Abstract
Purpose of Review
Our ability to detect effects of the landscape context on wildlife often depends on the spatial scale at which environmental variables are measured. Theory suggests that the scale at which the species most strongly responds to this context—its scale of effect—should depend on the type of biological response. This is hypothesized to occur because the temporal scale regulating the response is linked to the spatial scale at which the species interacts with its environment; i.e. the scale of effect should be larger for responses influenced by forces acting over longer time periods. Here I test the prediction that the scale of effect increases in the order: fecundity < abundance < occurrence < genetic diversity, using a quantitative review of studies that empirically estimated scales of effect.
Recent Findings
The scale of effect of a given environmental variable depended on the type of response in 70% of the 145 cases identified in this review. However, scales of effect did not increase in the predicted order. This is likely, at least in part, because some studies did not include a wide enough range of scales in their analyses to accurately estimate the scales of effect.
Summary
Future research is needed to test this prediction using study designs that allow for accurate estimation of scales of effect. Nevertheless, my results have implications for wildlife research and landscape management, suggesting that we cannot assume that a species responds to its landscape context at only one scale.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The landscape context is often important for understanding the effects of environmental variables (e.g. amount of forest cover, road density) on a biological response (e.g. abundance) [1]. However, our ability to detect those effects can strongly depend on the spatial scale at which environmental variables are measured (i.e. the landscape size or spatial extent). Empirical evidence suggests that the strength of the relationship between an environmental variable and a biological response varies with the scale of measurement, and significant relationships are often detected at only certain scales [2,3,4,5]. This suggests that our ability to detect an effect of the landscape context depends on whether we select the appropriate scale of measurement.
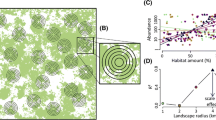
We do not usually know the actual scale at which a species responds to the landscape context. In the absence of this information we must rely on empirical methods to estimate the appropriate scale of measurement. Researchers typically estimate the appropriate scale in a ‘multi-scale study’ (Fig. 1) by: (1) measuring the response at multiple sampling sites within the study area; (2) measuring a given environmental variable at multiple scales, represented by different-sized square or circular areas centered on each sampling site; (3) estimating strength of effect of that environmental variable on the response (e.g. r2 from a linear regression model) at each scale; and (4) comparing the strength of relationship across scales, and selecting the scale at which the relationship is strongest. The scale where the relationship is strongest is often called the ‘scale of effect’ [5, 6].
Typical design of a multi-scale study, used to empirically estimate the scale at which a species responds to the landscape context. (a) The response (e.g. abundance) is measured at multiple sampling sites within the study area. (b) The environmental variable of interest (e.g. the proportion of the landscape in forest) is measured at multiple scales, represented by different-sized circular or square areas centered on each sampling site. (c) The strength of effect of that environmental variable on the response (e.g. r2 from a linear regression model) is measured at each scale. (d) The strength of relationship is compared across scales, and the scale of effect is designated as the scale at which the relationship is strongest
Different species tend to respond to the landscape context at different scales [2,3,4,5], and there have been a number of hypotheses proposed to explain why the scale of effect varies among species. The most prominent of these predict that the appropriate scale of measurement increases with the species’ home range/territory size, and that the scale increases with the species’ dispersal distance [7••]. Indeed, when researchers justify a species-specific choice of scale, it is based on its mobility [8•]. This assumes that the scale at which a species responds to the landscape context is related to the scale at which it perceives and interacts with the landscape.
Although there is some theoretical and empirical evidence to support the suggestion that the scale at which a species responds to environmental variables depends on its mobility (as reviewed in [7••]), this is clearly not the only factor that determines the scale of effect. For example, empirical studies have also shown that different environmental variables affect a given species’ response at different scales [9,10,11]. This idea that species respond to different environmental variables at different scales has been particularly influential in the field of habitat suitability modeling [12].
Although the influence of the environmental variable type on the scale of effect has been well documented, few have considered that the scale may also depend on the type of biological response (e.g. fecundity vs. abundance). For example, in their review of landscape ecology studies, Mayer and Cameron [13] found no evidence that authors considered the type of biological response when selecting the scale of measurement for environmental variables. However, simulation modeling supports the prediction that the scale of effect of a given environmental variable varies with the type of biological response measured for a given species (or species group) [14••].
