Abstract
The checkered snapper, Lutjanus decussatus, is an important species for fisheries in the Okinawan region. This study estimated the reproduction of this lutjanid species in the waters around Ishigaki Island. The main spawning season was estimated to be between June and October, since oocytes at the maturation stage and/or postovulatory follicles were found during the study period. In the main spawning season, high gonadosomatic index values were found around the time of the last quarter moon for each month from June to September. It is suggested that L. decussatus is a lunar-synchronized spawner off Ishigaki Island.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Snappers (Lutjanidae) are one of the important fisheries resources in tropical and subtropical waters such as the Caribbean and Indo-Pacific waters (Allen 1985). Several previous studies have clarified their spawning, settlement, feeding habits, age and growth (e.g., Newman et al. 2000a, b; Denit and Sponaugle 2004; Kritzer 2004; Heyman et al. 2005; Shimose and Tachihara 2005; Amezcua et al. 2006; Nanami and Yamada 2009). The spawning season in lutjanid species has been reported in several study sites (Grimes 1987; Kaunda-Arara and Ntiba 1997; Shimose and Tachihara 2005; Fry et al. 2009). Another aspect of fish reproduction is their lunar-synchronized gonadal development, which is common in coral reef fishes (Johannes 1978). Lunar-synchronized spawning has been reported for sciaenids (Aalbers 2008), serranids (Lee et al. 2002), siganids (Hoque et al. 1999; Harahap et al. 2001) and sparids (Saavedra and Pousao-Ferreira 2006). For lutjanid species, Heyman et al. (2005) demonstrated a lunar-synchronized spawning aggregation for Lutjanus cyanopterus on the Belize Barrier Reef. However, no studies have demonstrated lunar-synchronized reproductive activities for lutjanid species in Okinawan coral reefs.
The checkered snapper, Lutjanus decussatus, is one of the lutjanid species that is widely distributed in the western Pacific and eastern Indian Ocean (Allen 1985). The species is an important fisheries target in the Okinawan region (Masuda et al. 1984). Although some previous studies clarified their home range size and feeding behavior (Nanami and Yamada 2008a, b), little is known regarding the reproduction. The purpose of this study is to describe the seasonal and lunar-related changes in oocyte development for L. decussatus off Ishigaki Island, Okinawa.
Materials and methods
Biological data were collected from specimens purchased from commercial catches made off the coast of the Ishigaki Island, Okinawa, Japan (24°27′N, 124°13′E) between April 2007 and April 2008. Samples were obtained every week in order to be consistent with lunar cycles (new moon, first quarter moon, full moon and last quarter moon) except in bad weather conditions. A total of 172 female specimens were measured for fork length (FL, nearest 0.05 cm), whole body weight (g) and gonad (ovary) weight (nearest 0.01 g). Ovaries were removed from the specimens and preserved in 20% buffered formalin.
The gonadosomatic index (GSI) for females was calculated using the formula: gonad weight (g)/[whole weight (g) − gonad weight (g)] × 100. In order to clarify the temporal changes of oocyte development, small pieces of the ovaries were placed in 20% buffered formalin for 48 h and then kept in 70% ethanol. After dehydration using a series of ethanol, they were embedded in paraffin (m.p. 56–58°C, Histologie; Merck, Darmstadt, Germany). Embedded tissues were serially sectioned at 5–10 μm and stained with Mayer’s hematoxylin-eosin for microscopic observations. Oocyte development was classified as peri-nucleolus stage, oil-droplet stage, primary yolk stage, secondary yolk stage, tertiary yolk stage and maturation stage in accordance with Harahap et al. (2001) and Shimose and Tachihara (2006). Postovulatory follicles were also recorded. For analysis of oocyte composition, the most developed stage in the oocytes was regarded as the representation of the developmental stage of the ovary.
The spawning frequency was estimated in accordance with Ashida et al. (2008). In this estimation, the samples were obtained at the last quarter moon in June and August since the samples could be regarded as matured individuals with postovulatory follicles (see “Results”). The fecundity was estimated by using ten females with matured oocytes (see “Results”). For each ovary, five small pieces of the ovary (about 30 mg) were extracted. The oocytes of ≥0.4 mm in diameter were regarded as matured oocytes and counted under a Nikon profile projector at 10× magnification. Then, the fecundity was estimated in accordance with Murua et al. (2003).
