Abstract
The reproductive biology of fishes is crucial for providing reliable and scientifically sound recommendations for managing, monitoring, and evaluating fisheries regionally. This study aims to determine the reproductive characteristics of Lutjanus quinquelineatus(Bloch, 1790) in the Terengganu, southern South China Sea, Malaysia. A total of 527 specimens (255 males and 272 females) were collected using trawl net from April 2022 to March 2023. These specimens were measured, with total length ranging between 14.3 cm and 26.7 cm (mean ± SD: 19.1 ± 1.9 cm), and body weight ranging from 38.6 g to 316.8 g (mean ± SD: 119.1 ± 37.8 g). Sex ratio, gonadosomatic index (GSI), hepatosomatic index (HSI), spawning period, fecundity, condition factor (Kn), length at maturity (Lm) and the gonadal maturation stages were assessed in this study. No significant difference (χ2 = 0.54) was observed in the overall monthly sex ratio of male and female (1:1.07). The spawning season is determined by the GSI and gonad maturation stages, extending from February to June with peak periods in March. The evaluation of the relationship between the Kn and the HSI revealed that body energy and lipid storage in the liver may not be crucial factors for gonad development. The batch fecundity of 34 mature females ranged from 17.6 to 25.2 cm in length, with body weight ranging from 79.4 to 279.3 g, yielding 19,314 to 98,880 oocytes. The fecundity increased with gonad weight in contrast to the length and weight of fish. The lengths at maturity for males and females were 20.8 cm and 20.1 cm, respectively. This study offers indispensable information that will update the existing database and contribute to developing effective fishery management strategies in the specified area.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Snappers, belonging to the family Lutjanidae in the order Perciformes, have a crucial impact on tropical and subtropical ecosystems, serving crucial functions in both commercial and ecological aspects (Allen 1985; Messias et al. 2019). The genus Lutjanus has the majority of species in the Lutjanidae family (Allen 1985), and these species are highly valued and caught in fisheries around the world including Malaysia (Adibah and Darlina 2014). Snappers are a significant part of the local artisanal catch in their geographical spectrum (Allen 1985), with at least 26 species known from Malaysian waters (Froese and Pauly 2023). The five-lined snapper, Lutjanus quinquelineatus, is a small lutjanid fish found extensively in tropical and subtropical areas of the Indian Ocean and the western Pacific Ocean (Allen 1985). This species is consumed as a food source in tropical and subtropical regions. Despite its commercial and economic significance data regarding its biological aspects have been poorly documented in several regions (Mehanna et al. 2017; Araki and Tachihara 2021). The fundamental knowledge of life history, which includes spawning timing and duration, sex ratio, maturity stages, length maturity and fecundity, is essential for managing stocks and assessment of fisheries (Tsikliras et al. 2013; Sultana et al. 2023). Reproductive biomass is a crucial indicator for evaluating the condition of a harvested population and determining future fishing limits (Lucano-Ramirez et al. 2014); for instance, knowledge of the spawning biomass is necessary to estimate recruitment and length at maturity, which is crucial to deciding which size fish are captured for sustainable fisheries.Fish reproduction is sensitive to temperature variations. Rising temperatures and unusual ‘heat waves’ linked to climate change can affect reproductive performance in fish (Lema et al. 2024). Housh et al. (2024) reported that exposure to temperature exceeding a certain threshold negatively impacts fish gametic development and maturation, reducing spawning frequency and reproductive behavior. Numerous researchers have examined all of these variables, including sex ratio, spawning season, length at maturity, and fecundity to understand fish reproductive biology. For instance, Rahman et al. (2024) studied Lutjanus xanthopinnis, Fernandes et al. (2022) studied Lutjanus synagris, and Palla and Sotto (2021) explored Lutjanus vitta.
Despite the significance of the reproductive biology of fish in some nations, the lack or unavailability of fisheries data results in overfishing of the stocks and, in some cases, management failure (Alves and Minte-Vera 2012). A thorough understanding of the various reproductive features of fish species is crucial to offering reliable scientific advice for efficient fishery management in particular regions (Hossain et al. 2017; Khatun et al. 2019; Islam et al. 2021). Studying the reproductive behavior of snappers aids in their management and conservation (Lucano-Ramirez et al. 2013). Currently, there is a lack of information in the literature regarding the reproductive biology of studied fishes in Malaysia and the South China Sea and its reproductive biology has never been studied in the area of our study, which hampers the effective management of these populations. Therefore, the main objective of the present study is to investigate the reproductive traits of L. quinquelineatus in the Terengganu waters of the South China Sea, Malaysia. This study’s results will enrich the current database for this species and offer helpful scientific direction for future fisheries management planners.