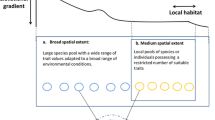
Furthermore, Jackson and Fahrig [14••] hypothesized that the scale of effect of a given environmental variable should be larger for biological responses that are influenced by forces acting over longer temporal scales than for biological responses influenced by forces acting over shorter temporal scales. This is based on the assumed link between the temporal scale regulating the biological response and the spatial scale at which the species interacts with its environment over that temporal scale. For example, fecundity is positively influenced by the availability of resources during and immediately before the breeding period [15, 16]. Thus, environmental variables likely have their strongest effects on fecundity at a small spatial extent, i.e. within the foraging habitat (Fig. 2a). In contrast, abundance at a given location is influenced not only by effects of local environmental conditions on birth and death rates, but also by the rates of immigration of individuals to that location. Thus the scale of effect for abundance should encompass the environmental context encountered by dispersers moving to that location (Fig. 2b). Occurrence, which depends on cumulative extinction and colonization events over a number of generations, and genetic diversity, which accumulates genetic contributions of past immigrants to the local gene pool, should have even larger scales of effect that encompass the environmental context experienced by multiple generations of dispersers (Fig. 2c-d). In summary, this leads to the prediction that the spatial scale of effect of a given environmental variable on a species’ biological response should increase in the following order: fecundity < abundance < occurrence < genetic diversity (as predicted by [7••]).
The scale of effect is predicted to increase in the following order: fecundity < abundance < occurrence < genetic diversity. (a) Fecundity is influenced by the availability of resources during/immediately before the breeding period and thus, environmental variables likely have their strongest effects on fecundity within the foraging habitat. (b) Abundance at a given location is influenced not only by effects of local environmental conditions on birth/death rates, but also by the rates of immigration of individuals to that location. Thus the scale of effect for abundance should encompass the environmental context encountered by dispersers moving to that location in a given season. (c) Occurrence depends on cumulative extinction and colonization events over a number of generations, and thus should have a larger scale of effect that encompasses the environmental context experienced by several generations of dispersers. (d) Genetic diversity accumulates genetic contributions of past immigrants to the local gene pool, and thus should have a scale of effect that encompasses the environmental context experienced by many generations of dispersers
In this paper I used a quantitative review of published multi-scale studies to address the following three questions:
-
(1)
Does the scale of effect of a given environmental variable depend on the type of biological response measured for a given species?
-
(2)
If the scale of effect does differ among response types, does it vary as predicted, i.e. does the scale of effect increase in the order: fecundity < abundance < occurrence < genetic diversity?
-
(3)
Does the degree of consistency with the above prediction depend on the design of the study to select the scale of effect? I include this final question because prior meta-analysis has shown that empirical estimates of the scales of effect can be strongly dependent on the design of the study to select those scales [8•]. If the degree of consistency with the above prediction depends on attributes of the study design known to (or suspected to) decrease the accuracy of scale selection, then these aspects of the study design could be obscuring differences in scales of effect driven by the biological response. In this case, the results of this analysis could be used to make recommendations to improve the design of future studies that test for differences in scales of effect between different biological response types.
Methods
Literature Search and Study Selection
I searched ISI Web of Science (Clarivate Analytics, USA; accessed December 2, 2016) for multi-scale studies that evaluated relationships between a given environmental variable and at least two of the four biological responses: fecundity, abundance, occurrence, genetic diversity. To identify multi-scale studies I used the following search string: buffer* OR “focal patch*” OR “focal point*” OR “landscape area*” OR “landscape size*” OR “spatial scale*” OR “spatial extent*”. To identify the subset of multi-scale studies that included at least two of the four biological responses, I combined the previous search string with all pairwise combinations of the following search strings for the four biological responses (fecundity AND abundance, fecundity AND occurrence, fecundity AND genetic diversity, etc.):
-
(1)
fecundity: fecundity OR reproduct*
-
(2)
abundance: abundance OR cover OR density OR prevalence
-
(3)
occurrence: incidence OR occupancy OR occurrence OR presence
-
(4)
genetic diversity: genetic*
I further limited each search to English-language articles published in the past 10 years (2006–2016) in the Environmental Sciences & Ecology subject area.
I reviewed the methods section of each article identified in my literature search, using the following criteria (modified from [8•]) to identify the relevant subset of articles. I included only articles that met the following seven criteria:
-
(1)
At least one environmental variable was measured at two or more scales around a central sampling site, so that a scale of effect could be selected using a multi-scale study design (Fig. 1).
-
(2)
The same scales (e.g. 0.5, 1.0, and 1.5 km) were used to estimate environmental variable values for all sample locations within a given study. For example, I excluded studies that measured environmental variables in a patch, landscape, and region, because the sizes of these vary among sample locations.
-
(3)
The source data (e.g. classified land cover map) for the environmental variable measurements was the same for all scales.
-
(4)
The resolution (or grain) of the data used to measure the environmental variable was the same for all scales, because the spatial grain of an environmental variable can also affect its relationship with a biological response [7••, 17].
-
(5)
The environmental variable was measured within the bounds of a given spatial extent (e.g. proportion of the area in wetland), rather than measuring the distance to a landscape feature (e.g. distance to the nearest wetland).
