Abstract
Etroplus suratensis (Bloch, 1790) is a highly exploited endemic cichlid fish species inhabiting the Cochin Estuary situated on the South-west coast of India. The study was conducted with the sample of 599 specimens with TL ranged 67–249 mm and TBW ranged 7–280 g. Females were predominant in the catches and the overall sex ratio was 1.00 : 1.18, which significantly deviated from theoretical 1 : 1 distribution with 45.9% males and 54.09% females. The pattern of gonadosomatic index and proportion of mature individuals suggest two peaks in the spawning season of E. suratensis occurred between May to August and November to January. The length at 50% maturity for males and females was estimated at 167 and 169 mm TL respectively. The absolute fecundity estimates vary between 2147 and 4432 eggs with an average of 3112 and the relative fecundity fluctuated between 18 and 23 eggs g–1 with an average of 21. The results of the present study provide option to develop sustainable fishery management practices such as closed season, mesh size regulations and development of fishing sanctuaries to conserve this commercially important cichlid species.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Cichlids (Perciformes) are one of the most species-diverse group of vertebrates (nearly 3000 fish species) constituting medium-sized, fast-growing and omnivorous fishes that are shoal forming and highly territorial in behaviour (Martine and Kristina, 2013) distributed in tropical and sub-tropical waters of Africa, Central and South America, Southern India and Sri Lanka (Nelson, 2006). Cichlids are strikingly recognized for their tremendous phenotypic diversity, speed of evolution and complex social behaviour including extreme parental care other than their huge economic importance in aquarium trade and aquaculture utilities (Martine and Kristina, 2013). This family is represented by only three endemic fish species in Southern India and Sri Lanka: Etroplus suratensis, E. canarensis and Pseudetroplus maculatus.
Etroplus suratensis (Bloch, 1790) commonly called as “pearlspot”, is a euryhaline cichlid endemic to rivers, estuaries, coastal lagoons and in natural and man-made freshwater habitats of Southern India and Sri Lanka (Munro, 1955; Baensch and Riehl, 1985; Padmakumar et al., 2012). It is the largest Indian cichlid and owing to its economically important in the inland fishery of Peninsular India, it has been declared as the state fish of Kerala (Padmakumar et al., 2012). E. suratensis has gained enormous acceptance both as a delicious and costly edible fish (cost 10 US dollars per kg in the international markets) and an ideal aquaculture species both in and out of its natural range (Padmakumar et al., 2009). Though included in the Least Concern category by IUCN (International Union for Conservation of Nature), a recent population decline has been recorded in fishery of E. suratensis in the home range due to habitat degradation, overexploitation, pollution and invasion of exotic species (Padmakumar et al., 2009; Abraham et al., 2020).
Although E. suratensis is a locally abundant and commercially important fish species worldwide, only a few information is available on the reproductive strategy of the species (Jayaprakas and Nair, 1981; Ward and Samatakoon, 1981; Costa, 1983; Bindu and Padmakumar, 2014). The goal of the study is to investigate the reproductive biology aspects such as sex ratio, maturity stages, spawning season, gonadosomatic index (GSI), size at first maturity (L50) and fecundity of E. suratensis from Cochin estuary situated on the South-west coast of India.
MATERIALS AND METHODS
A total of 599 fish individuals (275 males and 324 females) were collected monthly from Cochin Estuary during June 2012 to May 2013. The specimen had been caught by commercial fishermen conducting gill net fishing (20–80 mm mesh size). The fish samples were preserved in ice box and transferred to the laboratory for further analysis. The total length (TL) (to the nearest 0.01 cm) was measured using Vernier Caliper and body weight (TBW) (to the nearest 0.01 g.) using digital balance for all the fish samples.
The fishes were then dissected out to determine the sex by visual inspection of condition of the gonads. The sex and stage of maturity of each specimen were ascertained by the micro and macroscopic observation of gonads. Maturity stages were grouped by the method proposed by Holden and Raitt (1974).
