk)
-
C.
Detection bias:
-
C.
-
5.
Blinding of outcome assessors: “an independent assessor unaware of the patients and treatment was employed for the follow-up evaluation of all patients” (low risk)
-
D.
Attrition bias:
-
D.
-
6.
Outcome data: complete, “all patients completed the study” (low risk)
-
E.
Reporting bias:
-
E.
-
7.
Selective reporting: non-selective, all outcomes measured showed clear results (low risk)
-
8.
Other bias: none
-
3.
A conventional versus laser-assisted therapy of peri-implantitis: a 5-year comparative study
-
3.
Critical appraisal: [21]
-
A.
Selection bias:
-
1.
Randomization sequence generation: “patients were divided randomly”: unclear
-
2.
Allocation concealment: unclear
-
B.
Performance bias:
-
B.
-
3.
Blinding of participants: unclear
-
4.
Blinding of personnel: not applicable (low risk)
-
C.
Detection bias:
-
C.
-
5.
Blinding of outcome assessors: unclear
-
D.
Attrition bias:
-
D.
-
6.
Outcome data: complete (low risk)
-
E.
Reporting bias:
-
E.
-
7.
Selective reporting: none selective (low risk)
-
8.
Other bias: none
Results
Data synthesis included a qualitative analysis of the risk of bias assessment and narrative interpretation of the studies, lasers, parameters, and associated therapies, and main results of the studies evaluating the quality of the studies by evaluating the results of each article with regard to their risk of bias. However, a quantitative analysis was not warranted due to high heterogeneity between the included studies. In 2000, Bach et al. [21] did not present exact outcome data that can be quantitatively synthesized with the other two studies, and another study by Lerario et al. in 2015 [2] was questionable with regard to study design. The author classified it as “Retrospective” although its methodology seems prospective. The study was questionable in its bias assessment, having four unclear points and reporting bias due to selective reporting error. In the study by Lerario et al. in 2015 [2], 606 periodontally affected sites accounted for the test group, while 144 unaffected sites accounted for the control group, and their follow-up period occurred after 1 year. In the test group, the probing depth mean difference was 2.66 mm (± 1.07), while in the control group, it was 0.94 (± 1.13), and paired t testing showed a statistically significant difference between the two groups. This is why the authors recommended the use of a diode laser at this wavelength to treat peri-implantitis. In the second study by Arisan et al. [4], baselines were measured for both the test group and the control group, which were presented by ten patients having 48 implant sites, with 1- and 6-month follow-up periods. At baseline, the mean probing depth and marginal bone level values were the same between the two groups. However, after 6 months of follow-up, the laser group showed higher marginal bone levels (2.79, SD 0.48) than the control group (2.63, SD 0.53), and the mean probing depth in the test group was higher than that in the control group (4.54 SD 0.74 in the test group compared with 4.17 SD 0.41 in the control group), which, although not statistically significant, helped the authors conclude that a diode laser in adjunct to conventional therapy in the treatment of peri-implantitis did not prove beneficial. The third study by Bach et al. [21] actually failed to report measurable outcome data in terms of numbers that can be analyzed or presented statistically. Their sample size was 30 patients, 15 in each group with a similar number of implants in both groups and a follow-up period that extended up to 5 years. However, they assumed that it was more beneficial to add laser treatment to conventional therapy in the treatment of peri-implantitis.
Discussion
All reviewed studies stated that a diode laser seems to be a valuable therapeutic tool in combination with conventional methods for treating peri-implantitis [2, 4]. Only one study claimed that a diode laser did not add any additional positive influence [21].
In the qualitative analysis of articles included in this systematic review, blinding of participants was not performed, although it could have been made possible via directing the beam of the laser hand piece towards the site to be treated; thus, the patient could not tell the difference between whether they were receiving laser treatment or not. Thus, a study was considered high risk whenever such blinding was not found.
In the laser-related parameters of the studies included, one of the included studies by Lerario et al. [2] mentioned using a power of 1 W near the implant surface. Although this is a very high-power setting near the implant surface [33], the author claimed that this power setting was considered safe around peri-implant tissue, guided by another study that studied thermal changes caused by simulated surface decontamination of the bone implant interface by Kresiler et al. [29]. The later authors studied various power settings and claimed that the threshold temperature of 47 °C was only outreached after 30 s using the 1 W. Heat production during laser application on implant and bone causing temperature to rise above 47 °C for 60 s negatively affects living bone and compromises its regeneration [33,34,35,36,37].
