Abstract
Nowadays 37% of Earth’s ice-free land is composed by fragments of natural habitats settled in anthropogenic biomes. Therefore, we have to improve our knowledge about distribution of organisms in remnants and to understand how the matrix affects these distributions. In this way, the present study aims to describe the structure of the butterfly assemblages and determined how richness and abundance are influenced by the scale of the surrounding vegetation. General linear models were used to investigate how the type and scale of vegetation cover within a radius of 100–2,000 m around the sampling point explained butterfly diversity. After sampling ten forest fragments we found 6,488 individuals of 73 species. For all clades tested null models explain the species richness at the fragments better than other models when we include the effect of butterfly abundance as a covariate. Abundance of Satyrini, Brassolini, and Biblidinae were best predicted by small scales (100–200 m), and large scales were more suited for Charaxinae. The presence of pasture best explains the abundance of all groups except Charaxinae, which was best explained by early-regrowth forest. The abundance of different species and groups are correlated with different kinds of vegetation cover. However, we demonstrate that small scales (100–200 m) are more effective at explaining the abundance of most butterflies. These results strongly suggest that efforts to preserve insect diversity in forest fragments should take in account the immediate surroundings of the fragment, and not only the regional landscape as a whole. In general, actions of people living near forest fragments are as important to fruit-feeding butterflies as large scale actions are, with the former being seldom specified in management plans or conservation policies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wild undisturbed habitats have been the main topic of ecological research since the word “Ecology” was first used by Ernst Haeckel (1866). However, these habitats cover only 11% of the Earth surface (Ellis et al. 2010; Ellis and Ramankutty 2008) with more than 75% of Earth’s ice-free land exhibiting evidence of alteration as a result of human residence and land use (Ellis and Ramankutty 2008). Almost 39% has been converted into agricultural land and settlements and 37% of global land without such use, is settled in anthropogenic biomes (Ellis et al. 2010). Therefore, we should recognize the importance of studying fragmented landscapes which comprise more than one-third of earth’s surface.
In Brazil, the Atlantic forest is a severely fragmented and threatened biome that represents a biodiversity hotspot characterized by high levels of endemism and species diversity (Brown and Brown 1992; Morellato and Haddad 2000). In this biome more than 97% of the remnants are smaller than 250 ha which comprises almost half of the remaining area (Ribeiro et al. 2009). These remnants are spread over 150 million ha that cover 29º of latitudinal extension inside a mosaic of croplands, ranching areas, urban settlement and abandoned areas. The remaining areas comprise eight biogeographical sub regions with several endemic species (Ribeiro et al. 2009). In view of the great extension and diversity of this fragmented landscape, it is valuable to increase our understanding of local biota in these areas.
The fauna and flora of fragments is often different from that of continuous landscapes (Saunders et al. 1991; Cook et al. 2002; Ewers and Didham 2006). Forest fragmentation has been shown to affect a wide range of taxa including vertebrates, arthropods and woody plants (Tabarelli et al. 1999; Roslin 2002; Esch et al. 2005; Marsh et al. 2005; Yamaura et al. 2005; Pearson and Dawson 2005), with recent studies showing that fragmentation changed the composition of several insect groups (e.g., Kitahara and Fujii 1994; Magura et al. 2001; Hunter 2002; Cairns et al. 2005; Shahabuddin and Ponte 2005; Dover and Settele 2009; Collier et al. 2010).
Among the insect groups affected by forest fragmentation, butterflies are considered an ideal group for such studies (Bonebrake et al. 2010). They are relatively large, conspicuous, easy to sample, and have a well-known taxonomy. Accordingly, butterflies are among the most studied insects, offering great potential insights into patterns of diversity and conservation of insects and their habitats (DeVries 1987; DeVries et al. 1997; Brown 1991).
Habitat fragmentation can affect richness, abundance, diversity and composition of communities of butterflies (e.g., Summerville and Crist 2001; Cook et al. 2002; Horner-Devine et al. 2003; Koh and Sodhi 2004; Veddeler et al. 2005; Shahabuddin and Ponte 2005; Collier et al. 2010). Butterflies also respond to characteristics of vegetation structure (e.g., Shahabuddin and Terborgh 1999; Ramos 2000; Dover and Settele 2009) and may explore the surrounding matrix (Dennis 2004; Dennis and Hardy 2007). The traits of the surrounding matrix can be as important as remnant traits to fauna in fragmented landscapes, probably, because our view of habitat is different from that experienced by the studied taxon (Ricketts 2001; Vandermeer and Carvajal 2001; Prugh et al. 2008; Franklin and Lindenmayer 2009). Therefore, it is important to understand how the entire landscape mosaic can affect the butterfly fauna instead of focusing only on traits of the remaining patches (Dover and Settele 2009; Pocewicz et al. 2009; Ekroos and Kuussaari 2011).
Several factors across multiple scales could affect richness and abundance of butterflies in a given location and it is recognized that scale is very important for planning conservation strategies (Hamer and Hill 2000; Whittaker et al. 2001; Dungan et al. 2002; Clark et al. 2007). Although, many important ecological patterns are scale-dependent (Weibull et al. 2000; Cowley et al. 2001; Steffan-Dewenter et al. 2002; Debinski et al. 2001; Summerville et al. 2002; Krauss et al. 2003; MacNally et al. 2004; Ribas et al. 2005; Luoto et al. 2007) or influenced by the sampling and analyses scale (MacNally 1997; He and Gaston 2000; Dennis and Hardy 2001; Lennon et al. 2001; Whittaker et al. 2001; Schneider 2003), we have to understand which scale is most influential on faunal diversity patterns.