The following maturity stages were assessed in males and females.
Stage I (Immature). The ovaries are small, elongated, translucent and somewhat cylindrical with a reddish yellow hue and occupy less than a third of the body cavity. The ova are not distinct. Testes are long, thin, thread-like, translucent and light pinkish in hue.
Stage II (Developing). Ovaries slightly larger, pale yellow in colour occupying one third of the body cavity. Ova are distinct, which are spherical and partly opaque. Testes are slightly enlarged, opaque and flesh coloured.
Stage III (Ripening). Ovaries are larger, yellowish brown in colour and occupy half of the body cavity. Ova are quite distinct with a semi-transparent and distended ovarian wall. Testes are creamy white in colour and opaque.
Stage IV (Ripe). Ovaries are broader, distended, occupy more than half of the body cavity. Brownish with blood vessels and very distinct and larger ova, Testes are dull pinkish in hue. With gentle pressure milt oozes out.
Stage V (Spent). Ovaries are yellowish-brown, shrunken and flaccid with a few scattered blood patches. Testes are shrunken and dull reddish in colour.
Sex ratio was calculated on a monthly basis as the percentage of males to females (M : F) and a Chi-square (χ2) test was used to determine the significant differences in the frequency each sex (Snedecor and Cochran, 1967). GSI was calculated monthly for each fish according to Hossain and Ohtomi (2008) using the following formula: GSI = (gonad weight/body weight) ×100. This index was used to calculate development of gonad and spawning period of the fish. The size at L50 is defined as the length at which 50% of the population had reach sexual maturity and L50 was estimated using the ogive model (Rickey, 1995), M(L) = e(a+bL)/(1+e(a+bL)), where M(L) is the proportion of mature fishes, L—length class, and a and b are parameters (Remyamohan et al., 2018).
The fecundity was estimated from mature oocytes in both ovaries from a sample of 48 mature females during the spawning season. The ovaries of mature fishes were preserved in Gilson’s fluid to facilitate the separation of ova. The ovaries are weighted and from each of them sub-samples from the anterior, middle and posterior regions were weighed and the number of ova in each sub-sample was counted. Absolute fecundity was estimated by the gravimetric method using the following formula: F = nG/g, where F—absolute fecundity, n—number of eggs in the sub-sample, G— total weight of the ovary and g—weight of the sub-sample. Relative fecundity was estimated as the number of ova per unit of body weight (Bagenal and Braum, 1968).
RESULTS
Sex ratio. A total of 599 individuals (275 males and 324 females) of E. suratensis were collected with the TL ranged 67–249 mm and TBW ranged 7–280 g. The females TL ranged 76–249 mm and males range 67–246 mm. The overall sex ratio was 1.00:1.18, which significantly deviated from hypothetical distribution of 1 : 1 with 45.9% males and 54.09% females (Table 1). χ2 value was 4.01 (p < 0.01) indicating a significant difference between both the sexes. The month wise sex ratio indicated that females outnumbered males in most of the months except in August and May.
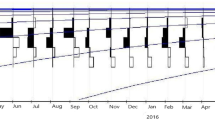
Spawning season. The fishes with immature (stage I) and developing (stage II) male present almost round year, although a high proportion of the fish in these stages were observed between February to October. Higher percentage of ripening fish (stage III) were observed from March to May and September to December. The ripe (stage IV) males have two peaks from May to August and November to January. The spent specimens (stage V) were detected from November to March and reappeared again in June–September (Fig. 1a). Immature (stage I) and developing (stage II) females of E. suratensis were present almost round the year, similar to their male counterpart. Ripening fishes (stage III) appeared in all months except in August and were recorded peaks during September to November and March to April. Ripe (stage IV) females were observed with its peaks from May to August and November to January. The spent females (stage V) appeared from November to March and June to September (Fig. 1b).
