Abstract
Purpose of Review
The continued, rapid development of novel molecular genetic tools is contributing to a better understanding of forest-associated fungi and their interactive roles within diverse forest ecosystems. This paper focuses on recent developments of DNA-based diagnostics/detection, phylogenetics, population genetics, genomics, and metagenomics tools that have been applied to forest-associated fungi to better understand their roles in forest ecosystems and provide key insights for managing forest health.
Recent Findings
With the advent of new molecular technologies, we can better understand the biology of forest fungi by examining their genetic code. By utilizing genomics, fungal pathogens’ biological functions can be deduced from its genomic content. Further, high-resolution marker systems allow the determination of a pathogen’s population genetics and genomics, which provides important insights into its global movement and genetic shifts in local pathogen populations. Such genetic information has diverse applications for forest management to improve forest health. Lastly, new technologies in metagenomics will enhance the abilities to detect, describe, and utilize the complex interactions among fungal pathogens/symbionts, host trees, and associated microbial communities to develop novel management strategies for forest ecosystems.
Summary
Continued development and applications of molecular genetic and genomic tools provide insights into the diverse roles of forest-associated fungi in forest ecosystems, but long-term, wide-scale research is needed to determine how ecological functions are influenced by complex ecological interactions among microbial communities, other forest ecosystem components, and the environment. Such approaches may foster a paradigm shift away from single microbial pathogens, decomposers, or symbionts interacting with a single host or substrate, and provide more holistic approaches toward understanding interactions among microbial communities that drive forest health processes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
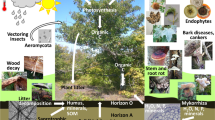
Fungi play diverse roles in forest ecosystems, such as pathogens, decomposers, beneficial symbionts, and biocontrol agents. They interact with each other and other ecosystem components in complex and multidimensional ways within forest ecosystems.
Fungal pathogens cause the majority of devastating diseases of forest trees, such as Armillaria root disease (caused by Armillaria spp.), white pine blister rust (caused by Cronartium ribicola), chestnut blight (caused by Cryphonectria parasitica), and Dutch elm disease (caused by Ophiostoma ulmi, O. novo-ulmi), and countless other forest diseases, which result in growth losses, mortality, and/or threats to life, limb, and property. Fungi are the causal agents of diverse forest diseases, including diseases of the root/butt, vascular system, outer stem/branches, wood, and foliage. Alternatively, fungi also play beneficial symbiotic roles in promoting forest health, such as ectomycorrhizae, arbuscular mycorrhizae, biocontrol agents, or endophytes/epiphytes that may confer resistance/tolerance against environmental stresses or pests [46•]. Furthermore, fungi are critical to decomposition and nutrient cycling processes within healthy forest ecosystems.
Because the literature on molecular genetics applications to forest-associated fungi is much too extensive for an all-inclusive review, our general goal is to provide a brief summary and selected examples of widely used approaches in (1) DNA-based identification and detection; (2) phylogenetics; (3) population genetics/genomics; (4) genomics/transcriptomics; and (5) metagenomics/metatransciptomics. Although molecular genetic tools are expected to rapidly evolve, this review is intended to provide a basic framework for understanding their applications to diverse fungi that are associated with forest diseases (e.g., foliar diseases, canker diseases, vascular diseases, root diseases, and/or wood rots) and other ecological roles in widely ranging forest ecosystems.
DNA-Based Identification and Detection
Accurate identification of fungi that are associated with ecological processes in forests, such as disease, symbioses, decomposition/nutrient cycling, and biological control is essential to understand the ecological roles of these diverse fungi. Fungal identification is sometimes difficult because diverse forest diseases can display similar symptoms, forest fungi with disparate ecological roles can display similar morphology, and obligate forest pathogens cannot be grown in culture. Determining appropriate disease management strategies are dependent on accurate identification of the pathogen, and other forest management strategies are dependent on knowing the ecological roles of key fungi that are not pathogens.
