Abstract
While several studies have demonstrated that roads can act as barriers to small mammal movement, the relationship between road density and small mammal abundance has not yet been investigated. In southeastern Ontario, Peromyscus leucopus (white-footed mice) suffer high over-winter mortality rates, resulting in small springtime populations and frequent local extinctions. Peromyscus leucopus movement is known to be inhibited by roads, which should result in lower rates of immigration into and recolonization of habitats in landscapes with high road density. We tested two predictions: (1) Forest sites situated in landscapes with high road densities have a higher chance of P. leucopus being absent during the early spring than forest sites situated in landscapes with low road densities and (2) P. leucopus populations during the summer are smaller in forest sites situated in landscapes with high road densities than in landscapes with low road densities. We sampled P. leucopus in focal patches within nineteen landscapes (7 rural, low-road-density landscapes; 7 rural, high-road-density landscapes; 5 urban landscapes). There was no significant relationship between road density and the presence/absence of P. leucopus during the early spring. We found a significant positive effect of road density on P. leucopus relative abundance during the summer, even when we excluded the urban landscapes and based the analysis on only the 14 rural landscapes. Our results suggest that any negative effect of roads on P. leucopus populations, created by their inhibition to moving across roads, is far outweighed by some positive effect of roads on P. leucopus abundance. We suggest that the two most likely explanations are that roads are positively correlated with an important as-yet-undetermined component of habitat quality, or that roads positively affect P. leucopus by negatively affecting their predators.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
North America’s continuous landscapes are becoming increasingly fragmented by roads. The network of paved roads in Canada has tripled from 100,000 km in 1959 to 300,000 km in 2001 (Forman et al. 2003). Forman (2000) estimated that about one-fifth of the United States land area is directly affected ecologically by the network of public roads and that this fraction is increasing. While road networks have both positive and negative ecological effects, the majority are negative and extend substantial distances beyond the road itself (Forman and Alexander 1998).
Movement of small mammals through heterogeneous landscapes to recolonize habitat patches can be critical for population persistence (Kozakiewicz 1993). Roads have been found to act as barriers to small mammal movement (Oxley et al. 1974; Kozel and Fleharty 1979; Garland and Brandley 1984; Mader 1984; Swihart and Slade 1984; Clarke et al. 2001), including movement of white-footed mice (Peromyscus leucopus), the focal species in our study. Oxley et al. (1974) found that P. leucopus were reluctant to travel onto roads, with only 8 of 254 marked individuals crossing roads, and Merriam et al. (1989) found movements of P. leucopus across roads to be uncommon, despite frequent and long movements adjacent to roads. In a translocation experiment, P. leucopus were found to be averse to crossing roads; the probability of successful return to the initial capture site decreased by approximately 50% for every road that lay between the translocation site and the initial capture site (R. McGregor, pers. comm.). These studies suggest that roads may effectively fragment P. leucopus populations, such that populations that would otherwise be large continuous populations are divided into smaller, partially isolated local populations.
With over-wintering mortality rates of 80–90% being common for P. leucopus in southeastern Ontario, many woodlots start the spring breeding season with only a handful (1–3) of reproductive females (Taylor 1978; Middleton 1979). Merriam and Wegner (1992) stated that 5 to 15% of P. leucopus populations in forest patches in the Ottawa area suffer local extinctions each year. Therefore, movement for rescue of populations from low numbers and for recolonization of local extinctions is important, making population subdivision by roads a large potential effect on P. leucopus populations. If a forest patch suffers a chance local extinction of P. leucopus over the winter, population build-up during the following summer can only begin after immigrants arrive at the patch. Such immigration will be delayed for patches situated in landscapes with high road densities in comparison to those in landscapes with few roads, due to the low movement rate of P. leucopus across roads. This should, in turn, delay the start of population build-up over the summer in the patches in landscapes with many roads, which should result in smaller populations in the fall in these forest patches. These smaller populations will, in turn, have a higher probability of chance local extinction over the following winter. Therefore, forest patches in landscapes with high road density should have both higher probabilities of P. leucopus local extinction over the winter and lower population abundances during the summer. While there have been numerous studies on the effects of roads on small mammal movement (including P. leucopus), the relationship between road density and the frequency of small mammal local extinctions and their abundance has not yet been investigated.
In this study we compared Peromyscus leucopus populations across landscapes with varying road densities to test two predictions: (1) Forest sites situated in landscapes with high road densities have a higher chance of P. leucopus being absent during the early spring (higher chance of local extinction over winter) than forest sites situated in landscapes with low road densities; and (2) P. leucopus populations during the summer are smaller in forest sites situated in landscapes with high road densities than in landscapes with low road densities.
Ecology and life history of P. leucopus
Peromyscus leucopus is a nocturnal, semi-arboreal small mammal weighing about 28 g. P. leucopus shows preference for shrubby, deciduous habitat with dense cover and particular features or microsites such as rocks and stumps (Barry and Franq 1980). It has a diverse, omnivorous diet that mainly consists of insects, fruit, seeds, assorted vegetation, and nuts. Minimum gestation period is 22 to 23 days and mean litter size in eastern Ontario is 5.0 (Morris 1986). Postnatal growth is largely completed within 2 months of birth.