Gonadosomatic index (GSI). Monthly changes of GSI for male and female E. suratensis are presented in Fig. 2. GSI (as the indicator of gonadal development) showed monthly variations and followed a similar pattern in both the sexes, although overall GSI values for female were higher than that of males. The mean GSI for female were lowest in April and steadily increased to a peak in July. The GSI value showed a declining trend from September onwards and reached the lowest during October and after that the GSI value increased with a peak in January. The values of GSI were higher during the spawning season, indicating E. suratensis has two breeding peaks from May to August and November to January.
Size at first maturity. The proportion of matured ovaries and testes in relation to size were plotted to calculate size at first maturity. The smallest mature male and females were 130 and 141 mm in TL respectively. The size L50 was calculated as 167 mm TL in males (Fig. 3a) and 169 mm TL in females (Fig. 3b).
Fecundity. The fecundity was estimated from mature oocytes in both ovaries from a sample of 48 mature females ranging from 152–249 mm TL and with a GSI varied from 2.2 to 4.8 during the spawning season. The absolute fecundity varied from 2147–4432 eggs with an average of 3112 ova (Table 2). The relative fecundity varied between 18 and 23 eggs/g with the average of 21.
DISCUSSION
The M : F ratio of E. suratensis was recorded as 1.00 : 1.18 showing a significant variation from the expected 1 : 1 ratio with a feeble advantage for the females. The dominance of females of E. suratensis over males has been reported from Pulikat Lake and Veli Lake in India (Prasadam, 1971; Jayaprakas et al., 1990). According to Bindu and Padmakumar (2014), there is a dominance of male E. suratensis in freshwater reaches of Vembanad Lake with a male to female ratio of 1.00 : 0.80 which differs from the results of the present study.
The monthly distribution of maturity stages showed that ripe stage of male and female E. suratensis showed a peak in May and ended in August and then declined and again showed an increasing trend from November which ended up in January. This means that E. suratensis in Cochin Estuary has two peak spawning seasons from May to August and November to January which supports the earlier findings that this cichlid species tends to breed twice a year in tropical waters (Jayaprakas and Nair, 1981; Ward and Samarakoon, 1981; Bindu and Padmakumar, 2014). Bindu and Padmakumar (2014) discerned that E. suratensis is a multiple spawner exhibiting two breeding peaks in upstream reaches of Vembanad Lake during August–September and February–April which is slightly deviated from the results of the present study and this may be due to the difference in the environmental characteristics. Spawning behaviour in E. suratensis may vary from place to place depending upon the prevailing environmental conditions such as salinity, turbidity and tidal amplitude in an area (Jayaprakas and Nair, 1981; Ward and Samarakoon, 1981; Bindu and Padmakumar, 2014).
GSI is an important tool to identify the breeding season of fishes and GSI increase with maturity and decreases with the depletion of gonadal activity after spawning (Kumar et al., 2003; Jan et al., 2014). The development of GSI of female fishes is important because the ovarian tissue need higher energy allocation for gonadal development when compared to testes (Yin, 1993; Encina and Granado-Lorencio, 1997). In the present investigation the GSI values reached at peak in the month of May to August and November to January, which indicate the spawning period of E. suratensis. Thus, it has been revealed E. suratensis breeds twice in year with a seasonal reproductive period from May–August and November–January for both sexes that coincides with the South west and North east monsoon in Kerala state, India. Most of the factors triggering spawning in tropical fishes are supposed to be associated with onset of monsoon and flooding. Fishes are thought to be sensitive to the rising water levels (Alikunhi and Rao, 1951; Kulkarni, 1971) Habitat expansion in the rainy season leads to decreased crowding and predation pressure (Alkins-koo, 2000). Better food availability, high dissolved oxygen and optimum temperature during rainy season are the other reported factors influencing the spawning of fishes (Qasim and Qayyum, 1961; Hails and Abdullah, 1982). Costa (1981) suggested the GSI value of E. suratensis vary accordingly with prevailing environmental conditions. Jayaprakas and Nair (1981) reported that GSI of E. suratensis was highest values in April–May and October–November. Bindu and Padmakumar (2014) recorded the highest GSI during February–April and June–October in freshwater reaches of Vembanad Lake.
