Abstract
To conserve and manage the bat population in an agricultural landscape, we investigated the relative importance of environmental factors on their occurrence and abundance in riparian zones at site and landscape scales. From May to October 2003, four bat species (Myotis daubentonii, M. frater, Plecotus auritus, and Eptesicus nilssonii) were mist-netted at 26 sampling sites in five streams in the Tokachi plain in central Hokkaido, northern Japan. The environmental factors of the two scales were measured at each sampling site. To clarify the most suitable landscape scale for bats, we also compared three spatial extents (250, 500, and 750 m) at each site. Generalized linear mixed models revealed that the occurrence and/or abundance of all four species, except for M. frater, was positively correlated with the percent cover of broadleaved deciduous forest and the height of bank-side tree canopy. Additionally, according to variation partitioning, although the value of the pure effect varied among species and measurement types, the site scale had a relatively larger pure effect than the landscape scale in many cases. However, in three of the five combined models, the proportion of confounding effect was much larger than that of the pure effect at the respective scales. Our study demonstrated that streams adjacent to a well-developed broadleaved deciduous forest are important for night habitats of many bat species in an agricultural landscape. Although focusing on the site scale may be important for bat conservation, we strongly stress the necessity for conservation and management plans at multiple scales.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The riparian zone, an interface between aquatic and terrestrial systems, harbors a wide variety of animal species (Gregory et al. 1991; Knopf et al. 1988; Naiman et al. 1993), and many of them are riparian specialists (Sabo et al. 2005). Despite their important role in maintaining regional biodiversity, riparian zones are severely degraded by human activities, including damming, river channelization, and human recreation (Brookes 1988; Miller et al. 2003; Rood et al. 1995; Scott et al. 2003). These habitat degradations are particularly serious in agricultural landscapes (Belanger and Grenier 2002; Nakamura and Yamada 2005; O’Neill et al. 1988). Therefore, conservation and management of the riparian zone is important for maintaining biological diversity in agricultural landscapes (Jobin et al. 2004).
The distribution of an organism is influenced by environmental factors that act at multiple spatial scales (Steffan-Dewenter et al. 2002; Thogmartin and Knutson 2007). These previous studies also indicate that evaluation of environmental factors at inappropriate spatial scales may provide insufficient information for management, and stressed the importance of the evaluation of the responsible environmental factors at multiple scales. At the same time, habitat selection by animals is influenced by environmental factors that operate at multiple spatial scales (Fletcher and Hutto 2008; Okland et al. 2006). Thus, clarifying the relative importance of environmental factors on each scale provides important bases for determining the allocation of conservation resources at the appropriate scale (Fletcher and Hutto 2008). Moreover, because environmental requirement of animals can vary depend on their abundance and occurrence (Heinanen et al. 2008), conservation planning should consider these differences. Because the riparian zone harbors a wide variety of animals, and different species or taxa respond differently to environmental conditions, preferable conditions for the habitat should be evaluated according to multiple taxa. However, although many mammalian species are considered to prefer the riparian zone (i.e., Helfield and Naiman 2006; Maisonneuve and Rioux 2001; Sepulveda et al. 2007), limited studies have focused on terrestrial mammals. Bats, relatively well-studied riparian-dwelling mammals, are one of the largest taxa, which accounts for a quarter of the whole mammalian species (Kunz and Fenton 2003). Bats are also bioindicators in various habitats, particularly in agricultural landscapes (Jones et al. 2009): they show wide geographic ranges, gradual responses to habitat degradation correlated with responses of other taxa (i.e., insects), rich trophic diversity, slow reproductive rates that can result in rapid population declines, and they are reservoirs of a wide range of emerging infectious diseases whose epidemiology may reflect environmental stress. Nevertheless, approximately 45% of bat species are on the verge of extinction, mostly because of anthropogenic disturbances (Hutson et al. 2001). Therefore, efficient conservation strategies are urgently required.
River and riparian habitats are some of the most important habitats for foraging, drinking, and commuting for a broad range of bat species throughout the world (Ciechanowski 2002; Holloway and Barclay 2000; Lloyd et al. 2006; Racey et al. 1998; Whitaker 2004). Therefore, protecting these aquatic–terrestrial interrelated ecosystems is a high priority for bat management and conservation (Fukui et al. 2006). The importance of the riparian habitat for bats has been studied from several perspectives (i.e., comparison of river and riparian habitats to others, stream channel morphology, quality of riparian vegetation, and so on; Akasaka et al. 2009b; Grindal et al. 1999; Ober and Hayes 2008). However, most of these previous studies on bats’ habitat selections were conducted on the site scale, and landscape studies are limited, with very few multiple-scale studies (but see Ober and Hayes 2008).