Peromyscus leucopus is near the northern edge of its range in eastern Ontario (Wegner 1995). Here, populations begin very low in spring and peak in the autumn with the breeding season extending from early May to mid-October (Wegner and Merriam 1990; Wegner 1995). Population density in this area peaks in August at 1–3.5/ha (Wegner and Merriam 1990; Smith and Speller 1970), which is an order of magnitude lower than peak densities in more southerly locations (Blem and Blem 1975; Gottfried 1979; Adler and Wilson 1987; Vessey 1987; Krohne et al. 1988). Estimates of home range size vary greatly; ranging from 0.2 to 1.6 ha (Speller 1968; Smith and Speller 1970; Wegner 1995).
Methods
Except where mentioned, all landscape data in our study (road density, forest cover, patch sizes and shapes; see below) were taken from NTDB (National Topographic Database) digital topographic maps (NTDB 1998), and all spatial analyses were conducted using ArcView3.2 (Environmental Systems Research Institute, Redlands, California).
Site selection
While P. leucopus are capable of living in a variety of habitats, they prefer to live and move in woodland habitat and avoid areas with little cover (Bendell 1961; Whitaker 1967; Hansen and Warnock 1978; Drickamer 1990; Wegner 1995; Seamon and Adler 1996). Therefore, sample sites were located in forest patches. Forest patches (focal patches) were selected within landscapes that varied in road density (km/km2). We defined each landscape as the area within a 2-km radius of each focal patch. This size of landscape was based on reported movement distances of P. leucopus. Although reports on long-distance movements by small mammals are relatively rare (Diffendorfer and Slade 2002), a few studies have documented Peromyscus sp. travelling over 1 km in less than a month (Murie and Murie 1931; Howard 1960; Bowman et al. 1999; Maier 2002). It has also been suggested that, in landscapes where habitat is sparsely distributed, small mammals can travel greater distances than predicted from their average home range size (Kozakiewicz et al. 1993).
In selecting focal patches, we attempted to maximize the variation in road density among the surrounding landscapes while controlling for landscape variables other than road density. We began by selecting only rural landscapes, containing no urban development, and approximately 20–35% forest. We chose 20–35% forest because it allowed the largest possible range of road density values, given the variation in forest cover and road densities in the Ottawa area. Landscapes also contained limited or no water (i.e., no rivers or lakes) and no railways, to avoid the possibility of additional barrier effects. We chose focal patches that were located at least 3 km apart to minimize overlap of the landscapes; focal patches were all greater than 1 ha in size and of similar forest type (deciduous/mixed deciduous).
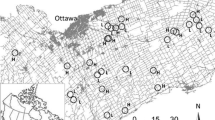
We selected 14 landscapes in the Ottawa region that spanned the largest possible range of road densities while meeting these criteria. It turned out, however, that due to the constraint that all the landscapes were rural, there was a relatively small difference in the road densities between the low-road-density landscapes and the high-road-density landscapes (0.42 to 0.85 km/km2 compared to 1.24 to 1.62 km/km2). We were concerned that if we found no effect of road density on mouse abundances across these sample sites, it could be because our landscapes did not contain a large enough range of road density values. Therefore, we added 5 urban landscapes. We followed as closely as possible the same criteria as for the other landscapes when selecting the urban landscapes, in that all of the urban landscapes contained approximately 20–35% forest, limited water systems/bodies, and focal patches were all greater than 1 ha in size and of similar forest type (deciduous/mixed deciduous). We defined urban landscapes as having at least 40% of the 2-km radius landscape covered by urban development (i.e., settlements and developed land, major transportation routes, etc.). Road densities in the 5 urban landscapes ranged from 4.28 to 9.48 km/km2. While the 14 rural landscapes were interspersed across the Ottawa region as much as possible, it was not possible to do the same for the urban landscapes (Fig. 1).
Distribution of landscapes studied across the Ottawa region. Circles represent 2-km radius landscapes and letters below correspond to landscape category. Key: rural low-road-density landscape (L); rural high-road-density landscape (H); urban landscape (U). Included on map is the urban landscape that was abandoned after the spring tracking phase (located in Kemptville). Excluded from the map is the amount of forest
We calculated road density as the total length of roads of all types (i.e., paved, gravel and/or dirt) within each 2-km radius landscape divided by the total area of the landscape (km/km2), and amount of forest within each 2-km radius landscape. All road types were included when determining road density because previous studies have reported that gravel and even narrow, seldomly used vehicle paths can restrict small mammal movements (Kozel and Fleharty 1979; Swihart and Slade 1984; Clarke et al. 2001).
Peromyscus leucopus sampling
We sampled P. leucopus in focal patches within each of the nineteen landscapes (7 rural, low-road-density; 7 rural, high-road-density; 5 urban) during the early spring (April 11–29) and summer (June 6–July 29) of 2005. We sampled P. leucopus using footprint tracking tubes (Merriam 1990). The proportion of tracking stations containing small mammal tracks has been shown to be a good estimate of relative abundance (Fahrig and Merriam 1985; Brown et al. 1996; Drennan et al. 1998; Glennon et al. 2002). In addition, relative to live trapping, footprint tracking reduces or eliminates the risks of stress and mortality in the study animals, reduces risk of exposure to diseases transmitted by rodents, and is less expensive and easier to deploy in the field, allowing for simultaneous sampling of small mammal populations in several areas.
We lined 30-cm lengths of 3.75 cm (inside diameter) plastic water pipe (PVC tubing) with a strip of white paper (28 × 7 cm). To the center of each paper, we stapled a 6 × 6-cm square of waxed paper with a smear of powdered carbon black (decolorizing) and paraffin oil (Nams and Gillis 2002). We tested various ratios of carbon black to paraffin oil to obtain the best consistency and chose a ratio of 1:3 (by weight). Each focal patch contained a 10 × 10 grid of tubes at 10 m spacing.