Size at first maturity is a crucial parameter in fisheries resource management and provides permissible mesh size in the fishing grounds for fish species (Renjithkumar et al., 2020). The present study recorded the size L50 of E. suratensis was 167 and 169 mm for males and females respectively indicating both males and females reach sexual maturity at almost the same TL. The present lengths were longer those reported by Jayaprakas and Nair (1981) from Veli Lake, India (140 and 144 mm for male and female fishes respectively). However, Bindu and Padmakumar (2014) reported that the fish attained L50 at 195 mm in males and 200 mm in females in freshwater reaches of Vembanad Lake. This difference may be attributed to prevailing environmental conditions or the characteristics of the individual population (Moyle and Cesh, 2004; Arula et al., 2017).
Information on fecundity of fishes is a crucial factor for successful fisheries management practices. The absolute fecundity of E. suratensis in the present study were ranged from 2147–4432 eggs with an average of 3112. Jayaprakas et al. (1990) estimated almost a similar value of fecundity for the fish (2108–3684 eggs) in Veli Lake, India. The variation in fecundity in fishes may depend on different factors such as size of the fish, water temperature, environmental condition, food availability and fishing (Bromage et al., 1990; Johnson et al., 1997). However, a high fecundity of E. suratensis was reported from fresh water reaches of Vembanad Lake that varied from 874 to 7554 eggs (Bindu and Padmakumar, 2014). In the present study, average relative fecundity was estimated to be 21 eggs/g. The relative fecundity in fishes are related to fish length, environmental factors and the conditions of the fish (Raitt and Hall, 1967). Bindu and Padmakumar (2014) found that in freshwater regions of Vembanad Lake, the relative fecundity of E. suratensis ranged between 4 to 51 eggs/g with an average of 16. The current reproductive characteristics provide various option for fishery managers such as spatial and temporal fishing closures to protect spawning fish, protection of immature fish via minimizing mesh size of fishing gear and creation of sanctuaries (non-fishing zone) in order to avoid the gradual collapse of this fish species in the immediate future.
REFERENCES
Abraham, R., De Alwis Goonatilake, S., Fernando, M., and Kotagama, O., Etroplus suratensis, in The IUCN Red List of Threatened Species, Version 04/ 2020, Glanz, 2019, no. e.T172368A60612143. https://doi.org/10.2305/IUCN.UK.20193.RLTS.T172368A60612143.en.
Alikunhi, K.H. and Rao, S.N., On the bionomics, development and growth of a Cauvery carp, Labeo kontius Jerdon, Rec. Indian Mus., 1951, vol. 49, pp. 157–174.
Alkins-Koo, M., Reproductive timing of fishes in a tropical intermittent stream, Environ. Biol. Fish., 2000, vol. 57, pp. 49–66. https://doi.org/10.1023/A:1007566609881
Arula, T., Shpilev, H., Raid, T., Vetemaa, M., and Albert, A., Maturation at a young age and small size of European smelt (Osmerus eperlanus): a consequence of population overexploitation or climate change? Helgol. Mar. Res., 2017, vol. 71, no. 7, pp. 1–9. https://doi.org/10.1186/s10152-017-0487-x
Baensch, H.A. and Riehl, R., Aquarien Atlas, Vol. 2: Mergus, Melle: Verlag Natur- Heimtierknd., 1985.
Bagenal, T.B. and Braum, E., Eggs and early life history, in Methods for Assessment of Fish Production in Fresh Waters, IBP Handb. Ser., no. 3, Ricker, W.E., Ed., Oxford: Blackwell, 1968, pp. 159–181.