Materials and Methods
Study Site and Sample Collection
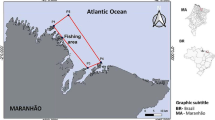
Samples were collected monthly basis from April 2022 to March 2023 from Pulau Kambing fish landing port (5°19’20.3"N 103°07’42.6"E) landed by fishermen, Terengganu waters of South China Sea, Malaysia (Fig. 1). A total of 527 specimens (255 males and 272 females) collected using trawl net with mesh size 38 mm and depth 20–80 m. The samples were stored in ice and transported to the Fisheries Science Laboratory, Universiti Malaysia Terengganu (UMT), for further examination and confirmation utilizing other systematic morphological characteristics specified by Allen (1985).
A map showing the study area (Rahman et al. 2023)
Morphometrics, Determination of Sex and Sex Ratio
Each specimen was measured for the total length (TL) nearest to 0.1 cm by using L-shaped board and electronic balance was used to record body weight (BW) nearest to 0.1 g (Fadzli et al. 2022). All specimens were dissected, and sex was determined for each individual. For each sample, the gonad was removed and its weight was measured to the nearest 0.0001 g using an electronic balance (ATX224 SHIMADZU). In the present study, the sex ratio was calculated by the proportion of both the sexes (male and female). The total number of both the sexes was used to calculate the monthly variations in sex ratio (Fakoya and Anetekha 2019).
Gonadal Histology and Identification of Maturity Stages
Fish gonad tissue that has been dissected was put in a histology cassette, and fixed in 10% Neutral Buffered Formalin. After 24 h, a small portion from the middle part of the fixed gonads was transferred to 70% ethanol and then dehydrated with a series of ethanol dilutions (Vacuum automatic tissue processor Leica TP1020) (Rahman et al. 2024). The gonads were embedded in paraffin using a Leica HistoCore Arcadia H, and sections were cut at a thickness of 5 μm using a Galileo SEMI Series 2 rotary microtome. Then, it was counterstained with hematoxylin-eosin and mounted on a glass slide using a cover slip. Lastly, photographs from histological slides were taken using a compound advanced research microscope (Nikon Eclipse 80i) (Rahman et al. 2024). For each individual, stages of gonad development were identified. Five gonadal maturity stages of L. quinquelineatus were identified based on macroscopic and histological examination of gonads described by Russell et al. (2003), Grandcourt et al. (2006), Brown-Peterson et al. (2011) and Fakoya and Anetekha (2019); Stage I-immature, Stage II-developing/regenerating, Stage III-spawning capable, Stage IV-actively spawning and Stage V-regressing.
Gonadosomatic Index, Hepatosomatic Index and Relative Condition Factor
Gonadosomatic index (GSI) is assessed monthly for both sexes (males and females) to understand the spawning season. The mean GSI for each month was calculated using the following formula:\(\:GSI=\){WG × (WB – WG)–1} × 100 WG is gonad weight and WB body weight, both in grams (Pacicco et al. 2023). A line graph was used to display the monthly mean GSI. The increasing peak of the GSI depicts the spawning season for this species. The monthly pattern of hepatosomatic index (HSI) were estimated by using the following equation revealed by Costa (2019) and Fadzli et al. (2022);\(\:\:HSI=\)(WL × WB–1) × 100 Where, WL is liver weight and WB is fish body weight in grams. Relative condition factor (Kn) for each specimen was estimated using the formula developed by Le Cren (1951), which is stated as follows;\(\:Kn=\)(WO × WC–1) where WO = observed weight (g) of studied fish and WC is the calculated fish weight resulting from length weight relationships (Rahman et al. 2023).
Length at Maturity
The length at which 50% of the population is sexually mature is known as the length at maturity (Lm). This was calculated based on the percentage of matured individuals (Stage III and IV) suggested by Palla and Sotto (2021) of 2-cm size class. A line drawn against the midpoint (TL) for matured males (N = 109) and females (N = 115) has been carried out based on (King 2007) utilizing the logistic equation as follows:
Where, RM is the mature individuals’ rate, rLm is the intercept a, ̵ r is the slope of line, r is ̵ b, L is the total length of fish. For the calculation of r and Lm, values of ln [(1 ̵ P)/P] plotting opposed to the midpoint of each size class as: and Lm = a/r.