Data were presence or absence of P. leucopus tracks in each tube, irrespective of the number of times a mouse, or possibly several mice, went through the tube. We placed each tube within 1 m of a marker flag, adjacent to a prominent microhabitat feature, such as a tree trunk or log to improve chances of P. leucopus tracking the tubes (Drickamer 1990). We checked tubes weekly for P. leucopus tracks and replaced tubes with newly prepared papers weekly.
Spring tracking
The purpose of the spring tracking was to determine whether forest patches situated in landscapes with high road densities have a higher chance of small mammals being absent during the early spring (following the high over-winter mortality period and before reproduction begins), than forest patches situated in landscapes with low road densities. Tracking began April 11th 2005, and was carried out for three consecutive weeks ending April 29th 2005. In this phase of the sampling, we were only interested in determining whether there were any P. leucopus present in each site. Therefore, we baited tubes with sunflower seeds to attract animals to them.
Summer tracking
The purpose of the summer tracking was to determine whether small mammal populations are smaller in forest patches situated in landscapes with high road densities than in landscapes with low road densities. Tracking began June 6th, 2005 and was carried out for eight consecutive weeks ending July 29th, 2005. We replaced one of the urban landscapes used in the spring tracking phase with another urban landscape for the summer tracking phase due to interference and damage to the sampling grid by an adjacent landowner. We did not bait tubes during the summer phase of tracking because we were looking for an estimate of the relative abundance of P. leucopus based on the number of tubes containing P. leucopus prints. When the tubes are baited there is a high chance that one mouse will visit numerous tubes to obtain food. In contrast, unbaited tubes are similar to a hollow log that a mouse might run through simply as part of its environment.
It has been shown that P. leucopus demonstrate a strong attraction to new objects (Sheppe 1965). To reduce any bias this may cause, tubes were left out in each site between the spring and summer tracking phases so that the mice would become familiar with the tubes in their habitat before the summer tracking phase started. Note it was not necessary to put tubes out before the spring tracking phase because during that phase we were only interested in the presence or absence of mice in each site. We placed tubes in the one replacement urban landscape two weeks before summer tracking commenced.
Focal patch characteristics
To assess whether local habitat variables differed between focal patches and for focal patch descriptions, we carried out vegetation surveys on each focal patch from July 13th to July 22nd 2005. At each focal patch, we randomly chose five points within the tracking grid. We conducted sampling using the point-quarter method (Krebs 1989) in a 10-m2 plot that was centred over each of the five randomly chosen points within the sampling grid. In each of the four quarters of the 10-m2 plot, we recorded the species and diameter of the nearest woody tree (>10 cm diameter at breast height (dbh)), and shrub density (stems <10 cm dbh and >1 m in height). We measured dbh at 1.3 m above ground for trees only. The occurrence of P. leucopus has been reported to be influenced by both the number of woody tree species and the density of shrub vegetation (Dueser and Shugart 1978).
Lee (2004) reported that in sites with large amounts of coarse woody debris, the density of P. maniculatus, a species closely related to P. leucopus, was higher, populations fluctuated less, survivorship was better, and the time of residency was longer than in sites with less coarse woody debris. During the vegetation survey, we measured the percent of ground covered by coarse woody debris along five transects, one in each of the 10-m2 plots, at each focal patch. We selected a distance between 0 (North corner of the 10-m2 plot) and 10 (East corner of plot) using a random number generator. After locating that distance along the northeast boundary of the 10-m2 plot, we ran a 10 m transect to the southwest boundary of the plot, using a tape measure as the transect line. We measured the total length of the transect directly over coarse woody debris greater than 5 cm in length and greater than 2 cm in diameter (Zollner and Crane 2003). To estimate the percent cover of coarse woody debris at each focal patch, we divided the summed segment lengths that intersected coarse woody debris from each of the five transects by the total summed transect lengths (50 m) and multiplied by 100.
Patch size and shape have been found to affect small mammal abundances in previous studies. To determine possible confounding effects (with road density) of these variables on P. leucopus relative abundance in this study, we determined the size and shape of each focal patch. We determined patch shape using a modified “Patton index” (Faeth and Kane 1978; Schmid-Holmes and Drickamer 2001; Barko et al. 2003), which calculates the deviation from circularity of each patch as P/[2(πA)1/2], where P represents the perimeter and A represents the area of the patch.
Footprint tracking tubes vs. live trapping
Tracking is not as common a method as live–trapping for estimating small mammal abundance, despite studies supporting its use (Fahrig and Merriam 1985; Brown et al. 1996; Drennan et al. 1998; Glennon et al. 2002). Therefore, we investigated the reliability of our method by carrying out live trapping to compare the relative abundances obtained by tracking tubes and those obtained from live trapping. We live trapped in three of the sites, one from each landscape type, for one night each (October 11th, 12th, and 15th 2005).
Data analysis
Although we attempted to control for possible confounding variables in our site selection process, there were several variables that might affect P. leucopus relative abundance that we were not able to control completely in the experimental design. These included the amount of forest in the landscape, the size of the focal patch, its shape (Patton index), the percentage of coarse woody debris, the number of tree species, and the density of shrubs in the focal patch. We wanted to rule out the possibility of these variables confounding our results. Given our sample size (19 landscapes), however, we could not include all these variables in a multiple regression analysis. Therefore, we used principle components analysis (PCA) to reduce the six variables to two composite variables (the first two principle components). Since one of the urban landscapes (Kemptville) sampled over the spring sampling period was exchanged for another urban landscape (Kanata) for the summer sampling period (due to damage to the focal patch), separate PCAs were conducted for the spring and summer tracking periods.