Bindu, L. and Padmakumar, K.G., Reproductive biology of Etroplus suratensis from the Vembanad wetland system, Kerala, Indian J. Geo-Mar. Sci., 2014, vol. 43, pp. 646–654.
Bromage, N., Hardiman, P., Jones, J., Springate, J., and Bye, V., Fecundity, egg size and total egg volume differences in 12 stocks of rainbow trout, Oncorhynchus mykiss Richardson, Aquacult. Res., 1990, vol. 21, pp. 269–284. https://doi.org/10.1111/j.1365?2109.1990.tb00465.x
Costa, H.H., Biological studies of the pearl spot Etroplus suratensis (Pisces: Cichlidae) from three different habitats in Sri Lanka, Int. Rev. Gesamten Hydrobiol., 1983, vol. 68, pp. 565–580. https://doi.org/10.1002/iroh.19830680408
Encina, L. and Granado-Lorencio, C., Seasonal changes in condition, nutrition, gonad maturation and energy content in barbel, Barbus sclateri, inhabiting a fluctuating river, Environ. Biol. Fish., 1997, vol. 50, no. 1, pp. 75–84. https://doi.org/10.1023/A:1007381414397
Hails, A.J. and Abdullah, Z., Reproductive biology of the tropical fish Trichogaster pectoralis (Regan), J. Fish. Biol., 1982, vol. 21, pp. 157–170. https://doi.org/10.1111/j.1095-8649.1982.tb03996.x
Holden, M.J. and Raitt, D.F.S., Manual of Fisheries Science, Part 2: Methods of Resource Investigation and Their Application, Rome: UN Food Agric. Org., 1974.
Hossain, Md.Y. and Ohtomi, J., Reproductive biology of the Southern Rough Shrimp Trachysalambria curvirostris (Penaeidae) in Kagoshima Bay, Southern Japan, J. Crustacean Biol., 2008, vol. 28, no. 4, pp. 607–612. https://doi.org/10.1651/07-2970.1
Jan, M., Jan, U., and Shah, G.M., Studies on fecundity and gonadosomatic index of Schizothorax plagiostomus (Cypriniformes: Cyprinidae), J. Threatened Taxa, 2014, vol. 6, no. 1, pp. 1449–1455.
Jayaprakas, V. and Nair, N.B., Maturation and spawning in the pearl spot Etroplus suratensis (Bloch), Proc. Natl. Acad. Sci., India, Sect. B, 1981, vol. 47, no. 6, pp. 828–836.
Jayaprakas, V., Nair, N.B., and Padmanabhan, K.G., Sex ratio, fecundity and length weight relationship of the Indian pearlspot, Etroplus suratensis (Bloch), J. Aquacult. Tropics, 1990, vol. 5, no. 2, pp. 141–148.
Johnson, L.L., Sol, S.Y., Ylitalo, G.M., Hom, T., French, B., and Olson, O.P., Reproductive injury in English sole (Pleuronectes vetulus) from the Hylebos Waterway, Commencement Bay, Washington, J. Aquat. Ecosyst. Stress Recovery, 1997, vol. 6, pp. 289–310. https://doi.org/10.1023/A:1009986329935
Kulkarni, C. V., Spawning habits, eggs and early development of Deccan mahseer, Tor khudree (Sykes), J. Bombay Nat. Hist. Soc., 1971, vol. 67, no. 3, pp. 510–521.
Kumar, A., Singh, I.J., and Ram, R.N., Annual reproductive cycle of male rohu, Labeo rohita (Ham), in Tarai region of Uttaranchal, Indian J. Fish., 2003, vol. 50, pp. 231–241.
Maan, M.E. and Sefc, K.M., Colour variation in cichlid fish: developmental mechanisms, selective pressures and evolutionary consequences, Semin. Cell Dev. Biol., 2013, vol. 24, pp. 516–528. https://doi.org/10.1016/j.semcdb.2013.05.003
Moyle, P.B. and Cesh, J.J., Fishes: An Introduction to Ichthyology, Upper Saddle River, NJ: Prentice Hall, 2004.