-
(6)
The study looked at the relationship between a given environmental variable (measured at two or more scales; see criterion 1) and at least two of the four target biological responses (fecundity, abundance, occurrence, genetic diversity), for at least one species or species group. For example, a study that looked at the relationship between forest amount within 0.5, 1.0, and 1.5 km and occurrence was included only if it also looked at the relationship between forest amount within 0.5, 1.0, and 1.5 km and the fecundity, abundance, or genetic diversity of the same species (or species group). Species richness was included as a multi-species measure of occurrence, but only if the other biological response(s) were aggregated over the same set of species (e.g. bat species richness vs. total bat abundance).
-
(7)
The size of the sample area was the same for each biological response in the study, and did not co-vary with the scale of measurement of the environmental variable.
Data Extraction for Quantitative Review
For each article that met the criteria defined above, I extracted the scale of effect reported by the author(s), or from tables or graphs in the study, for each relationship between a biological response and environmental variable that was measured at multiple scales. The scale of effect was typically estimated as the single scale at which the relationship between the response and environmental variable was strongest, selected from the set of possible scales tested by the author(s). The strength of relationship was typically measured as the lowest Akaike Information Criterion (AIC) value, highest r2, or largest │correlation coefficient│ (see Online Resource 1 for a full list of metrics). If the strength of relationship was identical at ≥ 2 scales, I recorded the mean scale of effect. For studies using a circular landscape, the scale was measured as the radius of that circular landscape. For square landscapes the scale was half the width of the square. I also classified whether the relationship was scale-sensitive or not. A relationship was scale-sensitive if the selected scale of effect improved model fit relative to at least one other tested scale (e.g. ΔAIC > 2, Δr2 > 0.01; Online Resource 1), or if the relationship at the selected scale was significantly better than the relationship at at least one other tested scale, in a statistical test performed by the author(s) (as in [8•]). Note that in some cases I was unable to assess whether a relationship was scale-sensitive, for example, because the authors included estimates of model fit for only the most supported scale. I classified the scale sensitivity in these cases as ‘unknown’.
I also recorded attributes of the study design to select the scale of effect, i.e. the smallest assessed scale, largest assessed scale, distance between adjacent scales, number of scales, and approach to scale selection. In cases where the distance between adjacent scales was not constant across all scales, I used the mean distance between adjacent scales. For example, if the author(s) tested relationships at 0.5, 1, 5, and 10 km scales, I recorded the distance as 3.2 km (averaging the distances between adjacent scales: 0.5, 4, and 5 km). I classified papers as using one of three approaches to scale selection. First, a common scale of effect could be estimated for all environmental variables by simultaneously modeling the relationships between a biological response and all environmental variables measured at the same scale (e.g. by multiple regression), repeated for each potential scale. The scale with the strongest overall model fit was then selected as the scale of effect (hereafter called the ‘multivariate-single-scale’ approach). Because the same scale was selected for all environmental variables based on the strength of the combined effect of these variables on the response, I recorded the selected scale only once, rather than including the same scale for each individual environmental variable. Second, a scale of effect could be estimated simultaneously for all environmental variables but without assuming a common scale of effect (the ‘multivariate-multi-scale’ approach). This was done by comparing the strengths of the multivariate relationships between the biological response and all environmental variables as described above, with the exception that here they compared among all possible combinations of the tested scales. For example, if authors were testing for effects of road density and forest amount on species richness at 1 and 2 km, they would model species richness as a function of: (1) roads(1 km) + forest(1 km), (2) roads(1 km) + forest(2 km), (3) roads(2 km) + forest(1 km), and (4) roads(2 km) + forest(2 km). Third, a scale of effect could be estimated independently for each environmental variable measured at the landscape scale by modeling the relationship between the biological response and each individual variable at every scale and selecting the scale at which the relationship was strongest for each variable (the ‘univariate’ approach). Studies that included only one environmental variable were included in this final category. I also recorded the study taxa (e.g. mammal, bird, amphibian) and the number of sampling sites included in the analysis.
Statistical Analysis
No studies estimated scales of effect for more than two biological responses for a given species (or species group) and environmental predictor. Therefore, to test my first prediction I used a Χ2 test of association to compare the number of times I found different scales of effect of a given environmental variable on a pair of biological responses to the number of times I found the same scale of effect. The prediction that the scale of effect of a given environmental variable depends on the biological response would be supported if different scales of effect were found significantly more often than the same scale of effect, at α = 0.05.
Second, I used a Χ2 test of association to test the prediction that scales of effect of a given environmental variable should increase in the following order: fecundity < abundance < occurrence < genetic diversity. Specifically, I compared the number of times I found that the difference in the scales of effect of a given environmental variable on a pair of response variables was in the predicted direction versus the number of times the difference was in the opposite direction. The prediction that the scale of effect increases in the order fecundity < abundance < occurrence < genetic diversity would be supported if differences in the scales of effect of a given environmental variable on a pair of response variables were in the predicted direction significantly more often than differences in the opposite direction.