We then performed a multiple stepwise logistic regression analysis to test whether the probability of presence of P. leucopus in a forest patch in the early spring was related to road density in the landscape surrounding the sites and the two principle components from the combined landscape and habitat variables. To test the prediction that relative abundance of P. leucopus in a forest patch decreases with road density in the surrounding landscape, we performed a multiple stepwise linear regression of relative abundance on road density along with the two principle components. We performed all statistical analyses using SPSS version 10.0.
Results
Focal patches were primarily mature, deciduous forest, dominated by white ash (Fraxinus americana), red maple (Acer rubrum), american elm (Ulmus americana), and sugar maple (Acer saccharum). Dominant vegetation varied among focal patches. Sixteen of the nineteen focal patches had greater than 75% deciduous tree species and two of the nineteen focal patches were dominated by eastern white cedar (mixed deciduous-evergreen forests; one in the rural low-road-density category and one in the urban category).
The first two components of the PCAs accounted for 51.1% of the total variation for the spring sampling period and 52.8% of the total variation for the summer sampling period, of the variation in the possible confounding variables (percentage of forest, focal patch size, focal patch shape, percentage of coarse woody debris cover, number of tree species, or shrub density). Shrub density was highly loaded on the first principal component (PC1) for both the spring (0.872) and summer tracking periods (0.865), and the percentage of coarse woody debris was highly loaded on the second principal component (PC2) for both the spring (0.868) and summer (0.766) periods.
Peromyscus leucopus tracks were not found in 5 of the 19 sites over the three week period during the early spring sampling (3 of the rural low-road-density landscapes; 1 rural high-road-density landscape; 1 urban). The multiple stepwise logistic regression analysis revealed no significant effects of road density, PC1 or PC2 on the presence of P. leucopus in a forest site during the early spring (Road Density: Wald χ2 = 0.493, P = 0.482, PC 1: Wald χ2 = 0.795, P = 0.372; PC 2: Wald χ2 = 0.016, P = 0.898; n = 19).
The two most frequently tracked species over the summer sampling period were P. leucopus and the short-tailed shrew (Blarina brevicauda), making up 78% and 16.9% of the total number of paper tracked respectively. Other species tracked included the meadow jumping mouse (Zapus hudsonius—2.5%), the woodland jumping mouse (Napaeozapus insignis—1.6%) and the red squirrel (Tamiasciurus hudsonicus), the meadow vole (Microtus pennsylvanicus), and the eastern chipmunk (Tamias striatus), which when combined comprised <1% of the total number of tracked papers in our sites.
Of the 5 sites that contained no P. leucopus tracks during the spring tracking phase, 4 were later found to contain tracks of P. leucopus during the summer tracking period. There were a total of 877 tracking papers containing P. leucopus tracks over the eight week summer tracking period. The multiple stepwise linear regression revealed no effect of PC1 or PC2, and a significant positive effect of road density on the relative abundance of P. leucopus across all 19 sites (Fig. 2A: F = 48.1, P < 0.001). When the urban landscapes were removed from the analysis, road density was again the only variable retained in the model, showing a significant positive relationship to the relative abundance of P. leucopus across the 14 rural landscapes (Fig. 2B: F = 5.79, P = 0.033).
(A) Simple linear regression of the relative abundance of Peromyscus leucopus on road density. There was a significant positive relationship between the relative abundance of P. leucopus and road density across all 19 sites (F = 48.070; P < 0.001; R 2 = 0.739), (B) Simple linear regression of the relative abundance of Peromyscus leucopus on road density for the 14 rural landscapes only. When the urban landscapes were removed from the analysis, there was still a significant positive relationship between the relative abundance of P. leucopus and road density in the 14 rural landscapes (F = 5.791; P = 0.033; R 2 = 0.326). Key: rural low road density (•); rural high road density (■); urban sites (▴)
Live trapping in the fall produced 3 individual P. leucopus in the rural low-road-density site, 8 in the rural high-road-density site, and 30 in the urban site, compared to 10, 72, and 149 tracked papers respectively over the summer tracking period in these three sites.
Post-hoc analyses
In contrast to our prediction, we found a positive relationship between P. leucopus relative abundance and road density. Due to this unexpected result, we carried out several post-hoc analyses to evaluate possible explanations for this positive relationship. There are many differences between urban landscapes and rural landscapes that could lead to higher P. leucopus densities in urban landscapes, where road density is highest (see Discussion). Therefore, in the post-hoc analyses we focussed on the 14 rural landscapes only, for which there was still a positive relationship between road density and P. leucopus relative abundance.
Road density at finer scales
We first evaluated the possibility that our results were an artifact of the spatial scale at which road density was measured (2.0-km radius around the P. leucopus sampling sites). We calculated road density at 1.5, 1.0, and 0.5-km radii from the sampling sites. We conducted the same logistic and simple linear regressions at each scale as we had conducted at the 2.0-km scale. There was no qualitative difference in the results for the different scales: at the 1.5, 1.0, and 0.5-km scales (i) there was no significant relationship between the presence of P. leucopus in the early spring and road density (respectively: Wald χ2 = 2.08, P = 0.149; Wald χ2 = 0.033, P = 0.856; Wald χ2 = 0.213, P = 0.645; n = 14), and (ii) there was a significant positive effect of road density on P. leucopus relative abundance during the summer (respectively: R 2 = 0.318, F = 5.59, P = 0.036; R 2 = 0.384, F = 7.48, P = 0.018; R 2 = 0.498, F = 11.91, P = 0.005; n = 14).