DNA-based identification of fungal pathogens relies on species-specific sequences of genomic regions, such as internal transcribed spacer (ITS) of rDNA, which is commonly used as a fungal barcode for species identification [79]. However, additional genomic regions, such as the small subunit (18S; SSU), large subunit (26S; LSU), and intergenic spacer (IGS) of rDNA, β-tubulin gene, translation elongation factor-1α (tef1) gene, ribosomal polymerase II (RPB2), Actin-1 (actin-1), glyceraldehyde 3-phosphate dehydrogenase (gpd), or a combination of different housekeeping DNA regions, are often used when the ITS region is inadequate to distinguish among closely related species. The most effective sequences for identification vary at the fungal species level, but are nearly identical at the population level within a species [79]. The utility of DNA regions for identification is frequently determined by phylogenetic analyses (see subsequent section). With well-studied fungal taxa, cursory identification can be attempted with sequence comparisons against a sequence database, such as GenBank (https://www.ncbi.nlm.nih.gov/genbank/) or others.
Numerous applications of DNA-based identification, which exemplify the utility with forest fungi, are much too extensive to cover comprehensively; however, a few examples demonstrate the diverse utility of DNA-based identification. After several decades devoted to controlling Ribes as a means to manage white pine blister rust, McDonald et al. [66] used ITS sequencing to determine that C. ribicola can also use non-Ribes species (Pedicularis sp. and Castilleja sp.) as alternate hosts to complete their lifecycle in northwestern USA. Stewart et al. [82, 83] used DNA-based characterization to determine that Fusarium commune, not Fusarium oxysporum, was the causal agent of damping-off of Douglas-fir (Pseudotsuga menziesii) seedlings in conifer nurseries; whereas, a subsequent report indicated that F. oxysporum has potential to protect Douglas-fir seedlings from root disease caused by F. commune [28]. Mmbaga et al. [68] used ITS sequences to distinguish between powdery mildew pathogens (e.g., Erysiphe pulchra, Phyllactinia guttata) on flowering dogwood (Cornus florida), and provided evidence that an emerging powdery mildew pathogen in the USA may have originated in Asia.
DNA-based identification can also be used to determine species diversity and global movement. These methods have played an essential role in identifying the myrtle rust pathogen, Austropuccinia psidii (formerly Puccinia psidii), and monitor pathogen spread and/or disease emergence in areas such as Hawaii, USA [89], Japan [47], Australia [14], South Africa [75], Indonesia [67], and Singapore [27]. Similarly, sequences of IGS, ITS, or tef1 are routinely used to identify Armillaria species from mycelial and/or rhizomorph samples (e.g., [4, 11, 41, 48, 49, 51,52,53, 58, 59, 69, 78]). This identification is critical to understanding the natural distribution of Armillaria species because many look morphologically similar in culture. Furthermore, DNA-based identification has been used to document areas where exotic Armillaria species were introduced to an area (e.g., [19, 20]). Lastly, DNA sequences were used as evidence that Heterobasidion sp. (subsequently named H. irregulare; [96]) from North America were introduced to Italy [35,36,37].
In contrast, rapid and sensitive detection methods are also needed to determine if a known pathogen is present in samples. Early detection is especially critical for invasive pathogens to prevent their establishment in new geographic areas. DNA-based identification can serve as a valuable tool for early detection of forest pathogens, and many tools have been developed for the detection of fungal pathogens of forest trees. Tzean et al. [88] developed an eight-oligonucleotide, microarray platform to simultaneously detect 17 Phellinus spp., including the invasive P. noxius, in field samples, such as roots, wood, and soil. Lamarche et al. [57•] used several different species-specific DNA sequences and real-time PCR to detect 10 different alien forest pathogens that represent an invasive threat to Canada. Also, real-time PCR methods also were developed for early detection of A. psidii, the invasive myrtle rust pathogen [7].
Phylogenetics
DNA-based phylogenetics of forest fungi examines evolutionary relationships within and among species. Such phylogenetic analyses typically rely on DNA regions similar to those used for identification, but more robust analyses can rely on more extensive genomic comparisons (phylogenomics). Phylogenetic approaches are also critical for recognizing cryptic species that are morphologically indistinguishable but genetically distinct, which is often reflected by an inability to mate in nature. Diverse mathematical inference methods are available to meet the demands of phylogenetic analyses, but these analyses typically result in a phylogenetic tree that allows evolutionary relationships to be visualized. Such analyses are a cornerstone of the phylogenetic species concept, on which fungal taxonomy is based.