Finally, I evaluated the degree to which consistency with the above prediction depended on the following attributes of the study design—the smallest assessed scale, largest assessed scale, distance between adjacent scales, number of scales, and approach to scale selection—as well as the categorized scale sensitivity and taxa. For this analysis I treated my comparisons of the scales of effect between biological responses as an ordinal variable with three levels: (1) the difference in the scales of effect of a given environmental variable for a pair of biological responses was opposite to the prediction (scales of effect increase in the following order: genetic diversity < occurrence < abundance < fecundity); (2) there was no difference in the scales of effect of an environmental variable for a pair of biological responses; and (3) the difference in the scales of effect of a given environmental variable for a pair of biological responses was consistent with the prediction. I used a cumulative link mixed effects model to model the relationships between this ordinal response variable and the seven predictors, including study identity as a random effect to account for repeated measurements within studies. I also weighted the analysis towards studies with more sampling sites (ln-transformed to normalize the distribution of weights), under the assumption that studies with a larger sample size produce more reliable estimates of the real-world conditions that sample represents. A weighted cumulative link mixed effects model estimates model parameters by maximizing the summed log-likelihood of weighted observations [18]. Thus this weighting meant that observations with more sample sites (a higher weight) affected the log-likelihood of the model more than observations with fewer sites (a lower weight). All predictors were modeled as fixed effects. The smallest assessed scale, largest assessed scale, and distance between adjacent scales were ln-transformed for analysis. All statistical analyses were done in R [19], using the ‘ordinal’ package [20] for the cumulative link mixed effects model. Data used in these analyses are available through Mendeley Data [21].
Results
Twenty-three of the 1512 articles I reviewed met my selection criteria (see Literature search and study selection, above). These studies provided sufficient data to make 145 comparisons of scales of effect between paired biological responses, i.e. they identified scales of effect of a given environmental variable on two of my four target biological responses (fecundity, abundance, occurrence, genetic diversity) for a given species or species group. The majority of these comparisons were between scales of effect for abundance vs. occurrence (111 / 145 comparisons), followed by fecundity vs. occurrence (27 / 145), fecundity vs. abundance (5 / 145), and abundance vs. genetic diversity (2 / 145). Mean scales of effect were 5.43 km (range 0.01–20.00 km, n = 32) for measures of fecundity, 2.41 km (range 0.05–50.00 km, n = 118) for measures of abundance, 3.13 km (range 0.01–32.00 km, n = 138) for measures of occurrence, and 1.5 km (range 1.50–1.50 km, n = 2) for measures of genetic diversity (Online Resource 2). See Table 1 for a full summary of the collected study design attributes.
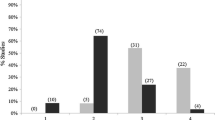
The scale of effect depended on the type of biological response. I found different scales of effect of a given environmental variable on a pair of response variables significantly more often (101 / 145 comparisons) than I found the same scale of effect (44 / 145 comparisons; Χ2 = 22.41, p < 0.001; Fig. 3).
Number of cases where the scale of effect of a given environmental variable was the same vs. different for a pair of biological responses, and the number of cases where differences in the scales of effect were in the predicted direction (scales of effect increased in the order: fecundity < abundance < occurrence < genetic diversity) vs. opposite direction (genetic diversity < occurrence < abundance < fecundity)
Although the scale of effect depended on the type of biological response, these scales of effect did not increase in the predicted order. Instead, I found that differences in the scales of effect were not significantly more likely to be in the predicted order (i.e. fecundity < abundance < occurrence < genetic diversity, 52 / 101 comparisons) than in the opposite direction (i.e. genetic diversity < occurrence < abundance < fecundity, 49 / 101 comparisons; Χ2 = 0.09, p = 0.85; Fig. 3).
However, the probability of detecting differences in the scales of effect in the predicted direction depended on the attributes of the study design to select the scales of effect. Specifically, I found that studies that had smaller minimum scales, larger maximum scales, smaller distances between adjacent scales, fewer tested scales, and a multivariate approach to scale selection were more likely to detect a difference in the scales of effect consistent with the prediction (Fig. 4a-e). However, only the largest assessed scale, distance between scales, and number of scales significantly affected this probability (Fig. 5).