Number of buildings
Buildings (i.e., houses, garages, barns and silos) may provide over-winter refuge against cold stress and lack of food for P. leucopus (Gill and Bonnett 1973). Since landscapes with higher road densities likely contain more buildings, this could produce an indirect positive relationship between road density and P. leucopus relative abundance. We determined the number of buildings in each of the 14 rural landscapes. There was a significant positive correlation between the number of buildings and road density (r = 0.547, P = 0.043). There was a marginally significant positive effect of the number of buildings on P. leucopus relative abundance (R 2 = 0.273, F = 4.52, P = 0.055).
Accessible habitat
We considered the possibility that in the high-road-density landscapes, roads restrict the white-footed mice within the forest patches, inhibiting emigration and causing higher local populations. To test this we measured the amount of accessible habitat within each of the rural landscapes, where accessible habitat is the amount of forest available to mice in the focal site without having to cross a road (F. Eigenbrod, pers. comm.). There was a significant negative correlation between the amount of accessible habitat and road density (r = −0.795, P = 0.001). There was no significant relationship, however, between accessible habitat and P. leucopus relative abundance (R 2 = 0.031, F = 0.381, P = 0.549).
Number of forest patches
Several studies have found that small mammal densities are higher in smaller patches (Yahner 1992; Nupp and Swihart 1996, 2000; Krohne and Hoch 1999; Schmid-Holmes and Drickamer 2001; Anderson et al. 2003), although this was not the case in our focal patches. We considered that if the landscapes with higher road densities contained more, smaller forest patches than landscapes with lower road densities, it could be possible that the landscapes with higher road densities may support higher overall abundances of mice, despite the total amount of forest being similar between landscape types. This would lead to an apparent positive effect of road density on P. leucopus relative abundance. We determined the number of forest patches within each of the 14 rural landscapes. There was a positive correlation between the number of forest patches and road density (r = 0.661, P = 0.010). There was no significant relationship, however, between P. leucopus relative abundance and the number of forest patches in the landscapes (R 2 = 0.155, F = 2.21, P = 0.163).
Agricultural land use
Wegner (1995) reported that P. leucopus occupied both grain and corn fields in agricultural landscapes in eastern Ontario, but were rarely captured in hay fields. We considered the possibility that if our high-road-density rural landscapes contained more grain and corn fields, this could lead to an apparent positive relationship between road density and P. leucopus abundance. Field type could not be determined from remotely sensed imagery, but had to be determined on the ground by speaking with individual farmers. For practical reasons we could not do this over the 2-km radius landscapes. We estimated the percentages of cereal grains (oats, wheat, barley), corn, and hay (timothy, alfalfa, clover) within 500-m radius buffers around each focal forest patch in the 14 rural landscapes. There was no significant correlation between road density and the combined percentage cover of grains and corn (r = 0.097, P = 0.741). There was also no relationship between P. leucopus relative abundance and percentage cover of grains and corn (R 2 = 0.031, F = 0.390, P = 0.544).
Multivariate analysis (Post-hoc variables)
To evaluate the possibility that several of the post-hoc explanations were acting together to create the positive relationship between road density and P. leucopus relative abundance, we combined the post-hoc variables (number of dwellings, number of forest patches, amount of accessible habitat, and percent grain + corn) using PCA. We then conducted a multiple regression of P. leucopus relative abundance on the first two principle components from the PCA. The first principle component and PC2 accounted for 77.4% of the total variation in number of dwellings, number of forest patches, the amount of accessible habitat, and the percent grain + corn. Neither PC1 nor PC2, however, was significantly related to the relative abundance of P. leucopus (PC 1: F = 1.58, P = 0.142; PC 2: F = 0.722, P = 0.485).
Discussion
We predicted that forest patches situated in landscapes with high road densities would have a lower chance of white-footed mice being present during the early spring than forest patches situated in landscapes with low road densities. We found no significant relationship, however, between road density and the presence of P. leucopus during the early spring, and this result was not affected by the scale over which road density was estimated. In fact, the trend in the data was opposite to our prediction (i.e., three of the five sites with no P. leucopus tracks during the early spring sampling were in rural low-road-density landscapes). This is the first study to test this hypothesis and as such there are no published reports to which we can compare our findings.
We also predicted that P. leucopus populations during the summer would be smaller in forest patches situated in landscapes with high road densities than in landscapes with low road densities. We found the opposite result, however: as road density increased, the relative abundance of P. leucopus increased (Fig. 2A). This result was not due to the possible confounding factors that we had considered during the design of the study (the percentage of forest in the landscape, focal patch size, focal patch shape, the percentage of coarse woody debris, the number of tree species, or the density of shrubs). After combing these variables using PCA, neither of the principle components was retained in the regression model. We conclude that road density does not have a negative effect on P. leucopus populations as we had predicted. In fact, our results suggest that there is a positive effect of road density on the relative abundance of P. leucopus.