To enhance the understanding of diversity patterns in fragmented landscapes and how these are influence by the surrounding vegetation matrix we aim to:
-
(a)
Describe the assemblage of fruit-feeding butterflies in an intensely fragmented landscape;
-
(b)
Determine which scale of the vegetation matrix is most important for describing the diversity of butterfly assemblages.
Methods
Study site
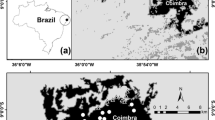
Field work was carried out in the municipality of São Luiz do Paraitinga, São Paulo State, Southeastern Brazil, near the scarps of the Serra do Mar mountain range (Fig. 1). The average altitude in this region is 740 m, and the relief consists of a complex landscape with rolling hills, steep escarpments, and deep, narrow valleys (MME 1983). The climate is humid without a marked dry season. Annual average temperature is 20.2°C (monthly min (July) 9.5°C, max (January) 28.6°C) and average annual rainfall is 1,340 mm (MME 1983).
The original vegetation of this area was dense humid forest (MME 1983). Over the past two centuries, forest fragmentation has drastically changed the landscape of the region (Dean 1997). At the beginning of the eighteenth century, human activity in this landscape replaced native rain forest with ranching areas and small crop fields. Around 1760, massive coffee plantations were planted in this region, and deforestation rates increased. Deforestation then accelerated with the decline of coffee plantations and the increase of dairy farming in the 1930s (Petrone 1959). Currently, most of the original vegetation is gone, and the remaining forest persists as small fragments surrounded by a matrix of crop fields, Eucalyptus plantations, ranching areas and abandoned pastures (Fig. 1). The percentage of remaining forest cover in this landscape is 10% of the total area, or approximately 140 ha.
Studied group
Tropical butterflies fall naturally into two adult feeding guilds (DeVries 1987). The first guild, those butterflies that feed primarily on flower nectar, includes most species in the six known butterfly families. The second guild, those whose adults gain virtually all of their nutritional requirements by feeding on rotting fruits or plant sap, is represented mainly by species in the subfamilies Biblidinae, Charaxinae, Satyrinae (including Satyrini, Brassolini and Morphini), and the tribe Coeini (Nymphalinae), all in the family Nymphalidae (sensu Wahlberg et al. 2009).
Sampling fruit-feeding butterflies offers some practical advantages, as they can be captured easily in traps containing rotting fruits and simultaneous sampling with standardized effort at different sites is feasible. After identification, most butterflies can be marked and released unharmed, so that recaptures can be evaluated with minimum handling (DeVries 1987). The richness of fruit-feeding butterflies represents 50–75% of the total richness of the Neotropical Nymphalidae (Brown 2005) and its richness is related to total butterfly richness in a given area (Brown and Freitas 2000a; Horner-Devine et al. 2003).
Sampling methods
To test the influence of the landscape context on butterfly diversity, 10 fragments varying from 11 to 83 ha (1—15.8 ha; 2—53.8 ha; 3—22.2 ha; 4—42.3 ha; 5—13.2 ha; 6—55.2; 7—11.0 ha; 8—17.4 ha; 9—83.4 ha and 10—17.9 ha, see Fig. 1) with similar structural characteristics (size, shape and normalized difference vegetation index (NDVI)) were chosen from two adjacent river basins of similar area according to matrix composition (Fig. 1). A sampling unit was placed in each fragment. The sampling unit consisted of five portable traps baited with a mixture of banana and sugar cane juice fermented for 48 h. The traps were arranged 30 meters apart along a linear transect, at least 30 m distant from the forest edge inside the fragment. Distances from edge and from water bodies, vegetation structure, terrain declivity and logistic aspects were considered (as much as possible) to minimize the heterogeneity in the captures and to maximize comparability rate between traps (Ribeiro and Freitas 2011; Ribeiro et al. 2008; Ribeiro et al. 2010).
Butterflies were sampled over 12 months from June 2004 to May 2005. In each month, the traps remained open in the field during eight consecutive days and were visited in intervals of 48 h. On each visit, the butterflies captured were identified, marked and released. Any specimens not identified were collected for subsequent identification. For more detailed information on the methods and study site see Ribeiro et al. (2008).
Data analyses
Butterfly assemblage description
We calculated indices of richness, diversity and similarity (Morisita–Horn, Sorensen). We calculated Chao2 and Jacknife for richness estimates for the assemblage of fruit-feeding butterflies in each fragment. These richness estimates were calculated with the software EstimateS 7.5 (Colwell 2005) to evaluate whether the samples adequately represent the total community. We used Fisher’s alpha as a diversity index because it is a robust, trustworthy index, little affected by sample size and broadly used in biodiversity studies (Magurran 2004). Fisher’s Alpha diversity indices were compared using bootstrap confidence intervals calculated by Past ® (Hammer et al. 2001). The critical values of α (Fisher’s Alpha) were corrected with the false discovery rate (FDR) procedure that diminishes the incidence of type I errors (reject the null hypothesis when it is true).
We used rarefaction curves to eliminate sample size influence in richness comparisons. Curves were calculated using an individual based approach (Gotelli and Graves 1996) to make comparisons of samples of different sizes possible (Gotelli and Colwell 2001) (Fig. 2).