Predicted effects of the study attributes (a–e), (f) scale sensitivity, and (g) taxonomic group on the probability of detecting differences in the scales of effect of a given environmental variable consistent with the prediction, i.e. scales of effect increased in the order: fecundity < abundance < occurrence < genetic diversity. Relationships were modeled by a cumulative link mixed effects model, including the study identity as a random effect and weighting the analysis towards studies with more sampling sites. In each plot, all other predictors are held constant: continuous predictors are held at their mean values, approach to scale selection = multivariate, number of scale-sensitive relationships = zero, and taxa = bird
Levels of support for effects of the study attributes, scale sensitivity, and taxa on the probability of detecting the predicted differences in the scales of effect, i.e. scales of effect increased in the order: fecundity < abundance < occurrence < genetic diversity. Relationships were modeled by a cumulative link mixed effects model. I included study identity as a random effect where the random effect of study identity was a normally-distributed variable with a mean = 0 and a model-estimated sd = 2.08, and weighted the analysis towards studies with more sampling sites. Parameter estimates for categorical predictors with n categories in a cumulative link mixed effects model are made for n-1 categories relative to the remaining category (e.g. for mammals, herptiles, and invertebrates relative to birds). Therefore, the estimated effect for the following categories—multivariate approach to the scale selection, zero scale-sensitive relationships, and birds—are set to zero in this figure. Parameters with 95% confidence intervals (CI) that do not cross zero have significant effects on the probability of detecting differences in the scales of effect of a given environmental variable consistent with the prediction
The probability of detecting the predicted differences in the scales of effect also depended on the scale sensitivity. Differences in the scales of effect were more likely to be in the predicted direction when the responses were scale-sensitive than when they were not (Figs. 4f and 5).
Discussion
This quantitative review shows that the scale of effect of a given environmental variable can—and often does—depend on what biological response you measure. I found different scales of effect of a given environmental variable on different biological responses (for a given species, study area, etc.) in 70% of the cases identified in this review. This suggests that scales of effect for a given species depend not only on the environmental variable you measure [9,10,11], but also on the type of species’ response. To my knowledge, this has been previously hypothesized [7••, 14••] but not tested using data from empirical studies.
Although this review clearly shows that scales of effect can differ among biological responses, it is less clear whether this occurs because the scale of effect of a given environmental variable increases with the temporal scale regulating the biological response. Overall, the scales of effect were not more likely to be in the predicted direction (scales of effect increasing in the order: fecundity < abundance < occurrence < genetic diversity) than in the opposite direction (genetic diversity < occurrence < abundance < fecundity). However, I found strong evidence that the probability of detecting effects in the predicted direction depended on the quality of the study design. In particular, studies were more likely to find differences in scales in the predicted direction when they included smaller minimum scales, larger maximum scales, and smaller distances between adjacent scales, although only the latter two effects were statistically significant. This may occur because studies that include a wider range of scales and have smaller distances between tested scales more accurately estimate the scale of effect than studies that include a narrow range of scales and large distances between tested scales. Indeed, in my review 37% (54 / 145) of cases had at least one estimated scale of effect at the smallest assessed scale and 43% (63 / 145) had at least one scale of effect at the largest assessed scale, suggesting that actual scales may have been smaller or larger than estimated in these cases, respectively. There is also theoretical support for this prediction: Jackson and Fahrig [14••] used an individual-based, spatially-explicit simulation model to show that scales of effect of habitat amount and fragmentation were smallest for abundance, intermediate for occurrence, and largest for genetic diversity. Thus it is possible that scales of effect do vary in the predicted order—with scales of effect for fecundity < abundance < occurrence < genetic diversity—but some studies were unable to detect this because of suboptimal study design.
The probability of detecting differences in scales of effect in the predicted direction was also higher when the species’ responses to an environmental variable were ‘scale-sensitive’, i.e. when the strength of relationship between a response and a given environmental variable varied with scale. A scale of effect for a response-environmental variable relationship is likely more accurate when the response is scale-sensitive than when it is not. In the latter case the selected scale of effect is less likely to be biologically relevant, either because the species is not affected by that environmental variable or because it responds similarly to the variable across the tested scales [5]. Alternatively, scale-insensitivity of relationships may indicate an issue with the design of the study to select the scales of effect; for example, effects of scale on the relationship may not be detected if the resolution of the land cover data used to estimate environmental variable values is larger than the distance between scales (resulting in high cross-scale collinearity).
Surprisingly, we also found that the probability of detecting the predicted differences in the scales of effect was lower in studies that tested more scales than in studies that tested fewer scales. This is counter-intuitive, because one would expect that studies with a wider range of scales and smaller distances between scales—which were more likely to detect differences in the predicted direction—would also have more tested scales than a study with a narrow range of scales and larger distances. However, the cross-study correlations between the number of scales and the smallest assessed scale, largest scale, and difference between scales in this review were relatively weak (Spearman ρ = −0.22, 0.25, and −0.25, respectively). Correlations were weak because studies with the largest assessed scales did not tend to also have the smallest scales and smallest distances between scales. This unexpected decline in the probability of detecting the predicted differences in the scales of effect with increasing number of scales may be explained, at least in part, by a relationship between the number of scales and the scale sensitivity of the responses. All of the cases with two scale-insensitive responses had the maximum number of tested scales and there were, on average, fewer tested scales in cases with more scale-sensitive responses (Online Resource 3).