Note, however, that the higher abundances found in the urban landscapes were not completely unexpected, and this was the reason that we had initially limited our site selection to rural landscapes. There is other evidence that urban forest patches may support greater abundances of small mammals, but some of the suggested reasons for these observations do seem speculative. For example, Barko et al. (2003) found a higher abundance of P. leucopus in sites in Illinois that were surrounded by a large percentage of urban habitats and a small percentage of upland deciduous forest than in sites surrounded by a large percentage of upland deciduous forest and a low percentage of urban habitats. They suggested that forest patches in urban areas are surrounded by unsuitable habitat, which may create island habitats from which small mammals do not emigrate, resulting in higher densities in these urban sites. Higher temperature in urban than rural areas (McDonnell et al. 1997) may also contribute to greater abundances of small mammals in urban forest patches. According to Gill and Bonnett (1973), the largest effect of increased urban heat occurs in winter, when the minimum temperatures are significantly higher in the city than in the rural areas. Mice in urban forest patches may benefit from these higher temperatures through reduced winter mortality. In addition, the large number of buildings in urban landscapes could be used as shelter over winter which could greatly increase survival of P. leucopus over winter, leading to higher abundances there. In our study, there were on average 4501 buildings in the urban landscapes compared to 79 in the rural high-road-density landscapes and 45 in the rural low-road-density landscapes. Along with increased shelter in urban landscapes, gardens, bird feeders, and fruit trees may provide additional food. Baker et al. (2003) suggested that the availability of good quality gardens likely more than balances the negative effects of higher predation and habitat fragmentation in urban areas, and that good quality gardens may even act as source populations for small mammals. Therefore, the higher densities in urban areas may not be a result of higher road densities, but may be related to other variables that differ between urban and rural sites.
When excluding urban landscapes, however, from our linear regression analysis, we still found a significant positive relationship between P. leucopus relative abundance and road density across only the 14 rural sites (Fig. 2B). This is the first study to test for this relationship and therefore the first to demonstrate this unexpected relationship. Comparisons between live trapping and tracking showed similar trends, indicating that tracking tubes were a reliable sampling method, and therefore our result was not an artefact of the sampling method.
Our post-hoc analyses did not satisfactorily explain the positive relationship between road density and P. leucopus relative abundance in the rural sites. First, the relationship remained positive when we evaluated road density at different scales. In fact, the relationship was even stronger when we evaluated road density within the 1-km and 0.5-km radius landscapes than within the 2-km radius landscapes. Second, while there were more buildings in landscapes with higher road densities, the relationship between P. leucopus relative abundance and the number of buildings was weaker than the relationship between P. leucopus relative abundance and road density for the rural landscapes (respectively, R 2 = 0.273 and R 2 = 0.326, n = 14). This suggests that the number of buildings alone did not cause an apparent increase in P. leucopus relative abundance with increasing road densities in rural landscapes. Third, while landscapes with low road density had significantly more accessible habitat than landscapes with high road density, there was no significant relationship between accessible habitat and P. leucopus relative abundance. Fourth, the crops surrounding the forest patches in the high-road-density rural landscapes were not more likely to contain grains and corn (nesting habitat) than the crops surrounding the forest patches in the low-road-density rural landscapes, and there was no relationship between P. leucopus relative abundance and the amount of these crops in the surrounding landscapes. Finally, there was no evidence that the positive relationship between road density and P. leucopus relative abundance was due to a combination of these four possibilities.
There is yet another possible explanation for the positive effect of road density on P. leucopus relative abundance, which we had no data to test. Peromyscus leucopus could experience a release from predation in areas with more roads. Predator populations may be susceptible to road effects, whether through road mortality, habitat destruction, or road avoidance. Predators of P. leucopus include owls, hawks, falcons, striped skunks, weasels, racoons, foxes, and coyotes. In a survey of road-killed vertebrates along a motorway in western France, Lodé (2000) found that the motorway had a considerable impact on rodent predators (21.7% of the vertebrate casualties). Bautista et al. (2004) found that some raptors avoid roads with high traffic loads. This could lead to lower predation pressure on rodents in landscapes with more high-traffic roads. Others have reported, however, the use of roads by raptors for hunting (Meunier et al. 2000) including in the Ottawa area (M. Runtz, pers. comm.). In contrast, nocturnal owls may avoid roadsides due to disturbance by traffic (M. Runtz, pers. comm.). Negative effects of roads could also occur for medium to large-sized mammalian predators (Mladenoff et al. 1999; Lodé 2000; Seiler et al. 2004; Hell et al. 2005) and also snakes (Shine et al. 2004; Andrews and Gibbons 2005), which could indirectly produce positive effects of roads on small mammals.
Our results cannot be generalized to say that roads have a positive effect on all small mammals. The next most abundant species tracked over our summer tracking period, however, the short-tailed shrew (Blarina brevicauda), showed the same general trend, with a higher relative abundance in rural high-road-density sites compared to rural low-road-density sites (103 vs. 66 tracks recorded, respectively).
What are the general implications of this study for predictions about roads and animal populations? Numerous studies have shown that roads can act as barriers to small mammal movement. We have found, however, that this negative effect of roads on movement does not necessarily translate into a negative effect on the population. Movement is only one component of population dynamics. While roads may fragment habitats, this subdivision may not be as important as indirect effects of roads on mortality and reproduction. We hypothesize that the positive effect of roads on P. leucopus populations is due to as-yet-undocumented correlations between roads and increased habitat quality and/or decreased predation, leading to increased reproduction and/or decreased mortality respectively in landscapes with high road density.