Rarefaction analyses of the assemblage of fruit-feeding butterflies in a fragmented landscape in São Luiz do Paraitinga São Paulo, Brazil. Each point represents one of the forest fragments sampled in the present study, following Fig. 1
Correlation between fruit-feeding butterfly assemblage and landscape characteristics
We analyzed the land-cover characteristics at different scales around the sampling unit in order to determine the influences of the surrounding matrix on the distribution of butterflies. This procedure allows us to identify the most influential landscape scales for the assemblage structure of fruit-feeding butterflies. Each scale was defined as a concentric circle of 100, 200, 300, 400, 500, 600, 700, 800, 900, 1000 and 2000 m radius around each sampling unit. In each scale, land-cover around the central point of each sample unit was classified in four categories using satellite (SPOT) images with resolution of 5 × 5 m (taken in 2002): Eucalyptus plantation, pasture, early-regrowth forest and secondary forest. These land-cover classes were identified in the circles by a supervised classification of the images. Segmentation routines with the Spring software (Câmara et al. 1996) were used to identify clusters of pixels with the same spectral signature. These clusters were then assigned to land-cover classes by the Battacharya algorithm using 143 training points of known cover classes, taken by direct verification in the study area. For each cover class we sampled at least 15 training points. The minimum final accuracy of the classification was 85%.
We used the proportion of each circle occupied by the four most important cover classes (pasture, Eucalyptus plantation, early-regrowth forest and secondary forest) as predictors of the abundance and richness of the most abundant and species-rich clades: Biblidinae, Charaxinae, Brassolini and Satyrini (these clades were chosen following earlier results of our research, see Ribeiro et al. 2010) except for Eucalyptus at 100 m radius, since this type of land cover have not enough variation to be tested by the model at this scale. We fitted generalized linear models (GLM, Dobson 1990), with each cover class at each scale as independent variables, and also a null model (absence of effects), for a total of twelve models. These single effect models were then used to evaluate the relative importance of cover and the circle radius of the surrounding vegetation using Akaike Information Criterion (AIC) weights. Models with additive effects of more than one cover class were not included because they always performed worse than the single effect models, and also because the high correlation among explanatory variables in many cases. We used the AIC corrected for small sample sizes (AICc) to rank the models according to their relative plausibility. The AICc is calculated for each model from their log-likelihoods, number of parameters and sample size, and the model with the lowest AICc is the best one among the candidates. The support of each alternative model relative to the best one is gauged by the differences in their AICc values (∆i). For large samples and independent observations, a value of ∆i lower than 2 indicates equally plausible models. From the AICc values we calculated the evidence weights of each model, which add up to one and thus express the relative quality of each model in a standardized scale. Weights of models that share a variable can be summed to express the overall importance of this variable: (Burnham and Anderson 2004). This is a valid use of the model selection approach that provides a direct way to estimate the relative importance of each variable (Burnham and Anderson 2002, 2004). Hence, the importance of each scale on the dependent variables were gauged by the sum of the weights of the three models that included cover classes at that scale as predictors. We used the same logic to gauge the importance of each cover class, irrespective of the scale. We used normal errors for abundances and Poisson errors for species richness. The logarithm of the number of recorded individuals in each patch was included as covariate in all models for richness, to control for the effects of abundance. We fit the models with the glm function of the R software (R Development Core Team 2009), and calculated AICc and weights with the bbmle R package (Bolker 2010).
We then used the scales that best explained butterfly abundances to perform a canonical correspondence analysis (CCA). We only used the butterfly species with N ≥ 10 in this analysis. The CCA was used to verify how each species or group of species respond to the different scales and land cover analyzed. The CCA was performed with PAST software (Hammer et al. 2001).
Results
Butterfly assemblage description
In all, 6,488 individuals of 73 species were sampled in four subfamilies (see Supplementary material S2); we had 1,884 recaptures with only two between fragments (captured in fragment 2 and recaptured in fragment 3, see Fig. 1). According to the richness estimators (Chao 2 = 80.56 ± 5.96; Jacknife 1 = 83.89 ± 3.46) the local assemblage of fruit-feeding butterflies was almost completely sampled, and the number of butterfly species that would be present in the community but were not sampled is between 2 to 14 species. The average similarity among all fragments was 77.4% for Sorensen and 78.0% for Morisita–Horn index. About a third of all captured butterflies (2,053 individuals) belong to two species typical of forest edges and clearings: Hamadryas epinome and Myscelia orsis (Biblidinae). For the landscape as a whole we recorded 12 singletons and 6 doubletons (Table 1). The most abundant species (more than 100 individuals recorded) included three in the subfamily Biblidinae (Hamadryas epinome, Hamadryas februa and Myscelia orsis), three in Charaxinae (Memphis appias, Memphis otrere and Fountainea ryphea), and nine in Satyrinae: eight in the tribe Satyrini (Godartiana muscosa, Moneuptychia soter, Forsterinaria quantius, Hermeuptychia hermes, Splendeuptychia doxes, Euptychoides castrensis, Paryphthimoides phronius and Forsterinaria necys) and one in Brassolini (Opoptera syme) (see Supplementary material S2).
The butterfly H. epinome was the most abundant in seven out of ten studied fragments. The biblidine M. orsis was the most abundant butterfly in fragment 7, while H. februa was the most abundant in fragment 10 and G. muscosa in fragment 8. In all fragments, the subfamilies Satyrinae (tribe Satyrini) and Biblidinae respectively were the two most abundant (see Supplementary material S2). Butterfly richness in the fragments ranged from 32 to 54 (Table 1). Individuals were not distributed evenly between the fragments: the number of individuals ranged between 328 in fragment 8 to 1165 in fragment 7 (Table 1). The distribution of individuals found in the study is significantly different from a uniform distribution (G = 524.5, P < 0.0001).
The results of the rarefaction showed that the richness of six fragments were inside the confidence interval for the total sampled assemblage, while the remaining four fragments presented lower species richness than expected (Fig. 2).