A quantitative analysis was not performed due to the high heterogeneity of the studies found. After initially excluding the study of Bach et al. in 2000 [21] due to its lack of exactness in reporting quantitative data in their outcomes in detailed measurable forms that can be added to the other studies, two studies were left: Lerario et al. [2] and Arisan et al. [4]. However, data between these two studies measuring values of probing depths at 6 months [2] and at 1 year [4] could not be added as they were found to have a large difference between the two time points, although the data were not quantitatively analyzed due to the questionable study design by Lerario et al. [2]. This study was controversial for being described as retrospective and was not randomized, and this finding was further confirmed by its qualitative analysis, which showed it was a high-risk study and thus was not a candidate for meta-analysis. This means that we are left with only one study [4], which had good data reporting; however, a quantitative analysis cannot be achieved by only one study.
As a result, a diode laser might be effective in the management of peri-implantitis. However, more clinical trials are required to ensure the clinical relevance of this approach.
In 2015, Lerario et al. [2] reported that they found statistically significant differences between the two groups after 1 year of follow-up according to a paired t test performed on the mean probing depth differences between the two groups. However, the study design is questionable since the authors mentioned it is of a retrospective study design, while the methodology indicates that it has the design of a prospective clinical trial. The authors also failed to explain the methodology in a logical sequence, which made the results questionable and non-reliable for use in statistical analysis. Their follow-up period of 1 year without any time intervals in the middle, such as a 6-month follow-up, was unjustified, even though it is the most common period used for the mean time of follow-up. Additionally, in the risk of bias assessment, they did not mention that any randomization was conducted nor was blinding accomplished for either the participants, personnel, or outcome assessors, and although the personnel applying the treatment cannot be blinded in this case, both the participants and the outcome assessor can, which makes this study a high-risk study in terms of bias. Moreover, there was selective reporting bias in that the plaque index values were said to be recorded but were not. This made the study of a poor quality in terms of the risk of bias assessment.
In the study by Arisan et al. [4], the authors could not prove the benefits of using a diode laser as an adjunct therapy to conventional treatment of peri-implantitis. This conclusion was guided by their statistical analysis of probing depth values and marginal bone loss at follow-up periods of 1 and 6 months. Although marginal bone loss is not one of the outcomes evaluated by this systematic review, it was noteworthy that marginal bone loss was greater in the test group of that study than in the control group that did not receive any laser therapy. This study is considered of low risk since random sequence generation was performed via a coin toss, and although allocation concealment or blinding of the participants and personnel was not performed, the outcome assessor was blinded, which adds to the values of these results. This was accomplished by not allowing the assessor to be knowledgeable of which groups the subjects were allocated to. Additionally, all patients completed the study, as mentioned by the authors, and all outcomes measured, as claimed by the authors at the beginning of the study, showed clear result reporting, so no reporting bias was assumed. This makes this study of low risk and of high quality—one whose results can be taken into account.
The third study by Bach et al. [21] failed to provide statistically measurable outcome data, which made their study outcomes questionable. It is noteworthy that within the 5 years of their follow-up period, only 1 of the 99 implants in the control group had to be removed compared with none in the test group, which led them to conclude that adding the diode laser to their treatment protocol made sense as it decontaminated the implant surfaces and enhanced the survival rates of the implants. However, the fact that their results were not presented numerically as they should have been and the the fact that the study was considered high risk in terms of risk of bias assessment lead to doubts regarding taking this conclusion for granted. In this study, the random sequence generation was unclear since the authors merely mentioned that “patients were divided randomly”. Additionally, whether allocation concealment and blinding of the participants and outcome assessors were accomplished or not was unclear, which adds more reasons why this study should be considered a high-risk study.
The lack of consistency between all three articles in terms of their outcomes, methods of reporting, and the risk of bias assessment prevented us (the authors of this systematic review) from reaching a conclusion that may support the use of a diode laser as an adjuvant to the conventional therapy of scaling and mechanical debridement in the treatment of peri-implantitis. Although diode laser treatment may help in enhancing the results due to its scientifically proven effect of decontamination of implant surfaces, the results of this systematic review could not prove these facts.