Next Steps
Further empirical study is needed to test the prediction that the scale of effect of a given environmental variable increases in the order: fecundity < abundance < occurrence < genetic diversity. In particular, studies including estimates of scales of effect for fecundity and genetic diversity were lacking in our data set, as were studies of some taxonomic groups (e.g. herptiles, plants). Such studies should be designed to minimize the chance of misidentifying the scale of effect. I suggest the following as a list of ‘best practices’ for such studies, expanding on the recommendations made by Miguet et al. [7••]:
-
(1)
Include a wide enough range to encompass the scales of effect for all responses. This is difficult to do without a priori knowledge of the scales of effect (which is the case for many species). However, theory suggests that scales of effect on abundance should be 4–9 times the median dispersal distance [6]. Thus one should test scales smaller than the species home range and scales larger than 4–9 times the median dispersal distance, to encompass scales for responses predicted to have smaller and larger scales of effect than abundance.
-
(2)
The distance between adjacent scales should be small enough to detect differences in scales among biological responses. The appropriate distance will depend, in part, on the spatial autocorrelation in the environmental variable of interest. This is because differences in the response between two adjacent scales can be detected only when there are differences in the measured environmental variable at these scales (i.e. the cross-scale correlation is < 1).Ideally, one would also consider the functional grain of a species, i.e. the smallest spatial scale at which a species perceives and responds to spatial heterogeneity in its environment [22]; however, in practice this information is likely unavailable for many species.
-
(3)
Select a set of study landscapes that have similar cross-landscape variability in environmental variable values when those values are measured at each scale. This is because, all else being equal, the estimated strength of relationship between a response and environmental variable will be weaker when there is less variability in the environmental variable values. Thus the scale of effect may not match the scale at which the species responds to the landscape context if the variance in the environmental variable is lower at that scale than at the other tested scales.
-
(4)
Use the finest-resolution data available for measuring environmental variables, as errors in measurement of environmental variables may reduce the ability to detect the scale of effect.
-
(5)
Check for non-linear relationships between a biological response and an environmental variable, because use of an inappropriate (linear) model when the true relationship is non-linear may lead to misidentification of the scale of effect.
Conclusions
Our ability to detect an effect of the landscape context on a species depends on whether we select the appropriate scale of measurement. The predictions that different species respond to the landscape context at different scales and that a given species may respond to different environmental variables at different scales have been well supported in previous studies. This is the first study to support the prediction that the scale at which a species responds to the landscape context also depends on what type of species’ response you measure, based on a review of published empirical studies.
These study results have implications for researchers looking to identify the appropriate scale of measurement for environmental variables. Estimation of scales of effect based on multi-scale analyses can be costly: it requires sampling in multiple landscapes, separated by perhaps larger-than-necessary distances (to incorporate all potential scales of effect), and replicate analyses at each of the potential scales. Thus research would be easier if the scale of effect could be predicted a priori. One way to do this is to use the selected scales of effect for your species from previously-published studies. However, the results of this quantitative review suggest that one should not extrapolate the previously-published scale of effect for one biological response to another. Furthermore, I found no clear support for the prediction that the scale of effect increases in the order: fecundity < abundance < occurrence < genetic diversity. This suggests that one cannot use knowledge of the relative differences in the temporal scale regulating the biological responses to extrapolate scales of effect from one response type to another. Thus multi-scale analyses, although costly, are currently the most reliable way to estimate the scale at which a species response is affected by the landscape context.
These study results also have implications for landscape management, suggesting that landscape management may fail to meet conservation objectives if the biological response considered when selecting the scale of management does not match the response of conservation concern. For example, landscape management to promote genetic diversity may be unsuccessful if the selected scale of management is smaller than the scale at which a species’ genetic diversity is affected by the landscape context, because human-caused landscape changes outside of the managed area could cause declines in genetic diversity. Our study suggests this could occur if the selected scale of management is based only on knowledge of the scale at which the species’ fecundity/abundance/occurrence responds to that landscape context. Therefore managers should be careful to consider what biological response is most appropriate to meet their management goals, and to select a scale appropriate for that response. One cannot assume that a species responds to its landscape context at only one scale.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Thornton DH, Branch LC, Sunquist ME. The influence of landscape, patch, and within-patch factors on species presence and abundance: a review of focal patch studies. Landsc Ecol. 2011;26:7–18.
Holland JD, Bert DG, Fahrig L. Determining the spatial scale of species’ response to habitat. Bioscience. 2004;54:227–33.
Eigenbrod F, Hecnar SJ, Fahrig L. The relative effects of road traffic and forest cover on anuran populations. Biol Conserv. 2008;141:35–46.
Ethier K, Fahrig L. Positive effects of forest fragmentation, independent of forest amount, on bat abundance in eastern Ontario, Canada. Landsc Ecol. 2011;26:865–76.