Correlation between fruit-feeding butterfly assemblage and landscape characteristics
Several authors (e.g., Baz and Garcia-Boyero 1995; Dapporto and Dennis 2008; Prugh et al. 2008) have shown that geographic distance and fragment area are poor predictors of butterfly occurrence, and this was the case for our data (see Supplementary material S1). Hence, we did not include these parameters in our landscape analyses.
Among the single-predictor models evaluated, the model with pasture cover proportion in the surrounding 100 m radius was the best description of the variation in abundance of Satyrini (weight = 0.610; Supplementary material S3). For Brassolini, abundance was best described by the pasture cover proportion in the surrounding 200 m (weight = 0.385; Supplementary material S3). The abundance of Biblidinae was best explained by the pasture cover at 200 m (weight = 0.202), but the null model was also plausible (ΔAICc = 1.5, weight = 0.0951, Supplementary material S3). For the Charaxinae, the cover of early-regrowth forest at the 1000 m radius was the best predictor, but with a low evidence weight (weight = 0.113). In this case, the null model was equally plausible (ΔAICc = 0.4, weight = 0.090) as well as the models with the effect of early-regrowth forest cover within 100, 900, and 2000 m (Supplementary material S3). For all clades tested null models explain the species richness at the fragments better than other models when we include the effect of butterfly abundance as a covariate (see Supplementary material S3).
When the evidence weights of all cover types are summed by scale (circle radius), the small scales (100–200 m) sum up the highest weights, and thus were the most important predictors for abundance of Satyrini, Biblidinae and Brassolini (Fig. 3a). The null models better explained the richness of Satyrini, Charaxinae, Brassolini and Biblidinae when we include the effect of butterfly abundance (Fig. 3b). The sum of relative weights by cover type showed that pasture was the most important predictor to abundance of Biblidinae, Brassolini and Satyrini, and early-regrowth forest to Charaxinae (Fig. 4a). The richness of all Clades tested was similarly predicted by each cover type and also by the null model (Fig. 4b).
Sum of relative weight of the linear models (a abundance and b richness) by scale (circle radius, see text) determined with the corrected AICc for four clades (Biblidinae, Brassolini, Charaxinae and Satyrini) of fruit-feeding butterflies in a fragmented landscape in São Luiz do Paraitinga, São Paulo, Brazil. The asterisks indicate exclusive models, i.e., models without any other plausible model according to AICc (the asterisk in b apply to all four clades, see text)
Sum of relative weight of the linear models (a abundance and b richness) by vegetation cover type determined with the corrected AICc for four clades (Biblidinae, Brassolini, Charaxinae and Satyrini) of fruit-feeding butterflies in a fragmented landscape in São Luiz do Paraitinga, São Paulo, Brazil. The asterisks indicate exclusive models, i.e., models without any other plausible model according to AICc
The first axis of CCA showed a clear separation among species related with pasture, forest and early-regrowth forest proportion (Fig. 5), with Taygetis tripunctata, Hermeuptychia hermes, Paryphthimoides phronius, Eteona tisiphone, Temenis laothoe, Moneuptychia soter and Taygetis laches abundance correlated with increasing proportion of pasture, and Taygetis acuta, Morpho helenor, Hamadryas fornax, Epiphile orea, Taygetis ypthima and Forsterinaria quantius with increasing forest cover. Only four species, namely Paryphthimoides poltys, Moneuptychia paeon, Memphis appias and Memphis moruus, were positively correlated with early-regrowth forest.
CCA of the assemblage of fruit-feeding butterflies in a fragmented landscape in São Luiz do Paraitinga, São Paulo, Brazil. Only species with N ≥ 10 individuals were included in the analyses. The environmental characteristics tested were the proportion of Pasture (P), forest (F) and early-regrowth forest (ERF) in the 100, 200 and 1000 m scales (circle radius). Code names are the initial letter of the genus and the two initial letters of the species (except for Pha = Pharneuptychia sp. and Tlc = Taygetis laches), following the species list in Supplementary material S2
Discussion
Butterfly assemblage description
The number of species sampled (73) is close to the estimated total number of species, indicating that the sampling was adequate to provide an accurate representation of the actual local assemblage (Magurran 2004).
The most abundant species sampled are all typical of forest edges, clearings and disturbed areas (Brown 1992). This finding indicates that human disturbance in fragments favoured organisms more tolerant to sunlight and drought, conditions that are typical of these environments. Moreover, the majority of species, including the most abundant, Biblidinae (H. epinome and M. orsis) and Charaxinae (especially species in the genera Memphis and Fountainea), have larvae that feed on plants typical of forest edges and early stages of succession (Brown 1992; Beccaloni et al. 2008).
Overall similarity among all fragments is high with respect to assemblage composition, structure, diversity, richness and species abundance. In general, these results are similar to those reported by Uehara-Prado et al. (2007) in a similar fragmented landscape of the Atlantic Plateau. This suggests that the fragmentation in the Atlantic Forest leads to homogenization of the fauna through this high degree of environmental disturbance (McKinney and Lockwood 1999). Despite the great similarity among the assemblages, four fragments had lower species richness than the total community (Fig. 2), indicating that richness is not evenly distributed through landscape, and the lower richness of some fragments is not a sampling effect (Ribeiro et al. 2008).