Batch Fecundity
Batch fecundity (BF) is the number of eggs released by each fish during a single spawning phase (Gonçalves et al. 2009). The oocytes from (stages III and IV) were used to determine the batch fecundity (BF) of mature females. The ovary was divided into three sub-samples obtained from the anterior, middle, and posterior and weighed to the nearest 0.1 g. Oocytes (N = 34) were separated from connective tissue and calculated using a dissecting microscope (OLYMPUS SZ51). Then, BF is estimated by the following (Fry et al. 2009): BF = (NES × WG) × WGS–1 where NES is the egg count in a subsample, WG is the weight of the (whole) gonad, and WGS is the weight of the subsample gonad.
Statistical Analysis
The data underwent analysis using Excel 2010 and PAST 4.09 (Hammer et al. 2001). Chi Square (χ2) analysis was used to assess the sex ratio for any variations beyond the expected 1:1. Straight-line analysis was used to determine length at maturity of males and females. The correlations between BF and the total length, body weight, and gonad weight were derived using regression analysis. The analysis also took into consideration a significance level of p < 0.05.
Results
Morphometric Measurement and Sex Ratio
The total length for males varied from 14.3 to 26.7 cm (mean ± SD: 19.3 ± 2.3 cm) and females ranged from 14.9 to 25.2 cm (19.1 ± 1.4 cm). Moreover, the body weight for males ranged from 38.6 to 316.8 g (121.2 ± 44.4 g) and females varied between 58.6 and 279.3 g (117.1 ± 30.2 g) respectively. The investigation of the monthly sex ratios for this species revealed that the ratios were evenly distributed. An overall sex ratio of 1: 1.07 (M: F) was found among the 527 fish examined, with 255 (48.39%) males and 272 (51.61%) females (Table 1). However, the statistical study indicated that there was no significant difference in the overall population’s sex ratio (χ2 = 0.54; P = 0.46) from expected (1:1) ratio.
Gonad Development and Identification of Maturity Stages
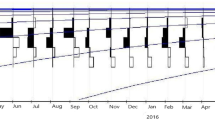
Five stages of gonad maturation were identified in L. quinquelineatus (Figs. 2 and 3), which include immature (stage I), developing/regenerating (stage II), spawning capable (stage III), actively spawning (stage IV) and regressing (stage V). In males with sectioned gonads, spawning occurs when spermatozoa are dominant and in the lobules of the sperm duct when spermatozoa are still present. In the ovaries, tertiary vitellogenic and hydrated oocytes signified approaching and continuing spawning, respectively. The macroscopic and histological investigation of the gonad revealed the monthly proportions of males and females in various gonadal stages of gonad development at Terengganu waters, Malaysia (Fig. 4). In addition, the reproductively active stages of gonads (spawning capable and actively spawning) for both sexes were observed in February to June (Fig. 4). This finding suggests that the studied fish species has an extended spawning season from February to June.
Microphotographs of histological slides for five gonadal maturity stages in male L. quinquelineatus. I-immature phase containing spermatogonia, II-developing/regenerating, III-spawning capable, IV- actively spawning phase containing enormous amounts of spermatozoa, V-regressing. Sg1-primary spermatogonia, Sc1-primary spermatocyte, Sc2-secondary spermatocyte, St-spermatid, Sz-spermatozoa, Lu-lumen, Scy-spermatocyst
Microphotographs of histological slides for five gonadal maturity stages in female L. quinquelineatus. I-immature, II-developing/regenerating, III-spawning capable, IV-actively spawning, V-regressing.PG-primary growth of oocytes, PVO-primary vitellogenin oocytes, SVO-secondary vitellogenic oocytes, TVO-tertiary vitellogenic oocytes HO-hydrated oocytes, AO-atretic oocytes, RO-residual oocytes, POF-post-ovulatory follicle
Gonadosomatic Index, Hepatosomatic Index, and Condition Factors
The mean GSI values for male and female individuals varied from 0.21 to 1.79 and 0.49 to 2.97, respectively. However, the monthly GSI trend for both sexes was consistent from February to June (males ranged: 0.98–1.79; females ranged: 1.23–2.97) with peaks in March (Fig. 5A and B), showing the spawning seasons of this species, which have their peak times in March. On the other hand, the mean monthly GSI for both sexes drastically declined from July to January (males ranged: 0.21–0.91; females ranged: 0.49–0.99) (Fig. 5A and B), which corresponds to the fish resting period.
On the contrary, the monthly HSI for males and females ranged from 0.32 to 1.23 (Fig. 5A) and 0.61 to 1.52 (Fig. 5B), respectively. Monthly changes in Kn and HSI were used to analyze the energy consumption patterns of fish during the reproductive phase. Furthermore, the monthly average Kn for both sexes exhibits a high level of uniformity. The monthly Kn for males and females varied from 1.00 to 1.04 and 1.00 to 1.05 (Fig. 5A and B), showing that they are growing in good health because of food availability and balanced ratio between predator and prey. The monthly values of Kn were minimally affected by the changes in GSI, which may be attributed to the plentiful availability of food (prey) in the study areas. Furthermore, there was a continuous linear relationship observed between GSI and HSI throughout each month.