In this study, to conserve the bat population in an agricultural landscape, we clarified the relative importance of environmental factors at the site scale and at the landscape scale in river and riparian habitats.
Materials and methods
Target bat species in this study
In this study, we focused on four bat species, Myotis daubentonii, M. frater, Plecotus auritus, and Eptesicus nilssonii, of which number of bats captured were large enough to analyze. Although M. macrodactylus was captured at eight sites, the captured sites belonged to only two streams. Therefore, we did not use M. macrodactylus for the further analyses. All four species except M. frater are widely distributed throughout Europe and Asia (Encarnacao et al. 2005; Maeda 2005a; Rydell 1993; Stebbings 1988). M. daubentonii gaffs prey from the water surface (trawling species; Ciechanowski 2002). Because M. daubentonii forages almost dominantly in aquatic habitats (Kalko and Schnitzler 1989), this species may be sensitive to river and riparian alterations. The common and critical component of their diet is Diptera, especially adults and larvae of Chironomidae, although the diet differs among sites (Boonman et al. 1998; Flavin et al. 2001; Vaughan 1997). In contrast to M. daubentonii, although M. frater are found in Russia and Asia, there are few observations, and captured records of this species are from Asia only (Endo 1967; Kawai 2006; Kondo 2005). These reports state that M. frater is able to use a broad variety of habitats, ranging from natural to human. However, most of the biology of this species is unknown. P. auritus is one of the true forest species that gleans; they predominantly forage and roost in forests and take surface-bound prey from the ground or the vegetation (Anderson and Racey 1991). This species can fly slowly and hover, and they forage in forests relatively near the roost (within about 1.5 m; Entwistle et al. 1996). Their main diet is composed of Lepidoptera and Diptera (Vaughan 1997). E. nilssonii has been suggested as an opportunist for the foraging habitat; they prefer various types of open areas, such as forest edges, areas above the forest, farmland, parks with trees, streams and lakes, and street lamps (Dejong 1994; Rydell 1990, 1992, 1993; Wermundsen and Siivonen 2008). The diet of the species is composed of Diptera, mainly Chironomidae, Lepidoptera, Heteroptera, Aphidomorpha, and Psyllomorpha (Gajdosik and Gaisler 2004).
Study site
The study was conducted at five small streams in the Tokachi plain in central Hokkaido, northern Japan. All stream widths ranged from 2 to 7 m and lengths from 4 to 15 km. The Tokachi plain is one of the major agricultural areas of Japan. Although >95% of the land in the Tokachi plain was originally covered with broadleaved deciduous forest, most has been altered to arable land and pastures since the onset of settlement in 1883. The dominant tree species of the remnant forest are Quercus dentate, Q. crispula, Betula platyphylla var. japonica, and planted Larix leptolepis. The mean temperature was 6.4°C (maximum and minimum temperatures of 30.8°C and −22.5°C, respectively), and total precipitation was 830.5 mm in 2003 (data provided by the Obihiro Climatological Observatory located within the study area). During the research period, the mean daily temperature was 14.3°C (maximum and minimum temperatures 30.8°C and 0°C, respectively).
Bat capturing
We set 26 sampling sites in five streams (three to nine sampling sites in each stream). All sampling sites were located at least 1 km apart. Although bats may travel within a large area, the distance among each sampling site would not distort our result because Moran’s I on the abundance and occurrence of the respective species was <0.05 and consistently not significant (P > 0.05). Bats were surveyed from May to October in 2003. We mist-netted each sampling site once every 2 months, or a total of three times. In each sampling site, three mist nets (each mist net was 2 × 6 m, with a 24-mm mesh size) were placed across streams for 2 h after sunset. We did not survey on rainy nights. Each capture was identified to species according to Maeda (2005b). Although Kondo (2003) revealed clear identification of M. gracilis and M. ikonnikovi, it was difficult to identify these two species in our study periods (in 2003). Thus, we grouped these two species as Myotis spp.