Conclusions
Our data from this systematic review do not support a recommendation for the use of a diode laser (810 nm) in the management of peri-implantitis.
To confirm this assumption, more clinical trials are recommended with long-term follow-up periods especially ones utilizing 810-nm laser treatment in combination with Erbium laser, to benefit from their combined effects on smear layers and biofilm as well as promoting healing and enhancing fibroblastic and osteoblastic activity.
References
Bevilacqua L, Milan A, Del Lupo V, Maglione M, Dolzani L (2018) Biofilms developed on dental implant titanium surfaces with different roughness: comparison between in vitro and in vivo studies. Curr Microbiol 75:766–772
Lerario F, Roncati M, Gariffo A, Attorresi E, Lucchese A, Galanakis A, Palaia G, Romeo U (2015) Non-surgical periodontal treatment of peri-implant diseases with the adjunctive use of diode laser: preliminary clinical study. Lasers Med Sci 31:1–6
Figuero E, Graziani F, Sanz I, Herrera D, Sanz M (2014) Management of peri-implant mucositis and peri-implantitis. Periodontol 2000, 66(1):255–273
Arisan V, Karabuda ZC, Arici SV, Topcuoglu N, Kulekci G (2015) A randomized clinical trial of an adjunct diode laser application for the nonsurgical treatment of peri-implantitis. Photomed Laser Surg 33:547–554
Klinge B (2012) Peri-implant marginal bone loss: an academic controversy or a clinical challenge? Eur J Oral Implantol 5:13–19
Lindhe J, Meyle J (2008) Peri-implant diseases: consensus report of the sixth European workshop on periodontology. J Clin Periodontol 35:282–285
Roncati M, Lucchese A, Carinci F (2013) Non-surgical treatment of peri-implantitis with the adjunctive use of an 810-nm diode laser. J Indian Soc Periodontol 17(6):812–815
Mizutani K, Aoki A, Coluzzi D, Yukna R, Wang C-Y, Pavlic V, et al Lasers in minimally invasive periodontal and peri-implant therapy. Periodontol 2000 [Internet]. Wiley/Blackwell (10.1111); 2016 Jun 1 [cited 2018 May 19];71(1):185–212
Salaria SK, Sharma I, Brar NK, Kaur S (2016) Diode laser and periodontal regeneration-assisted management of implant complications in anterior maxilla. Contem Clin Dent 9(1):114–119
Birang E, Ardekani MRT, Rajabzadeh M, Sarmadi G, Birang R, Gutknecht N (2017) Evaluation of effectiveness of photodynamic therapy with low-level diode laser in nonsurgical treatment of peri-implantitis. Laser Med Sci 8(3):136–142
Mettraux GR, Sculean A, Bürgin WB, Salvi GE (2016) Two-year clinical outcomes following non-surgical mechanical therapy of peri-implantitis with adjunctive diode laser application. Clin Oral Implants Res 27(7):845–849
Romeo U, Nardi GM, Libotte F, Sabatini S, Palaia G, Grassi FR (2016) The antimicrobial photodynamic therapy in the treatment of peri-implantitis. Int J Dent. [Internet]. Hindawi; 2016 Jun 26 [cited 2018 May 19]; 1–5
Tang E, Khan I, Andreana S, Arany PR (2017) Laser-activated transforming growth factor-β1 induces human β-defensin 2: implications for laser therapies for periodontitis and peri-implantitis. J Periodontal Res 52(3):360–367
Hakki SS, Tatar G, Dundar N, Demiralp B (2017) The effect of different cleaning methods on the surface and temperature of failed titanium implants: an in vitro study. Laser Med Sci 32(3):563–571
Santos CR, Tonetto MR, Presoto CD, Bandéca MC, Oliveira OB Jr, Calabrez-Filho S, Andrade MF (2012) Application of Er: YAG and Er,Cr:YSGG Lasers in cavity preparation for dental tissues: a literature review. World J Dent 3(4):340–343
Diaci J, Gaspirc B (2012) Comparison of Er: YAG and Er,Cr:YSGG lasers used in dentistry. J LAHA 1:1–13
Peters N, Tawse-Smith A, Leichter J, Tompkins G (2012) Laser therapy: the future of peri-implantitis management? Braz J Periodontol 22(1):26