Length at Maturity
Mature individuals ranged from 18 to 26.7 cm (21.3 ± 1.9 cm) for males and 17.3 to 25.2 cm (19.6 ± 1.4 cm) for females was observed. The calculated length at 50% sexual maturity (Lm) of L. quinquelineatus was 20.8 cm (N = 109) for males (Fig. 6A) and 20.1 cm (N = 115) for females (Fig. 6B). The relationship between mature and total length percentage had provided a coefficient of determination (R2), where R2 = 0.858 for males and R2 = 0.943 for females.
Batch Fecundity
A total of 34 mature female gonads have been evaluated for batch fecundity, TL varied from 17.6 to 25.2 cm (19.5 ± 1.7 cm), the BW ranged from 79.4 to 279.3 g (125.2 ± 39.5 g) and the GW ranged 1.74 to 9.6 g (3.9 ± 1.9 g). Each fish’s overall quantity of mature eggs ranged from 19,314 to 98,880 oocytes (43,836 ± 21,486 oocytes). The present study found that the fecundity of fish is positively correlated with the weight of their gonads, instead of length and weight of fish (Fig. 7). The strongest correlations were identified when fecundity compared with GW (R2 = 0.97).
Discussions
The current study gives firsthand knowledge of the reproductive biology of L. quinquelineatus in the southern South China Sea, Malaysia. In Malaysian waters, the maximum length was recorded at 26.7 cm, significantly less than the length reported by Mehanna et al. (2017) in the Red Sea at Hurghada, Egypt. The length disparities identified in this study may be attributed to various environmental factors allied to geographical variations (Smoliński and Berg 2022) or solely caused by the gear used for fishing. The study found that the monthly recorded and size-based sex ratio of the L. quinquelineatus population did not deviate significantly from the expected 1:1 ratio. Many researchers have made similar findings about various species of Lutjanus, namely Lutjanus goreensis (Fakoya and Anetekha 2019) and Lutjanus biguttatus (Longenecker et al. 2013) validate the results of the current study. It may also represent tendencies of monogamous mating (Teixeira et al. 2010; Trejo-Martínez et al. 2011). However, the findings of this study contradicted with same species from Okinawajima Island, Japan, reported by Araki and Tachihara (2021) resulting from variations in fishing pressure and alterations in life history traits. This might be also caused by several factors, including population adaption, sexual behaviour, availability of food, and environmental variables (Brykov et al. 2008; Vandeputte et al. 2012).
The spawning season of fish is dictated by an association of the GSI and the progression of gonadal development stages, which serves as a reliable measure of reproductive activity (Rizzo and Bazzoli 2020).The spawning season was estimated to be protracted from February to June, with a peak period in March based on GSI and gonad development stages (Figs. 4 and 5). According to Yaakob and Chau (2005), Malaysia’s northeast monsoon season continues from November to February. Spawning begins towards the end of the rainy season when the water temperature starts to rise from the lowest (February) and continues until mid-summer (June). The spawning season’s timing varied but was prolonged and aligned with the hottest water temperature reported by Araki and Tachihara (2021) and Mehanna et al. (2017) for L. quinquelineatus, and Rahman et al. (2024) revealed for L. xanthopinnis in Malaysia which consistent with the current study’s results. Tropical lutjanids typically engage in serial spawning with protracted spawning periods (Kritzer 2004; Marriott et al. 2007; Shimose and Tachihara 2005). Similar findings about the spawning season for Lutjanus species were observed in earlier studies conducted in different regions: May to September for L. quinquelineatus (Araki and Tachihara 2021) in Okinawa Island, April to August for L. fulviflammus (Shimose and Nanami 2015) in Yaeyama Island, June to September for L. fulvus (Shimose and Nanami 2014) in Okinawa Island, May to October for L. gibbus (Nanami et al. 2010a) in Ishigaki Island, June to October for L. decussatus (Nanami et al. 2010b) in Ishigaki Island. The protracted spawning period of L. quinquelineatus coincides with the findings of Araki and Tachihara (2021) and Mehanna et al. (2017) for the same species presented in Table 2.