Measurements of environmental factors
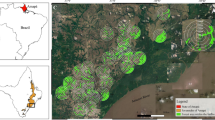
Environmental factors to explain the occurrence and abundance of bats were measured at site and landscape scales. We measured five site-scale environmental factors (stream width, presence of bank-side trees, shelter’s height, height of bank-side tree canopy, and volume of open space above stream) in each sampling site (Table 1). Every site scale environmental factor except the presence of bank-side trees was measured at the mist-netted points and those 10 m downstream and upstream. Although habitat uses of some of bats were affected by water-surface conditions, there were few rapid-surface areas, which the bats would avoid in our sampling sites. Thus, we did not measure water-surface conditions. On the landscape scale, we used the percentage cover of land-cover types as predictive environmental factors. Because there was no clear evidence to determine the occurrence and abundance of bats on the landscape scale, we applied three spatial extents (250, 500, and 750 m) as the landscape scale according to Akasaka et al. (2009a; Fig. 1). We used digital land-use maps and a geographical information system (GIS, ArcGIS9.2; ESRI, Redlands, CA, USA) to determine the percentage cover of the land-use types for areas within three selected extents from the sampling sites. Every sampling site was located by a hand-held Garmin WAAS-enabled Foretrex 101 GPS unit (Garmin International, Olathe, KS, USA) and plotted onto the digital land-cover map using the GIS. The land-cover types included Road, Urban, Arable, Grassland, Coniferous forest, Broadleaved deciduous forest, and Water body. The original land-use map was obtained from the latest vegetation/land-use map (scale of 1:25,000) on the Japan Integrated Biodiversity Information System (J-IBIS; Ministry of the Environment, 2000) and the National Spatial Data Infrastructure (Geographical Survey Institute, Government of Japan).
Data analysis
Factors determining the occurrence of bats
To clarify the relative importance of environmental factors on the sites and landscape scales to determine the occurrence of bats, we built three generalized linear mixed models (GLMM; Crawley 2002) based on three hypotheses. Firstly, we built a model based on the hypothesis that a bat occurrence can be explained only by site-scale environmental factors (site model). The explanatory variables were the five environmental factors measured (Table 1). The response variable was the presence or absence (1/0) of the bat in each sampling site. We used binomial error distribution and the logit link function and adopted a unique identification (ID) for each stream as a random effect for all the GLMM to model bat occurrence. Site-scale environmental factors that best explained bat occurrences were selected by a best-subset selection procedure (Burnham and Anderson 1998) based on Akaike’s Information Criterion (AIC). The model with the lowest AIC value was regarded as the best model. Secondly, a GLMM based on the hypothesis that bat occurrences can be explained only by landscape-scale environmental factors (landscape model) was built. The explanatory variables were percent covers of urban, arable, grassland, coniferous forest, and broadleaved deciduous forests within three spatial extents (250, 500, and 750 m) from each sampling site (Table 1). Because we did not use percent cover of road and water body as an explanatory variable, the sum of the percent cover of five land-cover type adopted was <100%. There was no significant correlation among each land-cover type (r < 0.5). The explanatory variable of each model was composed of one spatial extent variable. Thirdly, a GLMM based on the hypothesis that bat occurrences can be explained by both site- and landscape-scale environmental factors was built (combined model). We used the best predictive variables of both site and landscape models as explanatory variables of the combined model.
To reveal the relative importance of environmental factors in the two scales on bat occurrence, the pure effect of site and landscape environmental factors were respectively calculated by variation partitioning (Borcard et al. 1992; Meot et al. 1998) using the three above-mentioned models (site, landscape, and combined model). Variation partitioning can evaluate the relative importance among several groups of predictive variables on the same response variable. The pure effect of environmental factors on the respective scale was calculated by the following fractions, where “L” is the contribution of the landscape-scale model, “S” is the contribution of site-scale model, “C” is the contribution of the combined model, “a” is the pure effect of the site-scale model, “b” is the pure effect of the landscape-scale model, and “d” is the confounding effect of the site and landscape model.
When the confounding was calculated to be negative, the value is treated as zero. The contribution of each model was calculated as the percentage deviance explained [100 × (null deviance − residual deviance)/null deviance] (see Dobson 1999). All site-scale environmental factors, except for the presence of bank-side trees, were log-transformed, and all landscape factors were arcsine-transformed. We excluded arable land cover from the landscape-scale analysis on P. auritus because P. aurituss obviously did not use this land-cover type, and inclusion of this factor led to nonconvergence of the model. All statistical analyses were conducted using the statistical package R version 2.9.2 (R Development Core Team, 2008).
Factors determining the abundance of bats
We determined the environmental factors and their relative importance at two scales (site and landscape scales) that best explained the abundance of each bat species by the same procedures as the bat occurrence. In the analyses on abundance, we used only the sampling sites in which the respective bat species were present. The response variables were the abundance of bats in each sampling site. We used the highest number of the three captures at each sampling site. We used a Poisson error distribution and a log-link function and adopted a unique ID for each stream as the random effect for all GLMM in the abundance models. We did not use the percentage cover of arable land in the landscape scale analysis for P. auritus as the model on occurrence. We excluded E. nilssonii from the analysis due to the small number of observed sites.