Correlation between fruit-feeding butterfly assemblage and landscape characteristics
Our total estimated richness of fruit-feeding butterflies (Chao 2 = 80.56 + 5.96; Jacknife = 83.89 + 3.46) was similar to that of other areas of Atlantic Forest in São Paulo State with different degrees of fragmentation. Uehara-Prado et al. (2007) used similar methods and found an estimated richness of 83 species in the Morro Grande Reserve in Cotia, Southeastern Brazil (~10,000 ha). Similar figures (total richness, not estimates) were recorded for the extensive forests (>10,000 ha) in the Serra do Japi, Jundiai (96 species), in two forest fragments (~250 ha) in Campinas (82 and 100 species respectively), and in the Baixada Santista region (80 species) (Brown and Freitas 2003; Francini et al. 2011). This comparison shows that the fruit-feeding butterfly assemblages in this region of the Atlantic Forest had similar richness values, despite the total area of the sampled forest. These results may indicate that the butterflies species lost throughout the fragmentation process are replaced by others more able to exploit the surrounding matrix. This usually results in detectable changes in species composition, but not in diversity, as shown by previous authors (Cook et al. 2002; Ewers and Didham 2006; Uehara-Prado et al. 2007; Dover and Settele 2009).
The analysis of vegetation cover showed that the abundance of the fruit-feeding butterfly assemblages respond better to the immediate surroundings of the sampling unit (radius of 100–200 m, Fig. 3) than to the landscape in a larger scale (900–2,000 m, Fig. 3). Thus, we infer that the structure of butterfly assemblages is mainly influenced by the structure of the landscape in the immediate vicinity of the sampling point. In view of the fact that the landscape is not homogeneous, but rather a heterogeneous environment where floristic composition and structure change over small scales, we would expect that the distribution of butterflies would be non-random and follow the distribution of their larval and adult resources. Therefore the characteristics of the immediate surroundings could be as important in structuring the community of fruit-feeding butterflies as the characteristics of the regional landscape. Of the four analyzed groups, only Charaxinae presented responses to a larger scale (Fig. 3b). However, in two cases (Biblidinae and Charaxinae) the null model was also plausible. This could be related with local biotic and abiotic factors not evaluated in the present study such as average temperature. For instance, it is known that temperature can affect abundance (and consequently, richness) of sampled fruit-feeding butterflies in the study site (Ribeiro and Freitas 2010). The response to a larger scale in Charaxinae abundance could be explained by the higher dispersal capacity of Charaxinae when compared to Biblidinae, Satyrini and Brassolini (see Marini-Filho and Martins 2010), and they are probably using a larger area for foraging and reproduction. Our results for richness (with abundance effects statistically controlled) showed that in all cases, the null model was the best one according to AICc. This shows that richness is a simple function of total abundance in each fragment, and highly correlated with abundance as demonstrated by Gotelli and Colwell (2001). In this case, landscape structure affects diversity indirectly, through population sizes.
The most important land cover type to explain the abundance of Biblidinae, Satyrini and Brassolini was the percent of pasture cover, and for Satyrini and Brassolini the best models that explain the abundance of these butterflies was proportion of pasture present in 100 and 200 m of radius, respectively. These results probably reflect the great habitat availability for several Satyrini butterflies, which is the contact between forests and pastures (a similar result was found to a satyrine species in North America, see Pocewicz et al. 2009). These habitats not only could harbor more Satyrini, but also support a higher abundance of their larval host plants (mostly grasses, see Brown 1992 and Beccaloni et al. 2008); additionally, these areas of contact between forests and pastures can present supplementary resources for adults, such as rooting fruits and fermenting plant sap. In a similar way, most Brassolini use species of monocots as larval food plants (especially bamboos and palms), plants which are clearly favoured in the sunny forest edges formed in the contact zone between pastures and secondary forests.
The two species most related with pasture proportion in the CCA analyses (Fig. 5) were the abundant P. phronius and H. hermes (Supplementary material S2), which are both very tolerant to disturbance, occurring even in urban environments (Brown 1992). In contrast, some species increased with forest cover, such as F. quantius, T. acuta and H. fornax. Interestingly, the last two species were identified together with another 101 species of Atlantic Forest butterflies as requiring well preserved habitats to persist (Brown and Freitas 2000b). Among the species associated with early-regrowth forest, M. appias and M. moruus use species of Croton (Euphorbiaceae) and Lauraceae as host plants respectively, plants especially common in early stages of forest regeneration.
Our results clearly show that even if the regional landscape is important for defining the general composition and structure of butterfly assemblages, the immediate vicinity of a sampling point is decisive in explaining the subtle differences found among local assemblages, especially in Brassolini and Satyrini. Moreover, because these subfamilies are very abundant and rich, they have a preponderant effect on the structure of the overall butterfly assemblage. Even though several studies showed the importance of the surrounding matrix for the structure of butterfly assemblages (e.g., Weibull et al. 2000; Ricketts 2001; Prugh et al. 2008; Franklin and Lindenmayer 2009) our data show how different species and subfamilies are affected by different landscape components. The present study demonstrated that species with different biological needs are affected in different ways by the landscape context and the scale which affects each group can be different. This information can support the creation of more efficient sampling protocols, and can aid in choice of groups and species, sampling designs and statistical analysis that are most appropriate for use in protocols of bioindication, environmental impact analysis and for monitoring forest disturbance in the medium and long term.
Conclusions
To know the scale on which a pattern can be observed is of vital importance in ecology (Levin 1992), and the use of an inappropriate scale could lead to a neglect of critical aspects and processes. The present study yields results that can offer broader understanding of patterns of distribution of butterflies in fragmented landscapes. These results identify scales on which different vegetation covers can act to structure local assemblages. For example, a contact zone between forests and pastures could harbor more Satyrini species, while areas in early stages of regeneration and presenting several euphorbiaceous shrubs and vines can support most species of Charaxinae.