In the present study, mean relative condition factors (Kn) were greater or equal to 1 in both sexes, indicating they are physiologically stable and also revealed a balanced ratio between predators and prey in study regions. According to Jisr et al. (2018), if a fish species has a Kn value equal to or very close to 1, we consider that species to have a general fitness level. Muchlisin et al. (2017) claim that when Kn is 1, there is still a balance between prey and predators, the waterways are in good condition, and fish may flourish. In general, condition factors are typically influenced by a number of biotic and abiotic factors, such as the availability of food, the quality of the water, and the age, size, sex, and stage of gonad development (Kuriakose 2014). The correlation between Kn and GSI was not significantly varied over the course of each month indicating that the muscle weight may not be a greatly influenced on fish reproductive processes attributed to the plentiful availability of food. This study’s findings are consistent with Rahman et al.‘s (2024) studies regarding L. xanthopinnis in the same study locations. Furthermore, the pattern of HSI showed linear relationships with the monthly progression of GSI, suggesting that lipid storage in the liver is not a crucial factor to fish reproduction. The findings were confirmed by Fadzli et al. (2022), who observed that HSI had no significant impact on gonad maturation.
Understanding the length at maturity will help determine the size that should be fished for sustainable fisheries (Thulasitha and Sivashanthini 2013). The calculated length at 50% maturity (Lm) was 20.8 cm for males and 20.1 for females based on total length. These lengths are slightly different from what Araki and Tachihara (2021) reported for the same species from Okinawa‑Jima Island, Japan, where they revealed that lengths at maturity were 129.5 mm (12.95 cm) for males and 130.3 mm (13.03 cm) for females based on standard length. Variations in length maturity may be caused by differences in food available in different geographic locations (Oliveira et al. 2017). Compared to the average length for both sexes in the present investigation, L. quinquelineatus was captured at a smaller size than Lm. This indicates that fishing operations are depleting the fish populations in the study areas.
Fecundity is essential for the successful recruitment of fish species and the regulation of fish population (Fernandes et al. 2022). Our study on L. quinquelineatus in Malaysian waters revealed that the fecundity varied from 19,314 to 98,880 oocytes and showed a positive relationship with gonad weight compared to the length and weight of the fish. The calculated BF revealed in this study is lower than Palla and Sotto (2021) report and greater than that reported by Pradeep (2016). In addition, the relations between BF with TL, BW and GW were exponential, which is supported by the findings of Fadzli et al. (2022). Grimes (1987) imply that tropical lutjanids can be very fecund species. Aydin et al. (2019) reported for black sea turbot Psetta maxima, larger and older fish tend to be more fecund and produce more eggs. Age, the relationship of body size, population density, diet, water currents, nutrient effect and environmental variability could all contribute to the variation in fecundity (Bradshaw and McMahon 2008).
Conclusion
The scarcity of accurate biological and fishery data necessary for fisheries management is a common occurrence in developing nations. An understanding of reproductive biology is vital for sustainable management in Malaysia, where managers manage fisheries resources based on maturity length, reproductive period start and duration. These results could serve as a basis for effectively and sustainably managing reef fisheries. Based on our results, here are some management recommendations for the sustainable management of L. quinquelineatus in Malaysia. For example, the male Lm of L. quinquelineatus is 20.8 cm, while the female Lm is 20.1 cm. We suggest that fishing be banned during the peak spawning seasons and that catching fish smaller than the length at maturity be strictly discouraged for sustainable management.
Data Availability
No datasets were generated or analysed during the current study.