Results
We caught 293 individuals from seven species belonging to four genera (Table 2). Regarding the target species, we caught 116 M. daubentonii in 12 sites, 48 M. frater in nine sites, 43 P. auritus in eight sites, and 16 E. nilsonii in eight sites.
Factors determining the occurrence of bats
The occurrence of each bat species was explained by different environmental factors, whereas the most relevant spatial extent at the landscape scale was 750 m for all four species. On the site scale, the height of bank-side tree canopy was the best predictor explaining the occurrence of M. daubentonii (Table 3). M. daubentonii was likely to occur where the bank-side tree canopy was high. On the other hand, the best model of the landscape scale was positively correlated with the cover of broadleaved deciduous forest and negatively correlated with the urban cover (Table 3). The three models of M. daubentonii (best model of site and landscape models and the combined model) explained 43.25, 29.82, and 56.61% of the total deviation, respectively. Confounding in the two scales was 16.46% (Fig. 2), and the pure effect of each of the two scales was 26.79% (for the site scale) and 13.36% (for the landscape scale). The occurrence of M. frater was not related to any environmental factors in either scale. For P. auritus, both bank-side tree canopy at the site scale (deviance explained 8.50%) and percentage cover of broadleaved deciduous forest on the landscape scale (deviance explained 40.64%) were positively correlated with their occurrence (Table 3). The combined model of the P. auritus occurrence explained 49.14% of the total deviation, and confounding of the two scales was 0%, indicating that the pure effect of each of the two scales in this species was completely independent (Fig. 2). Although E. nilssonii did not correlate with any site-scale environmental factor, all land cover was positively correlated with the occurrence of this species (deviance explained 86.19%).
Factors determining the abundance of bats
Each bat species was related to a variety of environmental factors at both spatial scales. The abundance of M. daubentonii was best explained by the height of bank-side tree canopy at the site scale and the percentage cover of broadleaved deciduous forest within 250 m of spatial extent at the landscape scale (Table 3). Both variables were positively correlated with abundance (Table 3). The percentage deviance explained for each scale model and the combined model was 57.19% (site scale), 63.26% (landscape scale), and 63.61% (combined). Thus, confounding accounted for a greater percentage (56.84%) than the pure effect of the two scales (site scale 0.35%, landscape scale 6.42%; Fig. 2). The abundance of M. frater was not related to any of the environmental factors on the two scales. The abundance of P. auritus increased in the site with wide, open space above the stream (site-scale deviance explained 44.01%) and the low percentage cover of grassland (landscape-scale 500 m spatial extent; deviance explained 27.93%; Table 3). Deviance explained by the combined model was 44.65%. Confounding and the pure effect of site and landscape scales was 27.29, 16.7, and 0.64%, respectively (Fig. 2).
Discussion
Relative importance of site and landscape scales
The most suitable spatial extent for occurrence and abundance differed in all three bat species, which was related to more than one land-cover type for the landscape model (Table 3). Although previous studies often used either occurrence or abundance of the organisms to evaluate the suitable scale for preferable habitats (i.e., Bowman et al. 2001; Smith et al. 2008; Warren et al. 2005), their results were hardly distinguished from one another. Our results pointed out the need for clear discrimination of the measurements used and the importance of conducting assessments by both measurements when planning conservation programs. The selected spatial extent of the occurrence of M. daubentonii and P. auritus, of which the best model of both sites and landscape models included more than one environmental factor, was smaller than that of the abundance. Additionally, the site scale had a relatively larger pure effect than the landscape scale in many cases, although the volume of the pure effect varied among species and type of measurement. Habitat selection by bats could emphasize fine-scale selection of sites within a home range (Johnson 1980; Ober and Hayes 2008) because bats, similarly to birds, can exploit geographically disjunct habitat patches due to high mobility and relatively low energy costs during flight. These previous studies support our results, and our data also suggest that abundance, which provides more detailed information on habitat selection, is influenced more by a finer spatial extent than occurrence.
The models of three out of the five species focused their explanatory power on incorporating environmental factors on both site and landscape scales. This suggests that the distribution of bats is often influenced by environmental factors on at least two spatial scales. However, in three out of the five combined models, the proportion of the confounding effect was much larger than that of the pure effect of the respective scales (Fig. 2; Table 3). The large volume of the confounding effect that represents the interscale effect indicates that environmental factors on multiple scales interactively influence bat distribution, suggesting that a conservation program should take multiple scales into account (Fletcher and Hutto 2008; Nakamura et al. 2005).