Martin AE, Fahrig L. Measuring and selecting scales of effect for landscape predictors in species-habitat models. Ecol Appl. 2012;22:2277–92.
Jackson HB, Fahrig L. What size is a biologically relevant landscape? Landsc Ecol. 2012;27:929–41.
•• Miguet P, Jackson HB, Jackson ND, Martin AE, Fahrig L. What determines the spatial extent of landscape effects on species? Landsc Ecol. 2016;31:1177–94. A comprehensive review of hypothesized drivers of scales of effect and the support (or lack thereof) for each hypothesis. This is the first paper to formalize the prediction tested in my quantitative review.
• Jackson HB, Fahrig L. Are ecologists conducting research at the optimal scale? Glob Ecol Biogeogr. 2015;24:52–63. Meta-analysis evaluating the relative importance of a species’ traits vs. study design attributes for its estimated scale of effect. The main findings were that the strongest predictors of the scale of effect were the smallest and largest assessed scales.
Duren KR, Buler JJ, Jones W, Williams CK. An improved multi-scale approach to modeling habitat occupancy of northern bobwhite. J Wildl Manag. 2011;75:1700–9.
Coffey HMP, Fahrig L. Relative effects of vehicle pollution, moisture and colonization sources on urban lichens. J Appl Ecol. 2012;49:1467–74.
Patenaude T, Smith AC, Fahrig L. Disentangling the effects of wetland cover and urban development on quality of remaining wetlands. Urban Ecosyst. 2015;18:663–84.
McGarigal K, Wan HY, Zeller KA, Timm BC, Cushman SA. Multi-scale habitat selection modeling: a review and outlook. Landsc Ecol. 2016;31:1161–75.
Mayer AL, Cameron GN. Consideration of grain and extent in landscape studies of terrestrial vertebrate ecology. Landsc Urban Plan. 2003;65:201–17.
•• Jackson ND, Fahrig L. Landscape context affects genetic diversity at a much larger spatial extent than population abundance. Ecology. 2014;95:871–81. The first study to hypothesize that the scale of effect depends on the temporal scale regulating the measured biological response, and the primary basis for the prediction made in Miguet et al. [7].
Ruffino L, Salo P, Koivisto E, Banks PB, Korpimäki E. Reproductive responses of birds to experimental food supplementation: a meta-analysis. Front Zool. 2014;11:80.
Boggs CL, Ross CL. The effect of adult food limitation on life history traits in Speyeria mormonia (Lepidoptera: Nymphalidae). Ecology. 1993;74:433–41.
Mendenhall CD, Sekercioglu CH, Brenes FO, Ehrlich PR, Daily GC. Predictive model for sustaining biodiversity in tropical countryside. Proc Natl Acad Sci. 2011;108:16313–6.
Christensen RHB. A tutorial on fitting cumulative link models with the ordinal package [Internet]. 2015 [cited 2017 Dec 22]. p. 1–18. Available from: https://cran.r-project.org/web/packages/ordinal/vignettes/clm_tutorial.pdf.
R Core Team. R: A language and environment for statistical computing. Version 3.4.0. Vienna: R Foundation for Statistical Computing; 2017. https://www.r-project.org/
Christensen RHB. Ordinal - regression models for ordinal data. R package version 2015.6-28. 2015. http://www.cran.r-project.org/package=ordinal/.
Martin AE. Data from: The spatial scale of a species’ response to the landscape context depends on which biological response you measure. Mendeley Data, v1. 2017. https://doi.org/10.17632/sv444hpkk5.1.
Baguette M, Van Dyck H. Landscape connectivity and animal behavior: functional grain as a key determinant for dispersal. Landsc Ecol. 2007;22:1117–29.
Restani M, Davies JM, Newton WE. Importance of agricultural landscapes to nesting burrowing owls in the northern Great Plains, USA. Landsc Ecol. 2008;23:977–87.
Guerena KB, Castelli PM, Nichols TC, Williams CK. Spatially-explicit land use effects on nesting of Atlantic flyway resident Canada geese in New Jersey. Wildl Biol. 2014;20:115–21.
Crowe DE, Longshore KM. Nest site characteristics and nesting success of the western burrowing owl in the eastern Mojave Desert. J Arid Environ. 2013;94:113–20.
Kelly JP, Stralberg D, Etienne K, McCaustland M. Landscape influence on the quality of heron and egret colony sites. Wetlands. 2008;28:257–75.
Ghilain A, Belisle M. Breeding success of tree swallows along a gradient of agricultural intensification. Ecol Appl. 2008;18:1140–54.
Lockyer ZB, Coates PS, Casazza ML, Espinosa S, Delehanty DJ. Nest-site selection and reproductive success of greater sage-grouse in a fire-affected habitat of northwestern Nevada. J Wildl Manag. 2015;79:785–97.