Phylogenetic analyses are critical for determining evolutionary relationships among species and defining an organism’s taxonomic placement. Phylogenetic analyses were critical to the finding of an undescribed Armillaria species in Mexico [31, 32, 54•] (Fig. 1; [54•]). Recently, two exannulate Armillaria species (A. tabescens and A. ectypa) were assigned to the genus Desarmillaria, which was newly described on the basis of phylogenetic analyses of six genetic loci [55••]. The myrtle rust pathogen (formerly known as Puccinia psidii) was reassigned to the cryptic genus Austropuccinia, which was newly described on the basis of LSU-SSU-based phylogenetic analyses [5]. Two Japanese Heterobasidion species, H. annosum sensu lato and an undetermined Heterobasidion sp., were revealed based on phylogenetic analyses of three gene loci, tef1, gpd, and heat shock protein [72]. Subsequently, this undetermined but phylogenetically distinct Heterobasidion sp. was newly described as H. ecrustosum, based on both cultures and dried specimens [87].
Consensus phylogeny of coalescence-based Bayesian analyses estimated in Evolutionary Analysis by Sampling Trees (BEAST) under the strict clock with a GTR model of substitution on partial translation elongation factor 1-α consensus (50% strict) sequences of 43 phylogenic groups representing 242 Armillaria spp. isolates. Posterior support values > 0.5 are indicated at the nodes. Reprinted with permission from Klopfenstein et al. ([54•] Mycologia 109:75–91)
New species recognition is especially important when the ecology/biology (e.g., virulence, host range, climatic conditions) of the newly identified species varies from other species in the genus. Stewart et al. [82] revealed that damping-off in conifer nurseries is caused by F. commune, rather than F. oxysporum. Fusarium commune is difficult to morphologically distinguish from F. oxysporum. Although a few morphological characteristics (e.g., formation of polyphialides) can sometimes be used to separate F. commune and F. oxysporum, these characteristics are very subtle and occur only sporadically under specialized culture conditions. However, F. commune and F. oxysporum are distinct phylogenetically on the basis of three genetic loci (ITS, mtSSU, and tef1) [81, 82] and their ecological behavior (e.g., virulence) differs in forest nursery conditions [83]. Alamouti et al. [2] also used phylogenetics of 15 faster evolving genetic loci to discern a cryptic species, “Gs,” closely related to Grosmannia clavigera, a tree pathogen vectored by bark beetles (Dendroctonus spp.). The authors found host preference where G. clavigera was associated with Pinus ponderosa and Pinus jefferyi; whereas, Gs was associated primarily with Pinus contorta.
The emergence of next-generation sequencing provides an enormous DNA sequencing capacity for phylogenetics. For example, restriction-sited associated DNA sequencing (RAD-Seq) or genotype-by-sequencing (GBS) tools can provide hundreds of thousands of short anonymous sequence data that potentially add more depth of phylogenetic trees. In spite of a few obstacles (e.g., alignment, standardization of sequencing data, linked loci, and unavailable reference genome sequence), RAD-Seq has proven useful for phylogenetic studies in some plant species including California white oaks (Quercus section Quercus) [30•, 33••, 43]. Fitz-Gibbon et al. [33••] demonstrated that both reference-aligned and de novo assembly pipelines produce a reliable phylogenetic inference of California white oaks using RAD-Seq data. Although phylogenetic analyses of RAD-Seq datasets have not yet been reported for forest-associated fungi, this approach offers great potential for high-resolution analyses to infer phylogenetic relationships among forest-associated fungi.
Population Genetics/Genomics
DNA sequences provide the foundation for examining genetic variation within and among populations of forest fungi. Population genetic studies can identify populations that may respond similarly, monitor gene flow among populations, determine if fungal pathogens were introduced, examine the global movement of forest pathogens, and identify genetic shifts in local fungal populations. Current examples of DNA-based markers used for population genetic studies include microsatellites (simple sequence repeats; SSRs), amplified fragment length polymorphisms (AFLPs), RAD-Seq or GBS, single nucleotide polymorphisms (SNPs), and other DNA sequence-based methods. Each type of DNA-based marker has strengths and weaknesses that should be considered when addressing specific issues and situations associated with a proposed study. Diverse and numerous studies in population genetics have significantly influenced our understanding of fungal pathogen interactions within forest ecosystems, but only few examples can be presented here.