References
Adibah AB, Darlina MN (2014) Is there a cryptic species of the golden snapper (Lutjanus johnii)? Genet Mol Res 13(4):8094–8104
Allen GR (1985) FAO species catalogue: Vol. 6. Snappers of the world: An annotated and illustrated catalogue of Lutjanid species known to date (No. 6)
Alves DC, Minte-Vera CV (2012) Scientometric analysis of freshwater fisheries in Brazil: repeating past errors? Rev Fish Biol Fish 23:113–126. https://doi.org/10.1007/s11160-012-9282-6
Araki K, Tachihara K (2021) Age, growth, and reproductive biology of the five-lined snapper Lutjanus quinquelineatus around Okinawa-Jima Island, southern Japan. Fish Sci 87(4):503–512
Aydin I, Polat H, Sahin T (2019) Reproductive performance of wild and hatchery-reared black sea turbot, Psetta maxima, in the southern black sea coast. Turkish J Fisheries Aquat Sci 20(5):351–357
Bradshaw CJA, McMahon CR (2008) Fecundity. Encyclopedia of ecology, fivevolume set. Elsevier Inc, pp 1535–1543
Brown-Peterson NJ, Wyanski DM, Saborido-Rey F, Macewicz BJ, Lowerre-Barbieri SK (2011) A standardized terminology for describing reproductive development in fishes. Mar Coastal Fisheries 3(1):52–70
Brykov VA, Kukhlevsky AD, Shevlyakov EA, Kinas NM, Zavarina LO (2008) Sex ratio control in pink salmon (Oncorhynchus gorbuscha and chum salmon (O. Keta) populations: the possible causes and mechanisms of changes in the sex ratio. Russian J Genet 44:786–792
Costa AM (2019) Reproductive cycle of the blue jack mackerel, Trachuru Spicturatus (Bowdich, 1825), off the Portuguese continental coast. Aquat Living Resour 32:14
Fadzli MH, Jaafar TNAM, Ali MS, Nur NFM, Tan MP, Piah RM (2022) Reproductive aspects of the coastal trevally, Carangoides coeruleopinnatus in Terengganu Waters. Malaysia Aquaculture Fisheries 7(5):500–506
Fakoya KA, Anetekha MA (2019) Macroscopic gonad staging and reproductive seasonality in the Gorean snapper, Lutjanus goreensis a gonochoristic west African lutjanid. West Afr J Appl Ecol 27(1):1–22
Fernandes JFF, Freitas J, de Araújo SA, de Santana TC, Lobato RS, Figueiredo MB (2022) Reproductive biology of the lane snapper, Lutjanus synagris (Linnaeus 1758) (Perciformes, Lutjanidae), in the Maranhão continental shelf, Northeast of Brazil. Environ Biol Fish 105(8):1033–1050
Froese R, Pauly D (2023) FishBase. World wide web electronic publication. www Fishbase org, (10/2023)
Fry G, Milton DA, Van Der Velde T, Stobutzki I, Andamari R, Badrudin, Sumiono B (2009) Reproductive dynamics and nursery habitat preferences of two commercially important Indo-Pacific red snappers Lutjanus erythropterus and L. Malabaricus. Fish Sci 75:145–158
Gonçalves P, Costa AM, Murta AG (2009) Estimates of batch fecundity and spawning fraction for the southern stock of horse mackerel (Trachurus trachurus) in ICES Division IXa. ICES J Mar Sci 66(4):617–622
Grandcourt EM, Al Abdessalaam TZ, Francis F (2006) Age, growth, mortality and reproduction of the blackspot snapper, Lutjanus fulviflamma (Forsskål, 1775), in the southern Arabian Gulf. Fish Res 78(2–3):203–210
Grimes CB (1987) Reproductive biology of the Lutjanidae: a review. In: Polovina JJ, Ralston S (eds) Tropical snapper and groupers: biology and fisheries management. Westview, Boulder, pp 239–294
Hammer O, Harper DAT, Ryan PD (2001) Past: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontologia Electronica 4:1–9
Hossain MY, Hossen MA, Islam MS, Jasmine S, Nawer F, Rahman MM (2017) Reproductive biology of Pethiaticto (Cyprinidae) from the Gorai River (SW Bangladesh). J Appl Ichthyol 33(5):1007–1014
Housh MJ, Telish J, Forsgren KL, Lema SC (2024) Fluctuating and stable high temperatures differentially affect reproductive endocrinology of female pupfish. Integr Organismal Biology 6(1):obae003
Islam MA, Akhter F, Islam MM, Zahangir MM (2021) Comprehensive study on the reproductive biology of black pomfret (Parastromateus niger) in the Bay of Bengal, Bangladesh. 41:101556Regional Studies in Marine Science
Jisr N, Younes G, Sukhn C, El-Dakdouki MH (2018) Length-weight relationships and relative condition factor of fish inhabiting the marine area of the Eastern Mediterranean city, Tripoli-Lebanon. Egypt J Aquat Res 44(4):299–305