References
Adler GH, Wilson ML (1987) Demography of a habitat generalist, the white-footed mouse, in a heterogeneous environment. Ecology 68:1785–1796
Anderson CB, Cady AB, Meikle DB (2003) Effects of vegetation structure and edge habitat on the density and distribution of white-footed mice (Peromyscus leucopus) in small and large forest patches. Can J Zool 81:897–904
Andrews KM, Gibbons JH (2005) How do highways influence snake movements? Behavioural responses to roads and vehicles. Copeia 4:772–782
Baker PJ, Ansell RJ, Dodds PAA, Webber CE, Harris S (2003) Factors affecting the distribution of small mammals in an urban area. Mammal Rev 33:95–100
Barko VA, Feldhamer GA, Nicholson MC, Davie DK (2003) Urban Habitat: a determinant of white-footed mouse (Peromyscus leucopus) abundance in southern Illinois. Southeastern Nat 2:369–376
Barry RE Jr, Francq EN (1980) Orientation to landmarks within the preferred habitat by Peromyscus leucopus. J Mammal 61:292–303
Bautista LM, Garcia JT, Calmaestra RG, Palacin C, Martin CA, Morales MB, Bonal R, Vinuela J (2004) Effect of weekend road traffic on the use of space by raptors. Conserv Biol 18:726–732
Bendell JF (1961) Some factors affecting the habitat selection of the white-footed mouse. Can Field-Nat 75:244–245
Blem LB, Blem CR (1975) The effects of flooding on length of residency in the white-footed mouse, Peromyscus leucopus. Am Midl Nat 94:232–236
Bowman JC, Edwards M, Sheppard LS, Forbes GJ (1999) Record distance for a non-homing movement by a Deer Mouse, Peromyscus maniculatus. Can Field-Nat 113:292–293
Brown KP, Moller H, Innes J, Alterio N (1996) Calibration of tunnel tracking rates to estimate relative abundance of ship rats (Rattus Rattus) and mice (Mus musculus) in a New Zealand forest. New Zealand J Ecol 20:271–275
Clarke BK, Clarke BS, Johnson LA, Haynie MT (2001) Influence of roads on movements of small mammals. Southwestern Nat 46:338–344
Diffendorfer JE, Slade NA (2002) Long-distance movements in cotton rats (Sigmodon hispidus) and prairie voles (Microtus ochrogaster) in northeastern Kansas. Am Midl Nat 148:309–319
Drennan JE, Beier P, Dodd NL (1998) Use of track stations to index abundance of sciurids. J Mammal 79:352–359
Drickamer LC (1990) Microhabitat preferences of 2 species of deermice Peromyscus in a northeastern United-States deciduous hardwood forest. Acta Theriologica 35:241–252
Dueser RD, Shugart HH Jr (1978) Microhabitats in a forest-floor small mammal fauna. Ecology 59:89–98
Faeth SH, Kane TC (1978) Urban biogeography – City parks as islands for Diptera and Coleoptera. Oecologica 32:127–133
Fahrig L, Merriam G (1985) Habitat patch connectivity and population survival. Ecology 66:1762–1768
Forman RTT (2000) Estimate of the area affected ecologically by the road system in the United States. Conserv Biol 14:31–35
Forman RTT, Alexander LE (1998) Roads and their major ecological effects. Annu Rev Ecol Syst 29:207–231
Forman RTT, Sperling D, Bissonette JA, Clevenger AR, Cutshall CD, Dale VH, Fahrig L, France R, Goldman CR, Heanue K, Jones JA, Swanson FJ, Turrentine T, Winter TC (2003) Road ecology: science and solutions. Island Press, Washington
Garland T Jr, Bradley WG (1984) Effects of a highway on Mojave Desert rodent populations. Am Midl Nat 111:47–56
Gill D, Bonnett P (1973) Nature in the urban landscape. York Press Inc., Baltimore
Glennon MJ, Porter WF, Demers CL (2002) An alternative field technique for estimating diversity of small mammal populations. J Mammal 83:734–742
Gottfried BM (1979) Small mammal populations in woodlot islands. Am Midl Nat 102:105–112
Hansen LP, Warnock JE (1978) Response of two species of Peromyscus to vegetational succession on land strip-mined for coal. Am Midl Nat 100:416–423
Hell P, Plavy R, Slamecka J, Gasparik J (2005) Losses of mammals (Mammalia) and birds (Aves) on roads in the Slovak part of the Danube Basin. European J Wildlife Research 51:35–40
Howard WE (1960) Innate and environmental dispersal of individual vertebrates. Am Midl Nat 63:152–161
Kozakiewicz MA (1993) Habitat isolation and ecological barriers – the effect on small mammal populations and communities. Acta Theriologica 38:1–30
Kozakiewicz M, Kozakiewicz A, Lukowski A, Gortat T (1993) Use of space by bank voles (Clethrionomys glareolus) in a polish farm landscape. Landsc Ecol 8:19–24
Kozel RM, Fleharty ED (1979) Movements of rodents across roads. Southwestern Nat 24:239–248
Krebs CJ (1989) Ecological methodology. Harper Collins Publishers, New York
Krohne DT, Hoch GA (1999) Demography of Peromyscus leucopus populations on habitat patches: the role of dispersal. Can J Zool 77:1247–1253
Krohne DT, Merritt JF, Vessey SH, Wolff JO (1988) Comparative demography of forest Peromyscus. Can J Zool 66:2170–2176
Lee SD (2004) Population dynamics and demography of deermice (Peromyscus maniculatus) in heterogeneous habitat: Role of coarse woody debris. Polish J Ecol 52:55–62
Lodé T (2000) Effect of a motorway on mortality and isolation of wildlife populations. Ambio 29:163–166