Munro, I.S.R., The Marine and Fresh Water Fishes of Ceylon, Delhi: Biotech, 1955.
Nelson, J.S., Fishes of the World, New Jersey: Wiley, 2006.
Padmakumar, K.G., Bindu, L., and Manu, P.S., Captive breeding and seed production of Etroplus suratensis in controlled system, Asian Fish. Sci., 2009, vol. 22, pp. 51–60.
Padmakumar, K.G., Bindu, L., and Manu, P.G., Etroplus suratensis, the state fish of Kerala, J. Biosci., 2012, vol. 37, pp. 925–931. https://doi.org/10.1007/s12038-012-9271-x
Prasadam, R.D., Observations on the biology of the pearlspot Etroplus suratensis (Bloch) from the Pulicat Lake, Madras, J. Inland Fish. Soc. India, 1971, vol. 3, pp. 72–78
Qasim, S.Z. and Qayyum, A., Spawning frequencies and breeding seasons of some fresh water fishes with special reference to those occurring in the plains of Northern India, Indian J. Fish., 1961, vol. 8, no. 1, pp. 24–43.
Raitt, D.F.S. and Hall, W.B., On the fecundity of the red fish, Sebastes marinus (L.), ICES J. Mar. Sci., 1967, vol. 31, pp. 237–245.
Remyamohan, S., Harikrishnan, M., and Sherly, W.E., Reproductive biology of a Gobiid fish Oxyurichthys tentacularis (Valenciennes, 1837) inhabiting Ashtamudi Lake, S. India, J. Appl. Ichthyol., 2018, vol. 34, no. 5, pp. 1099–1107. https://doi.org/10.1111/jai.13739
Renjithkumar, C.R., Roshni, K., and Kurup, B.M., Reproductive biology of the endemic cyprinid fish Hypselobarbus thomassi (Day, 1874) from Kallada River in the Western Ghats, India, J. Appl. Ichthyol., 2020, vol. 36, no. 5, pp. 604–612. https://doi.org/10.1111/jai.14064
Rickey, M.H., Maturity spawning and movement of arrow tooth flounder, Arthereshes stomias, off Washington, Fish. Bull., 1995, vol. 93, pp. 127–138.
Snedecor, G.W., and Cochran, W.G., Statistical Methods, Ames, IA: Iowa State Univ. Press, 1967.
Ward, J.A. and Samarakoon, J.I., Reproductive tactics of the Asian cichlids of the genus Etroplus in Sril Lanka, Environ. Biol. Fish., 1981, vol. 6, no. 1, pp. 95–103. https://doi.org/10.1007/BF00001803
Yin, M., Ecology of Fishes, Beijing: Chin. Agric. Press, 1993.
ACKNOWLEDGMENTS
The authors are grateful to the Director, School of Industrial Fisheries, Cochin University of Science and Technology, Kochi, India for providing all necessary facilities for the research work. The authors are also thankful to local fishers for assisting in collection of fish samples.
Funding
The first author acknowledges the funding support of the Inspire Programme of the Department of Science and Technology (DST), Ministry of Science and Technology, India.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests. The authors declare that they have no conflicts of interest.
Statement on the welfare of animals. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Roshni, K., Renjithkumar, C.R. & Kurup, B.M. Reproductive Biology of the Endemic Fish Etroplus suratensis (Cichlidae) from a Tropical Estuary in Southern India. J. Ichthyol. 61, 460–466 (2021). https://doi.org/10.1134/S0032945221030097
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0032945221030097




 )—I, (
)—I, ( )—II, (
)—II, ( )—III, (
)—III, ( )—IV, (
)—IV, ( )—V.
)—V.