Using AFLPs with C. ribicola derived from diverse host populations with white pine blister rust in the western USA, Richardson et al. [73] found considerable genetic diversity despite the rust pathogen having been introduced ca. 100 years before. Furthermore, little population differentiation was apparent among six geographic locations (Idaho, Oregon, Colorado, and California, USA) with diverse aecial hosts [western white pine (Pinus monticola), whitebark pine (P. albicaulis), limber pine (P. flexilis), foxtail pine (P. balfouriana), bristlecone pine (P. aristata), and sugar pine (P. lambertiana)], except in a sugar pine plantation that was screened for major gene resistance (Cr1). That study indicated that the type of host resistance placed the strongest selection on the rust pathogen population, despite the diversity in aecial hosts, available telial hosts, and environment. A subsequent SNP-based analysis confirmed a strong differentiation between eastern and western populations of C. ribicola in North America, which reflects separate introduction processes [9•]. It was also found that locally distinct population structures were likely influenced by host connectivity, landscape features, and anthropogenic movement of this invasive pathogen [9•].
Microsatellite analyses of the myrtle rust pathogen (A. psidii, reported as P. psidii) demonstrated the existence of at least two distinct biotypes in Brazil, one associated with guava (Psidium spp.) and another associated with eucalypts (Eucalyptus spp.) and rose apple (Syzygium jambos) [38]. Furthermore, coalescence analyses indicated that the eucalypt-infecting A. psidii biotype in Brazil did not originate via host jump from guava following the introduction of eucalypts to Brazil, as was long-believed. Thus, the source of the eucalypt-infecting A. psidii biotype in Brazil remains unknown. Continued analyses on A. psidii populations in Australia, New Caledonia, China—Hainan, and Indonesia showed little variation among the microsatellite-based genotypes [63, 67, 77], whereas, a unique A. psidii genotype was associated with myrtle rust emergence in South Africa [76]. Subsequent microsatellite analyses have determined that a “pandemic” A. psidii biotype occurs in Costa Rica, Jamaica, Mexico, Puerto Rico, USA—Hawaii, and USA—Florida [84•], which contains the genotype that is found in Australia, New Caledonia, and Indonesia [63, 67] (Fig. 2, [84•]). Furthermore, bioclimatic modeling indicated that A. psidii biotypes were associated with a different predicted suitable climate space, which likely reflects distinct invasive threats to some geographic regions [84•].
Minimum-spanning network of Austropuccinia psidii microsatellite multilocus genotypes (MLGs) sampled from Brazil (BR), Costa Rica (CR), Jamaica (JM), Mexico (MX), Puerto Rico (PR), Uruguay (UR), and Florida (FL) USA, and Hawaii (HI) USA on 18 hosts. MLGs are represented by BAPS genetic clusters: C1 represents MLGs from Costa Rica on crimson bottlebrush (Callistemon lanceolatus), Jamaica, Mexico, Puerto Rico on rose apple (Syzygium jambos), and Hawaii, USA on koʻolau eugenia (Eugenia koolauensis), broad-leaved paperbark (Melaleuca quinquenervia), pōhutukawa (Metrosideros excelsa), ʻōhiʻa lehua (M. polymorpha), common myrtle (Myrtus communis), rose myrtle (Rhodomyrtus tomentosa), Java plum (S. cumini), rose apple, and Malay rose apple (S. malaccense); C2 represents MLGs collected from Brazil on eucalypts (Eucalyptus spp.) and rose apple and from Uruguay on eucalypts (Eucalyptus grandis and E. globulus); C3 represents one MLG collected from Brazil on eucalypts; C4 represents MLGs collected from Florida, USA on broad-leaved paperbark, twin berry (Myrcianthes fragrans), rose myrtle, and rose apple; C5 represents one MLG collected in Brazil on Java plum; C6 represents one MLG collected in Brazil on guava (Psidium guajava) and Brazilian guava (P. guineenese); C7 represents one MLG collected in Brazil on pitanga (E. uniflora); C8 represents MLGs collected from Jamaica on allspice (Pimenta dioica) and Uruguay on sweet flower (Myrrhinium atropurpureum); and C9 represents one MLG collected from Brazil on jabuticaba (Myrciaria cauliflora). Sizes of circles are proportional to MLG frequency. Connections are labeled with Bruvo genetic distances if different from 0.04, which corresponds to 1 mutational step at one locus. Broken lines connect MLGs that are separated by distances greater than 0.20. Reprinted with permission from Stewart et al. ([84•]. Forest Pathology, 48:e12378)
SNP-based analyses have been utilized to elucidate spread of invasive, introduced, and emerging pathogens. In studies of the ambrosia beetle (Platypus koryoensis)-vectored fungus (Raffaelea quercus-mongolicae) associated mortality of Mongolian oak (Quercus mongolica) in South Korea, Kim et al. [50•] used RAD-Seq to examine the population structure of the putative pathogen. Their analyses revealed low heterozygosity and no apparent population structure, which are consistent with the hypothesis that this putative pathogen was introduced to South Korea. Further, the population structure of the invasive ash dieback pathogen (Hymenoscyphus fraxineus) indicated that the pathogen can infect bark and survive saprophytically, and showed no differentiation between epidemic and post-epidemic populations and genetic diversity within the founding population in northeastern Europe was largely maintained in the front of the disease epidemic [12•].