Khatun D, Hossain M, Nawer F, Mostafa AA, Al-Askar AA (2019) Reproduction of Eutropiichthysvacha (Schilbeidae) in the Ganges River (NW Bangladesh) with special reference to potential influence of climate variability. Environ Sci Pollut Res 26(11):10800–10815
King M (2007) Fisheries biology, assessment and management. Blackwell Publishing Ltd., Oxford, UK, p 382
Kritzer JP (2004) Sex-specific growth and mortality, spawning season, and female maturation of the stripey bass (Lutjanus carponotatus) on the great barrier reef. Fish Bull 102:94–107
Kuriakose S (2014) Estimation of length weight relationship in fishes. Summer School on Advanced Methods for Fish Stock Assessment and Fisheries Management. Reprinted from the CMFRI, FRAD. Training Manual on Fish Stock Assessment and Management, p.150
Le Cren ED (1951) The length-weight relationship and seasonal cycle in gonad weight and condition in the perch (Perca fluviatilis). J Anim Ecol, 201–219
Lema SC, Luckenbach JA, Yamamoto Y, Housh MJ (2024) Fish reproduction in a warming world: vulnerable points in hormone regulation from sex determination to spawning. Philosophical Trans Royal Soc B: Biol Sci 379:20220516
Longenecker K, Langston R, Bolick H (2013) Rapid Reproductive Analysis and length-dependent relationships of Lutjanus biguttatus (Perciformes: Lutjanidae) from Papua New Guinea1. Pac Sci 67(2):295–301
Lucano-Ramírez G, Ruiz-Ramírez S, González-Sansón G, Ceballos-Vázquez BP (2014) Reproductive biology of the yellow snapper, Lutjanus argentiventris (Pisces, Lutjanidae), from the Mexican central Pacific. Ciencias Marinas 40(1):33–44
Lucano-Ramírez G, Ruiz-Ramírez S, Rojo-Vázquez JA, Lara-Mendoza RE, Aguilar-Betancourt CM, González-Sansón G (2023) Reproduction of Lutjanus guttatus (Perciformes: Lutjanidae) captured in the Mexican Central Pacific. Latin Am J Aquat Res 51(4):503–520
Marriott RJ, Mapstone BD, Begg GA (2007) Age-specific demographic parameters, and their implications for management of the red bass, Lutjanus bohar (Forsskal 1775): a large, long-lived reef fish. Fish Res 83(2–3):204–215
Mehanna SF, Baker TS, Soliman FM, Soliman HA (2017) Some biological aspects and population dynamics of the five-lined snapper, Lutjanus quinquelineatus (Family: Lutjanidae) from Red Sea off Hurghada, Egypt. Int J Fish Aquat Stud 5:321–326
Messias MA, Alves TI, Melo CM, Lima M, Rivera-Rebella C, Rodrigues DF, Madi RR (2019) Ethnoecology of Lutjanidae (snappers) in communities of artisanal fisheries in northeast Brazil. Ocean Coastal Manage 181:104866
Muchlisin ZA, Fransiska V, Muhammadar AA, Fauzi M, Batubara AS (2017) Length-weight relationships and condition factors of the three dominant species of marine fishes caught by traditional beach trawl in Ulelhee Bay, Banda Aceh City, Indonesia. Croatian J Fisheries 75(3):104–112
Nanami A, Kurihara T, Kurita Y, Aonuma Y, Suzuki N, Yamada H (2010a) Age, growth and reproduction of the humpback red snapper Lutjanus gibbus off Ishigaki Island, Okinawa. Ichthyol Res 57:240–244
Nanami A, Okuzawa K, Yamada H, Suzuki N, Aonuma Y (2010b) Reproductive activity in the checkered snapper, Lutjanus decussatus, off Ishigaki Island, Okinawa. Ichthyol Res 57(3):314–318
Oliveira MR, Nóbrega MF, Oliveira JEL, Chellappa S (2017) Reproductive Biology of Blue runner, Caranx crysos (Mitchell, 1815) from the Coastal Waters of Rio Grande do Norte, Brazil (Southwest Atlantic Ocean). J Aquac Mar Biol 5(6):00136
Pacicco AE, Brown-Peterson NJ, Murie DJ, Allman RJ, Snodgrass D, Franks JS (2023) Reproductive biology of yellowfin tuna (Thunnus albacares) in the northcentral US Gulf of Mexico. Fish Res 261:106620
Palla HP, Sotto FB (2021) Reproductive biology of brownstripe snapper Lutjanus vitta (Quoy and Gaimard, 1824) from West Sulu Sea, Philippines. Egypt J Aquat Res 47(1):67–73
Pradeep HD (2016), December Reproductive biology and histology of female bigeye snapper Lutjanus lutjanus (Bloch, 1790) off Madras coast along Southeast coast of India. In International Conference on Climate change adaptation and biodiversity: Ecological sustainability and resource management for livelihood security (ASA: ICCB-2016) (Vol. 8, p. 10)
Rahman MM, Ariffin NA, Seah YG, Jaafar TNAM, Habib A (2023) Length-weight relationships and relative condition factors of three coral-associated Lutjanus species from Terengganu waters of the South China Sea, Malaysia. Turkish J Zool 47(4):216–221