Results
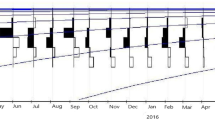
Figure 1a indicates the monthly changes in female GSI. The GSI was generally higher between April and October (GSI > 1.0) and lower between November and March (GSI < 1.0). Monthly changes in oocyte development and histological sections of ovaries are shown in Figs. 1b and 2, respectively. The tertiary yolk stage was observed during April and October. Since oocytes at the maturation stage were observed in June, September and October, and the postovulatory follicles were observed between June and August (Fig. 1b), the main spawning season was estimated to be between June and October. In contrast, only the peri-nucleolus stage and the oil-droplet stage were found between November and March.
Monthly changes in gonadosomatic index (GSI) ± SE (a) and oocyte composition (b) of female Lutjanus decussatus. Numbers above plots represent sample sizes. Since biological data were collected every week, monthly data were obtained from average values of weekly data. PNS peri-nucleolus stage, ODS oil-droplet stage, PYS primary yolk stage, SYS secondary yolk stage, TYS tertiary yolk stage, MA maturation stage. Numbers in parentheses indicate numbers of ovaries with the postovulatory follicles
Cross-section of ovaries in Lutjanus decussatus. Three stages in ovaries (peri-nucleolus stage, oil-droplet stage and primary yolk stage) (a); two stages in ovaries (secondary yolk stage and tertiary yolk stage) (b); maturation stage (c); postovulatory follicles (d). PNS peri-nucleolus stage, ODS oil-droplet stage, PYS primary yolk stage, SYS secondary yolk stage, TYS tertiary yolk stage, MA maturation stage, POF postovulatory follicles. Bar 100 μm
Clear lunar-synchronized GSI fluctuations of females were found during the main spawning season (June–October) (Fig. 3a), showing the highest GSI values around the last quarter moon of each month between June and September. In contrast, the highest GSI value was found around the new moon in October (Fig. 3a). Observations of oocyte development also support that the lunar-synchronized GSI fluctuation is related with spawning (Fig. 3b), since oocytes at the maturation stage were observed at the highest GSI values in June, September and October (Fig. 3b). Although oocytes at the maturation stage were not observed at the highest GSI values in July and August, the postovulatory follicles were observed in these 2 months (Fig. 3b).
Weekly changes in gonadosomatic index (GSI) ± SE (a) and oocyte composition (b) of female Lutjanus decussatus. Numbers above plots represent sample sizes. PNS peri-nucleolus stage, ODS oil-droplet stage, PYS primary yolk stage, SYS secondary yolk stage, TYS tertiary yolk stage, MA maturation stage. In a, lunar phase (last quarter moon) is indicated. Dotted line represents no samples as the typhoon approached Ishigaki Island. Numbers in parentheses indicate numbers of ovaries with the postovulatory follicles. In b, “P” indicates the existence of the postovulatory follicles
Spawning frequency ranged from 0.14 (August) to 0.22 (June). Fecundity ranged from 105,169 to 541,159 oocytes (average = 301,736 oocytes ± 42,234 SE, n = 10) in fish of 25.5 to 29.4 cm FL.
Discussion
It is suggested that the main spawning season of Lutjanus decussatus is between June and October (during 5 months). Several previous studies have demonstrated that gonadal development and/or spawning for lutjanid species occurs over a period of several months (Heyman et al. 2005; Shimose and Tachihara 2005). Grimes (1987) showed that the seasonality of reproduction for lutjanid species has two patterns: (1) a restricted season centered around summer and (2) more or less continuous year-round spawning with peaks of reproductive activity in the spring and fall. The results of the present study are consistent with the former case.