Our results make it clear that conservation efforts to preserve insect diversity in forest fragments should recognize the immediate surroundings of the fragment, and not only the regional landscape as a whole. This means that actions of the people living in the neighbourhood of fragments affect as much the community of fruit-feeding butterflies (but on a different level) as actions of large scale generally adopted in management plans and policies for conservation. Therefore, small scale actions should be included as one of the foci in conservation activities that seek to preserve diverse insect assemblages (with consequences for all other trophic levels) in forest fragments.
References
Baz A, Garcia-Boyero A (1995) The effects of forest fragmentation on butterfly communities in central Spain. J Biogeogr 22:129–140
Beccaloni GW, Hall SK, Viloria AL, Robinson GS (2008) Catalogue of the hostplants of the neotropical butterflies, vol 8. Monografias Tercer Milenio, Zaragoza
Bolker B (2010) Based on stats4 by the R Development Core Team. bbmle: tools for general maximum likelihood estimation. R package version 0.9.3
Bonebrake TC, Ponisio LC, Boggs CL, Ehrlich PR (2010) More than just indicators: a review of tropical butterfly ecology and conservation. Biol Conserv 143:1831–1841
Brown KS (1991) Conservation of the neotropical environments: insects as indicators. In: Collins NM, Thomas JA (eds) The conservation of insects and their habitats. Academic Press, San Diego, pp 350–404
Brown KS (1992) Borboletas da Serra do Japi: diversidade, habitats, recursos alimentares e variação temporal. In: Morellato LPC (ed) História Natural da Serra do Japi: Ecologia e Preservação de uma Área Florestal no Sudeste do Brasil. Editora Unicamp, Campinas, pp 142–186
Brown KS (2005) Geologic, evolutionary, and ecological bases of the diversification of neotropical butterflies: implications for conservation. In: Dick CW, Moritz G (eds) Tropical rainforest: past, present, and future. The University of Chicago Press, Chicago, pp 166–201
Brown KS, Brown GG (1992) Habitat alteration and species loss in Brazilian forests. In: Whitmore TC, Sayer SA (eds) Tropical deforestation and species extinction. Chapman and Hall, London
Brown KS, Freitas AVL (2000a) Atlantic forest butterflies: indicators for landscape conservation. Biotropica 32(4b):934–956
Brown KS, Freitas AVL (2000b) Diversidade de Lepidoptera em Santa Teresa, Espírito Santo. Bol Mus Biol Mello Leitao 11:71–116
Brown KS, Freitas AVL (2003) Butterfly communities of urban forest fragments in Campinas, São Paulo, Brazil: structure, instability, environmental correlates, and conservation. J Insect Conserv 6:217–231
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn. Springer, New York
Burnham KP, Anderson DR (2004) Multimodel inference: understanding AIC and BIC in model selection. Sociol Method Res 33:261–304
Cairns CE, Villanueva-Gutierrez R, Koptur S, Bray DB (2005) Bee populations, forest disturbance, and africanization in Mexico. Biotropica 37:686–692
Câmara G, Souza RCM, Freitas UM, Garrido J (1996) SPRING: integrating remote sensing and GIS by object-oriented data modelling. Comput Graph 20:395–403
Clark PJ, Reed JM, Chew FS (2007) Effects of urbanization on butterfly species richness, guild structure and rarity. Urban Ecosyst 10:321–337
Collier N, Gardner M, Adams M, McMahon CR, Benkendorff K, Mackay DA (2010) Contemporary habitat loss reduces genetic diversity in an ecologically specialized butterfly. J Biogeogr 37:1277–1287
Colwell RK (2005) EstimateS: statistical estimation of species richness and shared species from samples. Version 7.5. User’s guide. http://purloclcorg/estimates
Cook WM, Lane KT, Foster BL, Holt RD (2002) Island theory, matrix effects and species richness patterns in habitat fragments. Ecol Lett 5:619–623
Cowley MR, Thomas CD, Roy DB, Wilson RJ, Leon-Cortes JL, Gutierrez D, Bulman CR, Quinn RM, Moss D, Gaston KJ (2001) Density-distribution relationships in British butterflies. I. The effect of mobility and spatial scale. J Anim Ecol 70:410–425
Dapporto L, Dennis RLH (2008) Species richness, rarity and endemicity on Italian offshore islands: complementary signals from island-focused and species-focused analyses. J Biogeogr 35:664–674
Dean W (1997) A ferro e fogo. A história e a devastação da Mata Atlântica brasileira. Companhia das Letras, São Paulo
Debinski DM, Ray C, Saveraid EH (2001) Species diversity and the scale of the landscape mosaic: do scales of movement and patch size affect diversity. Biol Conserv 98:179–190
Dennis RLH (2004) Butterfly habitats, broad-scale biotype affiliations, and structural exploitation of vegetation at finer scales: the matrix revisited. Ecol Entomol 29:744–752
Dennis RLH, Hardy PB (2001) Loss rates of butterfly species with urban development. A test of atlas data and sampling artefacts at a fine scale. Biodivers Conserv 10:1831–1837
Dennis RLH, Hardy PB (2007) Support for mending the matrix: resource seeking by butterflies in apparent non-resource zones. J Insect Conserv 11:157–168
DeVries PJ (1987) The butterflies of Costa Rica and their natural history: Papilionidae, Pieridae, amd Nymphalidae. Princeton University Press, Princeton
DeVries PJ, Murray D, Lande R (1997) Species diversity in vertical, horizontal and temporal dimensions of a fruit-feeding butterfly community in an Ecuadorian rainforest. Biol J Linn Soc 62:343–364