Habitat preference of bats
The percent cover of broadleaved deciduous forest was positively correlated with at least the occurrence or the abundance of all of bat species, except for M. frater (Table 3). Our results were in line with studies that showed the preference of bats for streams that run through a broadleaved deciduous forest (Holloway and Barclay 2000; Lloyd et al. 2006). Both types of habitats are important for both aquatic and terrestrial insects, and these habitats harbor large quantities and diverse species of insects (Fukui et al. 2006). Broadleaved deciduous forests also provide safe sites against predators and wind (Russ and Montgomery 2002). Likewise, on the site scale, bats prefer a liner edge structure, especially the river–riparian forest ecotone, for commuting corridors and foraging ground (Verboom and Spoelstra 1999; Warren et al. 2000). Therefore, many bat species, including our target species, prefer streams surrounded by large areas of broadleaved deciduous forests and that have well-grown bank-side trees.
The percent cover of the broadleaved deciduous forest and the height of bank-side tree canopy were positively correlated to both occurrence and abundance of M. daubentonii (Table 3). Well-grown bank-side trees are important for foraging because M. daubentonii are a wind-sensitive species (Russo and Jones 2003). Because all previous studies on habitat selection of M. daubentonii were conducted on the site scale, our study is the first to clarify the positive association of M. daubentonii and the surrounding forest cover at the landscape scale and to delineate a relevant landscape extent within which the land-cover component influences the habitat selection of this species. M. daubentonii dominantly forages along the river; however, this species uses the forest under the cold, windy condition because insects are more abundant in the forest than above the stream under this condition (Vaughan et al. 1997). Moreover, to reduce predation risks, this species also forages inside the forest at dusk (Nyholm 1965). Because our survey period was 2 h after sunset, this period included dusk and evening twilight time. Therefore, we suggest that the stream surrounded by forest provides benefits for foraging. The negative association between M. daubentonii and urban land cover may be the consequence of the loss of forest.
The occurrence of P. auritus showed a positive correlation to the percent cover of the broadleaved deciduous forest and the height of the bank-side tree canopy (Table 3). Because P. auritus predominantly forages in forests, it is a true forest species and its echolocation structure and wing shape are well-adapted to forests (Norberg and Rayner 1987), which allows slow and maneuverable flights (including hovering). The well-developed broadleaved deciduous forest provides many surfaces from which the species can glean insects (Entwistle et al. 1996). Additionally, P. auritus uses linear features, such as edges of tree lines (i.e., hedgerow and rides through forests) and hedges for commuting routes between feeding sites (Entwistle et al. 1996). Consequently, we inferred that well-developed bank-side trees adjacent to the stream and the surrounding forests are preferred as a commuting site. Within forests, P. auritus was abundant in the wide, open area above a stream, with a low percentage of grassland cover. Open space, such as grassland, increases predation risk, especially for slow-flying bats (Entwistle et al. 1996). Additionally, the abundance of Lepidoptera, which is the main diet component for P. auritus, is very low in grasslands (Entwistle et al. 1996). These high risks of predation and low prey resources for P. auritus in the grassland seem to result in the negative relation between P. auritus and the proportion of grassland cover (Table 3). Although we are not able to find the reason for the correlation between bat abundance and the volume of open space above water from previously published studies, because P. auritus uses linear open space with trees for commuting (Entwistle et al. 1996), the correlation may be related to the commuting habitat.
The occurrence of E. nilssonii was not correlated with any site-scale environmental factors, but it was correlated with all factors on the landscape scale, including the percentage of cover of the broadleaved deciduous forest. This species uses a variety of habitat flexibly and, thus, is thought to be opportunist in night habitats (Haupt et al. 2006; Wermundsen and Siivonen 2008). Our results support these previous studies and suggest that E. nilssonii can forage extensively in this study area. In turn, both the occurrence and abundance of M. frater did not correlate with any environmental factors. M. frater has been observed in a wide range of habitats, such as around street lamps, above and surrounding houses, on arable land, and in forests (Endo 1967; Kawai 2006; Kondo 2005). We also suggest that M. frater is a generalist in the night habitat.
Conclusions and implications
Our study demonstrated that streams with adjacent well-developed broadleaved deciduous forests are important for the night habitats of many bat species in agricultural landscapes, although the underlying mechanisms are different among species. Although attention to site scale may be relatively important for bat conservation, we strongly emphasize the necessity for conservation and management plans to work on a multiple scale. In this study, we did not handle the explicit factors that indicate anthropogenic disturbances. However, the low percentage of cover of broadleaved deciduous forests can be interpreted as a high percentage of human-mediated land cover, because >95% of the study area was covered by broadleaved deciduous forests before settlement and development began in 1883 (Kikuchi 2002). In that sense, our results not only contribute to deepening the understanding of the mechanism that determines bat community but also provide valuable information on the degree to which the forthcoming anthropogenic forest plantation will damage bat communities. Using statistical models such as the ones we developed, a systematic a priori assessment of the impact of forest degradation, and assessment of the efficiency of conservation and management plans, are required when taking environmental factors into account on multiple spatial scales.