Giacomo P, Gian S, Riccardo B, Maurizio S, Cesare P (2017) Non-surgical treatment of peri-implantitis: a systematic review of the literature. J Anesth Clin Res 9:850
Heitz LA, Mombelli A (2014) The therapy of peri-implantitis: a systematic review. Int J Oral Maxillofac Implants 29:325–345
Albaker AM, ArRejaie AS, Alrabiah M, Abduljabbar T (2018) Effect of photodynamic and laser therapy in the treatment of peri-implant mucositis: a systematic review. Photodiagn Photodyn Ther 21:147–152
Bach G, Neckel C, Mall C, Krekeler G (2009) Conventional vs laser assisted therapy of peri-implantitis: a five year comparative study. Implant Dent 3:247–225
Gulati M, Govila V, Anand V, Anand B (2014) Implant maintenance: A clinical update. Int Sch Res Notices 9:85–94
Pirnat S (2007) Versatility of an 810 nm diode laser in dentistry: an overview. J Laser and Health Academy 4:1–9
Yousif A, Zwinger S, Beer F (2008) Investigation on Laser dental implant decontamination. J Laser Micro Nanoengineering 3:2–6
Ashnagar S, Nowzari H, Nokhbatolfoghahaei H, Yaghoub Zadeh B, Chiniforush N, Choukhachi ZN (2014) Laser treatment of peri-Implantitis: a literature review. Lasers Med Sc 5(4):153–162
Ting M, Craig J, Balkin BE, Suzuki JB (2018) Peri-implantitis: a comprehensive overview of systematic reviews. Oral Implantol 44(3):225–247
Mahato N, Wu X, Wang L (2016) Management of peri-implantitis: a systematic review. SpringerPlus. 5:105
Ramanauskaite A, Daugela P, Juodzbalys G (2016) Treatment of peri-implantitis: meta-analysis of findings in a systematic literature review and novel protocol proposal. Quintessence Int 47(5):379–393
Kreisler M, Haitham H, Bernd D (2002) Temperature changes at the implant-bone interface during simulated surface decontamination with an Er:YAG laser. Int J Prosthodont 15:582–587
Bombeccari GP, Guzzi G, Gualini F, Gualini S, Santoro F, Spadari F (2013) Photodynamic therapy to treat periimplantitis. Implant Dent [Internet]. [cited 2018 May 19];22(6):631–8
Thierbach R, Eger T (2013) Clinical outcome of a nonsurgical and surgical treatment protocol in different types of peri-implantitis: a case series. Quintessence Int 44:137–148
Spadari F, Bombeccari GP, Bosotti B, Marino R (2010) Photodynamic therapy on peri-implantitis: comparative effectiveness - an in vivo trial. Oral Dis 16:553
Geminiani A, Romanos G, Caton J (2010) Temperature rise during diode laser irradiation of implant surfaces
Eriksson AR, Albrektsson T (1983) Temperature threshold levels for heat-induced bone tissue injury: a vital-microscopic study in the rabbit. J Prosthet Dent 50:101–107
Eriksson AR, Albrektsson T, Magnusson B (1984) Assessment of bone viability after heat trauma. A histological, histochemical and vital microscopic study in the rabbit. Scand J Plast Reconstr Surg 18:261–268
Eriksson RA, Albrektsson T (1984) The effect of heat on bone regeneration: an experimental study in the rabbit using the bone growth chamber. J Oral Maxillofac Surg 42:705–711 Lasers Med Sci
Silva TSO, Lima LMS, Machado JIAG, Carvalho CMRS, Moura CDVS (2014) Influence of low-level laser on dental implant sites: a literature review. Dent Press Implantol 8(3):86–94. https://doi.org/10.14436/2237-650X.8.3.086-094.oar
Acknowledgements
Center for Oral and Dental Health Promotion (CODE HP), Oral and Dental Research Division, National Research Center (NRC).
Funding
Misr International University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This manuscript is of a systematic review which is exempted from the ethical committee approvals.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mattar, H., Bahgat, M., Ezzat, A. et al. Management of peri-implantitis using a diode laser (810 nm) vs conventional treatment: a systematic review. Lasers Med Sci 36, 13–23 (2021). https://doi.org/10.1007/s10103-020-03108-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-020-03108-w