Lunar-synchronized spawning of L. decussatus was also suggested since the GSI values of females were highest around the last quarter moon between June and September. In contrast, the spawning of four lutjanid species (Lutjanus argentimaculatus, Lutjanus cyanopterus, Lutjanus fulvus and Lutjanus griseus) occurred around the full moon in some tropical regions (Johannes 1978; Heyman et al. 2005), suggesting that the type of lunar periodicity of spawning is species-specific for lutjanid species. Some other coral reef fish species, such as siganids and serranids, also show lunar-synchronized spawning (Johannes 1978; Hoque et al. 1999; Harahap et al. 2001; Lee et al. 2002; Park et al. 2006). Johannes (1978) showed that the majority of tropical fishes that have lunar spawning rhythms spawn on or around the new or full moon. Grimes (1987) also showed that spawning of lutjanid species occurs around the new or full moon. However, some coral reef fishes have spawning rhythms around the first quarter moon (Hara et al. 1986) or last quarter moon (Johannes 1978). Although it is unclear what types of environmental cues act as stimuli for L. decussatus, water temperature, photoperiod and lunar cycle are reported to be cues of ovarian development for lutjanids (Grimes 1987). The highest GSI in October was found around the new moon; however, this might have been caused by the effects of the typhoon delaying the ovarian development.
Several lutjanid species show spawning aggregations at specific sites and/or lunar phases (Heyman et al. 2005). Such spawning aggregations are often targeted by commercial and recreational fisheries (Colin et al. 2003). Although it is not clear whether L. decussatus aggregates at specific sites for spawning, the species might form spawning aggregations since a lunar-synchronized ovarian development was found. If so, protection of spawning aggregations and/or conservation of spawning sites would also be useful for effective fishery management strategies for this species.
References
Aalbers SA (2008) Seasonal, diel, and lunar spawning periodicities and associated sound production of white seabass (Atractoscion nobilis). Fish Bull 106:143–151
Allen GR (1985) FAO species catalogue. Vol. 6: snappers of the world. An annotated and illustrated catalogue of lutjanid species known to date. FAO, Rome, p 208
Amezcua F, Soto-Avila C, Green-Ruiz Y (2006) Age, growth, and mortality of the spotted rose snapper Lutjanus guttatus from the southeastern Gulf of California. Fish Res 77:293–300
Ashida H, Tanabe T, Suzuki N, Fukui A, Tanaka S (2008) Spawning frequency and batch fecundity of skipjack tuna Katsuwonus pelamis in the tropical west-central Pacific Ocean. Nippon Suisan Gakkaishi 74:802–808
Colin PL, Sadovy YJ, Domeier ML (2003) Manual for the study and conservation of reef fish spawning aggregations. Society for the conservation of reef fish aggregations special publication No. 1 (version 1.0), pp 1–98 + iii
Denit K, Sponaugle S (2004) Growth variation, settlement, and spawning of gray snapper across a latitudinal gradient. Trans Am Fish Soc 133:1339–1355
Fry G, Milton DA, Van Der Velde T, Stobutzki I, Andamari R, Badrudin, Sumiono B (2009) Reproductive dynamics and nursery habitat preferences of two commercially important Indo-Pacific red snappers Lutjanus erythropterus and L. malabaricus. Fish Sci 75:145–158
Grimes CB (1987) Reproductive biology of the Lutjanidae: a review. In: Polovina JJ, Ralston S (eds) Tropical snapper and groupers: biology and fisheries management. Westview, Boulder, pp 239–294
Hara S, Duray MN, Parazo M, Taki Y (1986) Year-round spawning and seed production of the rabbitfish, Siganus guttatus. Aquaculture 59:259–272
Harahap AP, Takemura A, Nakamura S, Rahman MS, Takano K (2001) Histological evidence of lunar-synchronized ovarian development and spawning in the spiny rabbitfish Siganus spinus (Linnaeus) around the Ryukyus. Fish Sci 67:888–893