Dobson AJ (1990) An introduction to generalized linear models. Chapman and Hall, London
Dover J, Settele J (2009) The influences of landscape structure on butterfly distribution and movement: a review. J Insect Conserv 13:3–27
Dungan JL, Perry JN, Dale MRT, Legendre P, Citron-Pousty S, Fortin M-J, Jakomulska A, Miriti M, Rosenberg MS (2002) A balanced view of scale in spatial statistical analysis. Ecography 25:626–640
Ekroos J, Kuussaari M (2011) Landscape context affects the relationship between local and landscape species richness of butterflies in semi-natural habitats. Ecography 34:001–007
Ellis EC, Ramankutty N (2008) Putting people in the map: anthropogenic biomes of the world. Front Ecol Environ 6:439–447
Ellis EC, Goldewijk K, Lightman D, Ramankutty N (2010) Anthropogenic transformation of the biomes: 1700 to 2000. Glob Ecol Biogeogr 19:589–606
Esch S, Klinkhamer PGL, Meijden EVD (2005) Do distances among host patches and host density affect the distribution of a specialist parasitoid? Oecologia 146:218–226
Ewers RM, Didham RK (2006) Confounding factors in the detection of species responses to habitat fragmentation. Biol Rev 81:117–142
Francini RB, Duarte M, Mielke OHH, Caldas A, Freitas AVL (2011) Butterflies (Lepidoptera, Papilionoidea and Hesperioidea) of the “Baixada Santista” region, coastal São Paulo, Southeastern Brazil. Rev Bras Entomol 55:55–68
Franklin JF, Lindenmayer DB (2009) Importance of matrix habitats in maintaining biological diversity. Proc Natl Acad Sci USA 106:349–350
Gotelli NJ, Colwell RK (2001) Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecol Lett 4:379–391
Gotelli NJ, Graves GR (1996) Null models in ecology. Smithsonian Institution Press, Washington
Haeckel EHPA (1866) Generelle Morphologie der Organismen. Georg Reimer, Berlin
Hamer KC, Hill JK (2000) Scale-dependent consequences of habitat modification for species diversity in tropical forests. Conserv Biol 14:1435–1440
Hammer O, Harper DAT, Ryan PD (2001) Past: paleontological statistics software package for education and data analysis. Palaeontol Electron 4:1–9
He F, Gaston KJ (2000) Occupancy-abundance relationships and sampling scales. Ecography 23:503–511
Horner-Devine MC, Daily GC, Ehrlich PR, Boggs CL (2003) Countryside biogeography of tropical butterflies. Conserv Biol 17:168–177
Hunter MD (2002) Landscape structure, habitat fragmentation, and the ecology of insects. Agri Forest Entomol 4:156–166
Kitahara M, Fujii K (1994) Biodiversity and community structure of temperate butterfly species within a gradient of human disturbance: An analysis based on the concept of generalist vs. specialist strategies. Res Popul Ecol 36(2):187–199
Koh LP, Sodhi NS (2004) Importance of reserves, fragments and parks for butterfly conservation in a tropical urban landscape. Ecol Appl 14:1695–1708
Krauss J, Steffan-Dewenter I, Tscharntke T (2003) How does landscape context contribute to effects of habitat fragmentation on diversity and population density of butterflies? J Biogeogr 30:889–900
Lennon JJ, Koleff P, Greenwood JJD, Gaston KJ (2001) The geographical structure of British bird distributions: diversity, spatial turnover and scale. J Anim Ecol 70:966–979
Levin SA (1992) The problem of pattern and scale in ecology. Ecology 73:1943–1976
Luoto M, Virkkala R, Heikkinen RK (2007) The role of land cover in bioclimatic models depends on spatial resolution. Glob Ecol Biogeogr 16:34–42
MacNally R (1997) Monitoring forest bird communities for impact assessment: the influence of sampling intensity and spatial scale. Biol Conserv 82:355–367
MacNally R, Fleishman E, Bulluck LP, Betrus CJ (2004) Comparative influence of spatial scale on beta diversity within regional assemblages of birds and butterflies. J Biogeogr 31:917–929
Magura T, Kodobocz V, Tothmeresz B (2001) Effects of habitat fragmentation on carabids in forest patches. J Biogeogr 28:129–138
Magurran AE (2004) Measuring biological diversity, 2nd edn. Blackwell Science, Oxford
Marini-Filho OJ, Martins RP (2010) Nymphalid butterfly dispersal among forest fragments at Serra da Canastra National Park, Brazil. J Insect Conserv 14:401–411
Marsh DM, Milam GS, Gorham NP, Beckman NG (2005) Forest roads as partial barriers to terrestrial salamander movement. Conserv Biol 19:2004–2008
McKinney ML, Lockwood JL (1999) Biotic homogenization: a few winners replacing many losers in the next mass extinction. Trends Ecol Evol 14:450–453
MME (1983) Ministério das Minas e Energia.Projeto Radam Brasil. Programa de Integração Nacional. Levantamento de RecursosNaturais, vol 10. Publication Division, Rio de Janeiro
Morellato LPC, Haddad CFB (2000) Introduction: the Brazilian Atlantic forest. Biotropica 32:786–792
Pearson RG, Dawson TP (2005) Long-distance plant dispersal and habitat fragmentation: identifying conservation targets for spatial landscape planning under climate change. Biol Conserv 123:389–401
Petrone P (1959) A região de São Luiz do Paraitinga: estudo de geografia humana. Rev Bras Geogr 21:239–336
Pocewicz A, Morgan P, Eigenbrode SD (2009) Local and landscape effects on butterfly density in northern Idaho grasslands and forests. J Insect Conserv 13:593–601