References
Akasaka M, Takamura N, Mitsuhashi H, Kadono Y (2009a) Effects of land use on aquatic macrophyte diversity and water quality of ponds. Freshw Biol. doi: 10.1111/j.1365-2427.2009.02334.x (in press)
Akasaka T, Nakano D, Nakamura F (2009b) Influence of prey variables, food supply, and river restoration on the foraging activity of Daubenton’s bat (Myotis daubentonii) in the Shibetsu River, a large lowland river in Japan. Biol Conserv 142:1302–1310. doi:10.1016/j.biocon.2009.01.028
Anderson ME, Racey PA (1991) Feeding-behavior of captive Brown long-eared bats, Plecotus-Auritus. Anim Behav 42:489–493
Belanger L, Grenier M (2002) Agriculture intensification and forest fragmentation in the St. Lawrence valley, Quebec, Canada. Landscape Ecol 17:495–507. doi:10.1023/A:1021443929548
Boonman AM, Boonman M, Bretschneider F, van de Grind WA (1998) Prey detection in trawling insectivorous bats: duckweed affects hunting behaviour in Daubenton’s bat, Myotis daubentonii. Behav Ecol Sociobiol 44:99–107. doi:10.1007/s002650050521
Borcard D, Legendre P, Drapeau P (1992) Partialling out the spatial component of ecological variation. Ecology 73:1045–1055
Bowman J, Forbes G, Dilworth T (2001) Landscape context and small-mammal abundance in a managed forest. For Ecol Manag 140:249–255. doi:10.1016/S0378-1127(00)00315-7
Brookes A (1988) Channelized rivers. Wiley, Chichester
Burnham PB, Anderson DR (1998) Model selection and multimodel inference: a practical information-theoretic approach. Springer, Berlin
Ciechanowski M (2002) Community structure and activity of bats (Chiroptera) over different water bodies. Mamm Biol 67:276–285. doi:10.1078/1616-5047-00042
Crawley M (2002) Statistical computing: an introduction to data analysis using S-plus. Wiley, London
Dejong J (1994) Habitat use, home-range and activity pattern of the northern bat, Eptesicus-Nilssoni, in a Hemiboreal Coniferous Forest. Mammalia 58:535–548
Dobson A (1999) An introduction to generalized linear models. Chapman, London
Encarnacao JA, Kierdorf U, Holweg D, Jasnoch U, Wolters V (2005) Sex-related differences in roost-site selection by Daubenton’s bats Myotis daubentonii during the nursery period. Mammal Rev 35:285–294. doi:10.1111/j.1365-2907.2005.00066.x
Endo K (1967) Hunting flies of Myotis frater kaguyae (in Japanese). J Mammal Soc 3:64–67
Entwistle AC, Racey PA, Speakman JR (1996) Habitat exploitation by a gleaning bat, Plecotus auritus. Philos T Roy Soc B 351:921–931
Flavin DA, Biggane SS, Shiel CB, Smiddy P, Fairley JS (2001) Analysis of the diet of Daubenton’s bat Myotis daubentonii in Ireland. Acta Theriol 46:43–52
Fletcher RJ, Hutto RL (2008) Partitioning the multi-scale effects of human activity on the occurrence of riparian forest birds. Landscape Ecol 23:727–739. doi:10.1007/s10980-008-9233-8
Fukui D, Murakami M, Nakano S, Aoi T (2006) Effect of emergent aquatic insects on bat foraging in a riparian forest. J Anim Ecol 75:1252–1258. doi:10.1111/j.1365-2656.2006.01146.x
Gajdosik M, Gaisler J (2004) Diet of two Eptesicus bat species in Moravia (Czech Republic). Folia Zool 53:7–16
Gregory SV, Swanson FJ, McKee WA, Cummins KW (1991) An ecosystem perspective of riparian zones. Bioscience 41:540–551
Grindal SD, Morissette JL, Brigham RM (1999) Concentration of bat activity in riparian habitats over an elevational gradient. Can J Zool 77:972–977
Haupt M, Menzler S, Schmidt S (2006) Flexibility of habitat use in Eptesicus nilssonii: does the species profit from anthropogenically altered habitats? J Mammal 87:351–361
Heinanen S, Ronka M, von Numers M (2008) Modelling the occurrence and abundance of a colonial species, the arctic tern Sterna paradisaea in the archipelago of SW Finland (in English). Ecography 31:601–611
Helfield JM, Naiman RJ (2006) Keystone interactions: Salmon and bear in riparian forests of Alaska. Ecosystems 9:167–180. doi:10.1007/s10021-004-0063-5