Heyman WD, Kjerfve B, Graham RT, Rhodes KL, Garbutt L (2005) Spawning aggregations of Lutjanus cyanopterus (Cuvier) on the Belize Barrier Reef over a 6 year period. J Fish Biol 67:83–101
Hoque MM, Takemura A, Matsuyama M, Matsuura S, Takano K (1999) Lunar spawning in Siganus canaliculatus. J Fish Biol 55:1213–1222
Johannes RE (1978) Reproductive strategies of coastal marine fishes in the tropics. Environ Biol Fish 3:65–84
Kaunda-Arara B, Ntiba MJ (1997) The reproductive biology of Lutjanus fulviflamma (Forsskål, 1775) (Pisces: Lutjanidae) in Kenyan inshore marine waters. Hydrobiologia 353:153–160
Kritzer JP (2004) Sex-specific growth and mortality, spawning season, and female maturation of the stripey bass (Lutjanus carponotatus) on the Great Barrier Reef. Fish Bull 102:94–107
Lee YD, Park SH, Takemura A, Takano K (2002) Histological observations of seasonal reproductive and lunar-related spawning cycles in the female honeycomb grouper Epinephelus merra in Okinawan waters. Fish Sci 68:872–877
Masuda H, Amaoka K, Araga C, Uyeno T, Yoshino T (1984) The fishes of the Japanese archipelago. Tokai University Press, Tokyo
Murua H, Kraus G, Saborido-Rey F, Witthames PR, Thorsen A, Junquera S (2003) Procedures of estimate fecundity of marine fish species in relation to their reproductive strategy. J Northw Atl Fish Sci 33:33–54
Nanami A, Yamada H (2008a) Size and spatial arrangement of home range of checkered snapper Lutjanus decussatus (Lutjanidae) in an Okinawan coral reef determined using a portable GPS receiver. Mar Biol 153:1103–1111
Nanami A, Yamada H (2008b) Foraging rates and substrate selection in foraging activity of checkered snapper Lutjanus decussatus (Lutjanidae) in an Okinawan coral reef. J Fish Biol 73:1484–1488
Nanami A, Yamada H (2009) Seasonality, lunar periodicity of settlement and microhabitat association of juvenile humpback red snapper Lutjanus gibbus (Lutjanidae) in an Okinawan coral reef. Mar Biol 156:407–414
Newman SJ, Cappo M, Williams DMcB (2000a) Age, growth and mortality of the stripey, Lutjanus carponotatus (Richardson) and the brown-stripe snapper, L. vitta (Quoy and Gaimard) from the central great Barrier Reef, Australia. Fish Res 48:263–275
Newman SJ, Cappo M, Williams DMcB (2000b) Age, growth, mortality rates and corresponding yield estimates using otoliths of the tropical red snappers, Lutjanus erythropterus, L. malabaricus and L. sebae, from the central Great Barrier Reef. Fish Res 48:1–14
Park YJ, Takemura A, Lee YD (2006) Lunar-synchronized reproductive activity in the pencil-streaked rabbitfish Siganus doliatus in the Chuuk Lagoon, Micronesia. Ichthyol Res 53:179–181
Saavedra M, Pousao-Ferreira P (2006) A preliminary study on the effect of lunar cycles on the spawning behaviour of the gilt-head sea bream, Sparus aurata. J Mar Biol Assoc UK 86:899–901
Shimose T, Tachihara K (2005) Age, growth and maturation of the blackspot snapper Lutjanus fulviflammus around Okinawa Island, Japan. Fish Sci 71:48–55
Shimose T, Tachihara K (2006) Age, growth, and reproductive biology of the Waigieu seaperch Psammoperca waigiensis (Perciformes: Latidae) around Okinawa Island, Japan. Ichthyol Res 53:166–171
Acknowledgments
We express our grateful thanks to S. Sakihara and T. Sakihara for their assistance in collecting samples, M. Mukai and S. Mamiya for the laboratory work, and the staff of Ishigaki Tropical Station, Seikai National Fisheries Research Institute for their support in the present study. Constructive comments on the manuscript from C. P. Norman, K. Yoseda and two anonymous reviewers were much appreciated. We also thank the Yaeyama Fishermen’s Cooperative Association for permission to collect the samples.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Nanami, A., Okuzawa, K., Yamada, H. et al. Reproductive activity in the checkered snapper, Lutjanus decussatus, off Ishigaki Island, Okinawa. Ichthyol Res 57, 314–318 (2010). https://doi.org/10.1007/s10228-010-0155-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10228-010-0155-5