Rytwinski T, Fahrig L. Effect of road density on abundance of white-footed mice. Landsc Ecol. 2007;22:1501–12.
Herrera JM, Ploquin EF, Rodriguez-Perez J, Obeso JR. Determining habitat suitability for bumblebees in a mountain system: a baseline approach for testing the impact of climate change on the occurrence and abundance of species. J Biogeogr. 2014;41:700–12.
Griffis-Kyle KL, Kyle S, Jungels J. Use of breeding sites by arid-land toads in rangelands: landscape-level factors. Southwest Nat. 2011;56:251–5.
Akasaka T, Akasaka M, Yanagawa H. Relative importance of the environmental factors at site and landscape scales for bats along the riparian zone. Landsc Ecol Eng. 2010;6:247–55.
DeCatanzaro R, Chow-Fraser P. Relationship of road density and marsh condition to turtle assemblage characteristics in the Laurentian Great Lakes. J Great Lakes Res. 2010;36:357–65.
Cozzi G, Muller CB, Krauss J. How do local habitat management and landscape structure at different spatial scales affect fritillary butterfly distribution on fragmented wetlands? Landsc Ecol. 2008;23:269–83.
Rizkalla CE, Swihart RK. Community structure and differential responses of aquatic turtles to agriculturally induced habitat fragmentation. Landsc Ecol. 2006;21:1361–75.
Jankowiak L, Skorka P, Lawicki L, Wylegala P, Polakowski M, Wuczynski A, et al. Patterns of occurrence and abundance of roosting geese: the role of spatial scale for site selection and consequences for conservation. Ecol Res. 2015;30:833–42.
Flick T, Feagan S, Fahrig L. Effects of landscape structure on butterfly species richness and abundance in agricultural landscapes in eastern Ontario, Canada. Agric Ecosyst Environ. 2012;156:123–33.
de Boer WF, van de Koppel S, de Knegt HJ, Dekker JJA. Hibernation site requirements of bats in man-made hibernacula in a spatial context. Ecol Appl. 2013;23:502–14.
Ribeiro DB, Batista R, Prado PI, Brown KS Jr, Freitas AVL. The importance of small scales to the fruit-feeding butterfly assemblages in a fragmented landscape. Biodivers Conserv. 2012;21:811–27.
Schneider C, Ekschmitt K, Wolters V, Birkhofer K. Ring-based versus disc-based separation of spatial scales: a case study on the impact of arable land proportions on invertebrates in freshwater streams. Aquat Ecol. 2011;45:351–6.
Lyra-Jorge MC, Ribeiro MC, Ciocheti G, Tambosi LR, Pivello VR. Influence of multi-scale landscape structure on the occurrence of carnivorous mammals in a human-modified savanna, Brazil. Eur J Wildl Res. 2010;56:359–68.
Haenke S, Scheid B, Schaefer M, Tscharntke T, Thies C. Increasing syrphid fly diversity and density in sown flower strips within simple vs. complex landscapes. J Appl Ecol. 2009;46:1106–14.
Cardoso P, Aranda SC, Lobo JM, Dinis F, Gaspar C, Borges PAV. A spatial scale assessment of habitat effects on arthropod communities of an oceanic island. Acta Oecol. 2009;35:590–7.
Schotthoefer AM, Rohr JR, Cole RA, Koehler AV, Johnson CM, Johnson LB, et al. Effects of wetland vs. landscape variables on parasite communities of Rana pipiens: links to anthropogenic factors. Ecol Appl. 2011;21:1257–71.
Pérez-García JM, Sebastián-González E, Alexander KL, Sánchez-Zapata JA, Botella F. Effect of landscape configuration and habitat quality on the community structure of waterbirds using a man-made habitat. Eur J Wildl Res. 2014;60:875–83.
Acknowledgements
I thank the members of the Carleton University Geomatics and Landscape Ecology (GLEL) Friday Discussion Group, the section editor, and two anonymous reviewers for their comments on earlier versions of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
I have no conflicts of interest to declare.
Human and Animal Rights and Informed Consent
This article does not contain any studies involving human subjects. This article references one study with non-human animal subjects performed by A.E.M. [5]. This work was done in accordance with the guidelines of the Canadian Council on Animal Care, and was approved by the Carleton University Animal Care Committee.
Additional information
This article is part of the Topical Collection on Scale-Measurement, Influence, and Integration
Electronic supplementary material
ESM 1
(DOCX 177 kb)
Rights and permissions
About this article
Cite this article
Martin, A.E. The Spatial Scale of a Species’ Response to the Landscape Context Depends on which Biological Response You Measure. Curr Landscape Ecol Rep 3, 23–33 (2018). https://doi.org/10.1007/s40823-018-0030-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40823-018-0030-z