Holloway GL, Barclay RMR (2000) Importance of prairie riparian zones to bats in southeastern Alberta. Ecoscience 7:115–122
Jobin B, Belanger L, Boutin C, Maisonneuve C (2004) Conservation value of agricultural riparian strips in the Boyer River watershed, Quebec (Canada). Agric Ecosyst Environ 103:413–423. doi:10.1016/j.agee.2003.12.014
Johnson DH (1980) The comparison of usage and availability measurements for evaluating resource preference. Ecology 61:65–71
Jones G, Jacobs DS, Kunz TH, Willig MR, Racey PA (2009) Carpe noctem: the importance of bats as bioindicators. Endanger Species Res 8:93–115
Kalko EKV, Schnitzler HU (1989) The Echolocation and hunting behavior of Daubenton Bat, Myotis-daubentonii. Behav Ecol Sociobiol 24:225–238. doi:10.1007/BF00295202
Kawai K (2006) Faunal survey of bats in Tokachi Station of the National Livestock Breeding Center(NLBC), Hokkaido. Bull Asian Bat Res Inst 5:1–8
Kikuchi S (2002) Transition and presence state of forest in Tokachi plain. Donguri-bank, Obihiro (in Japanese)
Knopf FL, Johnson RR, Rich T, Samson FB, Szaro RC (1988) Conservation of riparian ecosystems in the United States. Wilson Bull 100:272–284
Kondo N (2005) Bats survey in Hamanaka town, Hokkaido. Bull Asian Bat Res Inst 3:1–6
Kondo N, Sasaki N (2005) An external taxonomic character suitable for separating live Myotis ikonnikovi and M. mystacinus. Mammal Study 30:29–32
Kunz TH, Fenton MB (2003) Bat ecology. University of Chicago Press, Chicago
Lloyd A, Law B, Goldingay R (2006) Bat activity on riparian zones and upper slopes in Australian timber production forests and the effectiveness of riparian buffers. Biol Conserv 129:207–220. doi:10.1016/j.biocon.2005.10.035
Maeda K (2005a) Chiroptera. Tokai University Press, Tokyo (in Japanese)
Maeda K (2005b) Key to the species of Japanese Chiroptera. Tokai University Press, Tokyo (in Japanese)
Maisonneuve C, Rioux S (2001) Importance of riparian habitats for small mammal and herpetofaunal communities in agricultural landscapes of southern Quebec. Agric Ecosyst Environ 83:165–175. doi:10.1016/S0167-8809(00)00259-0
Meot A, Legendre P, Borcard D (1998) Partialling out the spatial component of ecological variation: questions and propositions in the linear modelling framework. Environ Ecol Stat 5:1–27. doi:10.1023/A:1009693501830
Miller JR, Wiens JA, Hobbs NT, Theobald DM (2003) Effects of human settlement on bird communities in lowland riparian areas of Colorado (USA). Ecol Appl 13:1041–1059. doi:10.1890/1051-0761(2003)13[1041:EOHSOB]2.0.CO;2
Naiman RJ, Decamps H, Pollock M (1993) The role of riparian corridors in maintaining regional biodiversity. Ecol Appl 3:209–212
Nakamura F, Yamada H (2005) Effects of pasture development on the ecological functions of riparian forests in Hokkaido in northern Japan. Ecol Eng 24:539–550. doi:10.1016/j.ecoleng.2005.01.010
Nakamura F, Inahara S, Kaneko M (2005) A hierarchical approach to ecosystem assessment of restoration planning at regional, catchment and local scales in Japan. Landscape Ecol Eng 1:43–52
Norberg UM, Rayner JMV (1987) Ecological morphology and flight in bats (Mammalia, Chiroptera)—wing adaptations, flight performance, foraging strategy and echolocation. Philos Trans R Soc B 316:337–419
Nyholm ER (1965) Zur OE kologie von Myotis mystacinus (Leisl.) und M. daubentonii (Leisl.) (Chiroptera) (in French). Ann Zool Fenn 2:77–123
O’Neill RV, Krummel JR, Gardner RH, Sugihara G, Jackson B, DeAngelis DL, Milne BT, Turner MG, Zygmunt B, Christensen SW, Dale VH, Graham RL (1988) Indices of landscape pattern. Landscape Ecol 1:153–162
Ober HK, Hayes JP (2008) Influence of vegetation on bat use of riparian areas at multiple spatial scales. J Wildlife Manage 72:396–404
Okland RH, Bratli H, Dramstad WE, Edvardsen A, Engan G, Fjellstad W, Heegaard E, Pedersen O, Solstad H (2006) Scale-dependent importance of environment, land use and landscape structure for species richness and composition of SE Norwegian modern agricultural landscapes. Landscape Ecol 21:969–987. doi:10.1007/s10980-006-0005-z