Prugh LR, Hodges KE, Sinclair RE, Brashares JS (2008) Effect of habitat area and isolation on fragmented animal populations. Proc Natl Acad Sci USA 105:20770–20775
R Development Core Team (2009) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Ramos FA (2000) Nymphalid butterfly communities in an amazonian forest fragment. J Res Lepid 35:29–41
Ribas CR, Sonrinho TG, Schoereder JH, Sperber CF, Lopes-Andrade C, Soares SM (2005) How large is large enough for insects? Forest fragmentation effects at three spatial scales. Acta Oecol 27:31–41
Ribeiro DB, Freitas AVL (2010) Differences in thermal responses in a fragmented landscape: temperature affects the sampling of diurnal, but not nocturnal fruit-feeding Lepidoptera. J Res Lepid 42:1–4
Ribeiro DB, Freitas AVL (2011) Large-sized insects show stronger seasonality than small-sized ones: a case study of fruit-feeding butterflies. Biol J Linn Soc 104:820–827
Ribeiro DB, Prado PI, Brown KS, Freitas AVL (2008) Additive partitioning of butterfly diversity in a fragmented landscape: importance of scale and implications for conservation. Divers Distrib 14:961–968
Ribeiro DB, Prado PI, Brown KS Jr, Freitas AVL (2010) Temporal diversity patterns and phenology in fruit-feeding butterflies in the Atlantic forest. Biotropica 42:710–716
Ribeiro MC, Metzger JP, Martensen AC, Ponzoni F, Hirota MM (2009) Brazilian Atlantic forest: how much is left and how is the remaining forest distributed? Implications for conservation. Biol Conserv 142:1141–1153
Ricketts TH (2001) The matrix matters: effective isolation in fragmented landscapes. Am Nat 158:87–99
Roslin T (2002) Who said that size is all that matters? Trends Ecol Evol 17:10–11
Saunders DA, Hobbs RJ, Margules CR (1991) Biological consequences of ecosystem fragmentation: a review. Conserv Biol 5:18–32
Schneider C (2003) The influence of spatial scale on quantifying insect dispersal: an analysis of butterfly data. Ecol Entomol 28:252–256
Shahabuddin G, Ponte CA (2005) Frugivorous butterfly species in tropical forest fragments: correlates of vulnerability to extinction. Biodivers Conserv 14:1137–1152
Shahabuddin G, Terborgh JW (1999) Frugivorous butterflies in Venezuelan forest fragments: abundance, diversity and the effects of isolation. J Trop Ecol 15:703–722
Steffan-Dewenter I, Munzenberg U, Burger C, Thies C, Tscharntke T (2002) Scale dependent effects of landscape context on three pollinator guilds. Ecology 83:1421–1432
Summerville KS, Crist TO (2001) Effects of experimental habitat fragmentation on patch use by butterflies and skippers (Lepidoptera). Ecology 82:1360–1370
Summerville KS, Veech JA, Crist TO (2002) Does variation in patch use among butterfly species contribute to nestedness at fine spatial scales. Oikos 97:195–204
Tabarelli M, Mantovani W, Peres CA (1999) Effects of habitat fragmentation on plant guild structure in the montane Atlantic forest of southeastern Brazil. Biol Conserv 91:119–127
Uehara-Prado M, Brown KS, Freitas AVL (2007) Species richness, composition and abundance of fruit-feeding butterflies in the Brazilian Atlantic forest: comparison between a fragmented and a continuous landscape. Glob Ecol Biogeogr 16:43–54
Vandermeer J, Carvajal R (2001) Metapopulation dynamics and the quality of the matrix. Am Nat 158:211–220
Veddeler D, Schulze CH, Steffan-Dewenter I, Buchori D, Tscharntke T (2005) The contribution of tropical secondary forest fragments to the conservation of fruit-feeding butterflies: effects of isolation and age. Biodivers Conserv 14:3577–3592
Wahlberg N, Leneveu J, Kodandaramaiah U, Peña C, Nylin S, Freitas AVL, Brower AVZ (2009) Nymphalid butterflies diversify following near demise at the cretaceous/tertiary boundary. Proc Roy Soc Biol Sci B 276:4295–4302
Weibull AC, Bengtsson J, Nohlgren E (2000) Diversity of butterflies in the agricultural landscape: the role of farming system and landscape heterogeneity. Ecography 23:743–750
Whittaker RJ, Willis KJ, Field R (2001) Scale and species richness: towards a general, hierarchical theory of species diversity. J Biogeogr 28:453–470
Yamaura Y, Katoh K, Fujita G, Higuchi H (2005) The effect of landscape contexts on wintering bird communities in rural Japan. For Ecol Manag 216:187–200
Acknowledgments
We thank Marcio Uehara-Prado for help with the first steps of the study. Niklas Wahlberg, Carly Bishop, Matthew Williams and the anonymous referee made valuable suggestions in the last version of the manuscript. We also thank the land owners who permitted field work within their properties. DBR thanks FAPESP for a fellowship (grant #03/11697-0). AVLF thanks FAPESP (grant 04/05269-9), and the Brazilian Research Council—CNPq (fellowship 300282/2008-7). PIP acknowledges the FAPESP and CNPq (fellowship #303878/2008-8). This project is part of the BIOTA-FAPESP program (grants #98/05101-8 to AVLF and #02/08558-6 to PIP). This publication is part of the RedeLep “Rede Nacional de Pesquisa e Conservação de Lepidópteros” SISBIOTA-Brasil/CNPq (563332/2010-7), and BIOTA-FAPESP Program (11/50225-3).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ribeiro, D.B., Batista, R., Prado, P.I. et al. The importance of small scales to the fruit-feeding butterfly assemblages in a fragmented landscape. Biodivers Conserv 21, 811–827 (2012). https://doi.org/10.1007/s10531-011-0222-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10531-011-0222-x