Mader HJ (1984) Animal habitat isolation by roads and agricultural fields. Biol Conserv 29:81–96
Maier TJ (2002) Long-distance movements of female White-footed Mice, Peromyscus leucopus, in extensive mixed-wood forest. Can Field-Nat 116:108–111
McDonnell MJ, Pickett STA, Groffman P, Bohlen P, Pouyat RV, Zipperer WC, Parmeler RW, Carreiro MM, Medley K (1997) Ecosystem processes along an urban-to-rural gradient. Urban Ecosystems 1:21–36
Merriam G (1990) Ecological processes in time and space of farmland mosaics. In: Zonneveld IS, Forman RTT (eds) Changing landscapes: an ecological perspective. Springer-Verlag, New York, pp 121–33
Merriam G, Wegner J (1992) Local extinctions, habitat fragmentation, and ecotones. In: Hansen AJ, di Castri F (eds) Landscape boundaries: consequences for biotic diversity and ecological flows. Springer-Verlag, New York, pp 150–159
Merriam G, Kozakiewicz M, Tsuchiya E, Hawley K (1989) Barriers as boundaries for metapopulations and demes of Peromyscus leucopus in farm landscapes. Landsc Ecol 2:227–235
Meunier FP, Verheyden C, Jouventin P (2000) Use of roadsides by diurnal raptors in agricultural landscapes. Biol Conserv 92:291–298
Middleton J (1979) Insular biogeography in a rural mosaic: the evidence of Peromyscus leucopus. Dissertation, Carleton University
Mladenoff DJ, Sickley TA, Wydeven AP (1999) Predicting gray wolf landscape recolonization: logistic regression models vs. new field data. Ecol Appl 9:37–44
Morris DW (1986) Proximate and ultimate controls on life-history variation: the evolution of litter size in white-footed mice (Peromyscus leucopus). Evolution 40:169–181
Murie OJ, Murie A (1931) Travels of Peromyscus. J Mammal 12:200–209
Nams VO, Gillis EA (2002) Changes in tracking tube use by small mammals over time. J Mammal 84:1374–1380
Nupp TE, Swihart RK (1996) Effect of forest patch area on population attributes of white-footed mice (Peromyscus leucopus) in fragmented landscapes. Can J Zool 74:467–472
Nupp TE, Swihart RK (2000) Landscape-level correlates of small-mammal assemblages in forest fragments of farmland. J Mammal 81:512–526
Oxley DJ, Fenton MB, Carmody GR (1974) The effects of roads on populations of small mammals. J Appl Ecol 11:51–59
Schmid-Holmes S, Drickamer LC (2001) Impact of forest patch characteristics on small mammal communities: a multivariate approach. Biol Conserv 99:293–305
Seamon JO, Adler GH (1996) Population performance of generalist and specialist rodents along habitat gradients. Can J Zool 74:1130–1139
Seiler A, Helldin JO, Seiler C (2004) Road mortality in Swedish mammals: results from a drivers’ questionnaire. Wildlife Biol 10:225–233
Sheppe WA (1965) Characteristics and uses of Peromyscus tracking data. Ecology 46:630–634
Shine R, Lemaster M, Wall M, Langkilde T, Mason R (2004) Why did the snake cross the road? Effect of roads on movement and location of mates by garter snakes (Thamnophis sirtalis parietalis). Ecology and Society 9:9–21
Smith DA, Speller SW (1970) The distribution and behaviour of Peromyscus maniculatus gracilis and Peromyscus leucopus noveboracensis (Rodentia: Cricetidae) in a southeastern Ontario woodlot. Can J Zool 48:1187–1199
Speller SW (1968) Habitat selection and behaviour in two sympatric species of Peromyscus in south-eastern Ontario. Dissertation, Carleton University
SPSS for Windows, Rel 10.0.0 (1999) Chicago: SPSS Inc
Swihart RK, Slade NS (1984) Road crossing in Sigmodon hispidus and Microtus ochrogaster. J Mammal 65:357–360
Taylor DG (1978) The population biology of white-footed mice in an isolated and a non-isolated woodlot in south-eastern Ontario. Dissertation, Carleton University
Vessey SH (1987) Long-term population trends in white-footed mice and the impact of supplemental food and shelter. Am Zool 27:879–890
Wegner J (1995) Habitat distribution, spatial dynamics and reproduction of a forest rodent (Peromyscus leucopus) in an agricultural landscape. Dissertation, Carleton University
Wegner JF, Merriam G (1990) Use of spatial elements in a farmland mosaic by a woodland rodent. Biol Conserv 54:263–276
Whitaker JO Jr (1967) Habitat relationships of four species of mice in Vigo County, Indiana. Ecology 48:867–872
Yahner RH (1992) Dynamics of a small mammal community in a fragmented forest. Am Midl Nat 127:381–391
Zollner PA, Crane KJ (2003) Influence of canopy closure and shrub coverage on travel along coarse woody debris by eastern chipmunks (Tamias striatus). Am Midl Nat 150:151–157
Acknowledgments
We thank Mark Forbes and Francis Pick and two anonymous reviewers for their helpful comments on an earlier draft. This study was supported by a Natural Sciences and Engineering Research Council of Canada (NSERC) scholarship to T.R. and an NSERC research grant to L.F.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rytwinski, T., Fahrig, L. Effect of road density on abundance of white-footed mice. Landscape Ecol 22, 1501–1512 (2007). https://doi.org/10.1007/s10980-007-9134-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10980-007-9134-2