Racey PA, Swift SM, Rydell J, Brodie L (1998) Bats and insects over two Scottish rivers with contrasting nitrate status. Anim Conserv 1:195–202. doi:10.1111/j.1469-1795.1998.tb00029.x
Rood SB, Mahoney JM, Reid DE, Zilm L (1995) Instream flows and the decline of riparian cottonwoods along the St-Mary river. Alberta Can J Bot-Rev Can Bot 73:1250–1260
Russ JM, Montgomery WI (2002) Habitat associations of bats in Northern Ireland: implications for conservation. Biol Conserv 108:49–58. doi:10.1016/S0006-3207(02)00089-7
Russo D, Jones G (2003) Use of foraging habitats by bats in a Mediterranean area determined by acoustic surveys: conservation implications. Ecography 26:197–209. doi:10.1034/j.1600-0587.2003.03422.x
Rydell J (1990) Behavioural variation in echolocation pulses of the northern bat, Eptesicus nilssonii. Ethology 85:103–113
Rydell J (1992) Exploitation of insects around streetlamps by bats in Sweden. Funct Ecol 6:744–750
Rydell J (1993) Eptesicus nilssonii. Mammal Species 430:1–7
Sabo JL, Sponseller R, Dixon M, Gade K, Harms T, Heffernan J, Jani A, Katz G, Soykan C, Watts J, Welter A (2005) Riparian zones increase regional species richness by harboring different, not more, species. Ecology 86:56–62
Scott ML, Skagen SK, Merigliano MF (2003) Relating geomorphic change and grazing to avian communities in riparian forests. Conserv Biol 17:284–296. doi:10.1046/j.1523-1739.2003.00466.x
Sepulveda MA, Bartheld JL, Monsalve R, Gomez V, Medina-Vogel G (2007) Habitat use and spatial behaviour of the endangered Southern river otter (Lontra provocax) in riparian habitats of Chile: conservation implications. Biol Conserv 140:329–338. doi:10.1016/j.biocon.2007.08.026
Smith KM, Keeton WS, Donovan TM, Mitchell B (2008) Stand-level forest structure and avian habitat: scale dependencies in predicting occurrence in a heterogeneous forest. For Sci 54:36–46
Stebbings RE (1988) Conservation of European bats. Christopher Helm, London
Steffan-Dewenter I, Munzenberg U, Burger C, Thies C, Tscharntke T (2002) Scale-dependent effects of landscape context on three pollinator guilds. Ecology 83:1421–1432
Thogmartin WE, Knutson MG (2007) Scaling local species-habitat relations to the larger landscape with a hierarchical spatial count model. Landscape Ecol 22:61–75. doi:10.1007/s10980-006-9005-2
Vaughan N (1997) The diets of British bats (Chiroptera). Mammal Rev 27:77–94
Vaughan N, Jones G, Harris S (1997) Habitat use by bats (Chiroptera) assessed by means of a broad-band acoustic method. J Appl Ecol 34:716–730
Verboom B, Spoelstra K (1999) Effects of food abundance and wind on the use of tree lines by an insectivorous bat, Pipistrellus pipistrellus. Can J Zool 77:1393–1401
Warren RD, Waters DA, Altringham JD, Bullock DJ (2000) The distribution of Daubenton’s bats (Myotis daubentonii) and pipistrelle bats (Pipistrellus pipistrellus) (Vespertilionidae) in relation to small-scale variation in riverine habitat. Biol Conserv 92:85–91. doi:10.1016/S0006-3207(99)00062-2
Warren TL, Betts MG, Diamond AW, Forbes GJ (2005) The influence of local habitat and landscape composition on cavity-nesting birds in a forested mosaic. For Ecol Manag 214:331–343. doi:10.1016/j.foreco.2005.04.017
Wermundsen T, Siivonen Y (2008) Foraging habitats of bats in southern Finland. Acta Theriol 53:229–240
Whitaker JO (2004) Prey selection in a temperate zone insectivorous bat community. J Mammal 85:460–469
Acknowledgments
We are grateful to D. Fukui, and K. Kawai for their helpful advice on this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Akasaka, T., Akasaka, M. & Yanagawa, H. Relative importance of the environmental factors at site and landscape scales for bats along the riparian zone. Landscape Ecol Eng 6, 247–255 (2010). https://doi.org/10.1007/s11355-010-0105-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11355-010-0105-4