Rahman MM, Ariffin NA, Seah YG, Jaafar TNAM, Fadzli MH, Habib A (2024) Reproductive features of data-deficient yellowfin snapper, Lutjanus Xanthopinnis (Actinopterygii: Eupercaria: Lutjanidae), from east-coast of Peninsular Malaysia: implications for sustainable fisheries management. Acta Ichthyol Piscat 54:63–74
Rizzo E, Bazzoli N, Urbinati EC (2020) J Cyrino) pp. 287–313
Russell DJ, McDougall AJ, Fletcher AS, Ovenden JR, Street R (2003) Biology, Management and Genetic Stock Structure of Mangrove Jack, (Lutjanus argentimaculatus) in Australia, 1–189
Shimose T, Nanami A (2014) Age, growth, and reproductive biology of blacktail snapper, Lutjanus fulvus, around the Yaeyama Islands, Okinawa, Japan. Ichthyol Res 61:322–331
Shimose T, Nanami A (2015) Age, growth, and reproduction of blackspot snapper Lutjanus Fulviflammus (Forsskål 1775) around Yaeyama Islands, southern Japan, between 2010 and 2014. J Appl Ichthyol 31(6):1056–1063
Shimose T, Tachihara K (2005) Age, growth and maturation of the blackspot snapper Lutjanus fulviflammus around Okinawa Island, Japan. Fish Sci 71:48–55
Smoliński S, Berg F (2022) Varying relationships between fish length and scale size under changing environmental conditions–multidecadal perspective in Atlantic herring. Ecol Ind 134:108494
Sultana T, Mazumder SK, Kubra J, Nishad N, Khalil SMI, Das SK, Khan MAR, Hasan MT (2023) A multidisciplinary method to assess the reproductive biology of Mystus bleekeri. Aquaculture Fisheries 8(3):280–287
Teixeira SF, Duarte YF, Ferreira BP (2010) Reproduction of the fish Lutjanus analis (mutton snapper; Perciformes: Lutjanidae) from Northeastern Brazil. Rev Biol Trop 58(3):791–800
Thulasitha WS, Sivashanthini K (2013) Reproductive characteristics of doublespotted queenfish, Scomberoides lysan (Actinopterygii: Perciformes: Carangidae), from Sri Lankan waters: implications for fisheries management. Acta Ichthyol Piscat 43(1):7
Trejo-Martínez J, Brulé T, Mena‐Loría A, Colás‐Marrufo T, Sánchez‐Crespo M (2011) Reproductive aspects of the yellowtail snapper Ocyurus chrysurus from the southern Gulf of Mexico. J Fish Biol 79(4):915–936
Tsikliras AC, Stergiou KI, Froese R (2013) Editorial note on reproductive biology of fishes. Acta Ichthyol Piscat 43(1):1–5
Vandeputte M, Quillet E, Chatain B (2012) Are sex ratios in wild European sea bass (Dicentrarchus labrax) populations biased? Aquat Living Resour 25(1):77–81
Yaakob OMAR, Chau QP (2005) Weather downtime and its effect on fishing operation in Peninsular Malaysia, vol 42. JurnalTeknologi, pp 13–26. A
Acknowledgements
The authors sincerely thank Muhammad Haniff Bin Mohd Yusoff and Mohd Sharol Ali, Lab Officer at the Faculty of Fisheries and Food Science at Universiti Malaysia Terengganu, for assistance during lab work.
Funding
This work was funded by the Talent and Publication Enhancement-Research Grant (UMT/TAPE-RG2023/Vot 55484) sponsored by Universiti Malaysia Terengganu.
Author information
Authors and Affiliations
Contributions
Ying Giat Seah: Writing original draft & supervision; Md Moshiur Rahman: Writing original draft, formal analysis, field and lab work; Nur Asma Ariffin: Review & editing; Auni Nabila Kamrozaman: Filed and lab work, Review & editing; Tun Nurul Aimi Mat Jaafar: Review & editing; Mohammad Asmat Ullah: Review & editing; Ahasan Habib: Conceptualization, Data curation, Supervision, Fund acquisition, Review & editing.
Corresponding author
Ethics declarations
Ethical Statement
Dead specimens were collected from local fishermen. This species is not included on the IUCN’s threatened or endangered list. There was no need for a permit, and the study was not subject to any ethical restrictions. All authors have read, understood, and complied with the statement to the extent necessary.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Seah, Y.G., Rahman, M.M., Ariffin, N.A. et al. Reproductive Traits of Five-Line Snapper, Lutjanus quinquelineatus (Bloch, 1790) (Actinopterygii: Perciformes: Lutjanidae), from Southern South China Sea, Malaysia. Thalassas (2024). https://doi.org/10.1007/s41208-024-00748-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41208-024-00748-5