Using a combination of microsatellite and SNP-based analyses, the dissemination, population structure, and evolution of the brown root rot pathogen, Phellinus noxius, has been elucidated. Chung et al. [17] examined mechanisms of P. noxius spread in Taiwan using microsatellites. Their study showed that tree-to-tree spread of P. noxius is largely clonal, but basidiospore-derived spread has resulted in little differentiation among populations in Taiwan. A similar approach has been applied for P. noxius populations in Japan, and it was found that P. noxius populations are genetically distinct on the Ryukyu and Ogaswawara islands of Japan [1]. These results suggest that P. noxius on the two island chains had different origins. Further sequencing at the whole genome level found that P. noxius in Asia Pacific is comprised of two lineages, both of which are extremely genetically diverse [18•].
Genomics
Sequencing of the complete genome (whole genome sequencing) determines the DNA sequences contained in the nuclear (chromosomal) and mitochondrial DNA of a fungal isolate. The availability of genome sequences for forest fungi has increased rapidly with the continued improvement in sequencing technologies [3]. As more fungal genomes are available, researchers can more easily determine the possible biology of pathogens by determining the function of genes found within each genome.
The genomic sequence of Phanerochaete chrysosporium, a lignocellulose-degrading, wood-rot fungus, was an early example of genomic sequencing of a forest-associated fungus, which provided baseline data for subsequent sequencing of filamentous basidiomycetous fungi [65]. Since then, the genomic sequencing of wood-degrading fungi has rapidly increased [70], which has allowed large-scale genome comparisons with diverse applications, such as bioenergy production and bioremediation, and defines the genetic mechanisms behind different modes of wood decay (e.g., [34]). In addition, genomic sequencing has been completed for diverse other forest-associated fungi, including Grosmannia clavigera [25], Melampsora larici-populina [29, 40], Heterobasidion spp. [16, 71], Armillaria spp. (e.g., [21, 80]), Dothistroma septosporum (Mycosphaerella pini) [23•], Diplodia pinea [6], Ophiostoma novo-ulmi [22], Mycosphaerella populorum/M. populicola [24••], and others. Aims of the genomic sequencing of forest fungi are ultimately related to forest management and risk assessment through understanding pathogenicity/symbiosis, adaptability, fungus-host/substrate interactions, etc. A few more detailed examples are presented below.
A poplar (Populus spp.) leaf rust pathogen (Melampsora larici-populina) provided the first available genomic sequence for a tree pathogen, and genomic sequencing was conducted by a whole genome shotgun method [29]. The genome analysis of this pathogen identified genes related to obligate biotrophy and host infection, and subsequent analyses identified genes encoding candidate effectors in the rust pathogen [39]. Insights into the ectomycorrhizal symbiosis of Laccaria bicolor were obtained by genomic sequencing, which identified gene sets involved in rhizosphere colonization, symbiosis, and nutrient cycling, but noting the absence of genes encoding enzymes to degrade polysaccharides contained in plant cell walls [64]. It is suggested that the genome comparison of symbiotic (L. bicolor) and pathogenic (M. larici-populina) basidiomycota fungi interacting with poplar can provide insights into pathogenicity/symbiosis mechanisms in evolutionary processes.
When the genome of a poplar canker pathogen (Mycosphaerella populorum) was compared to a closely related poplar leaf pathogen (M. populicola), relatively few genomic changes were found [24••]. Especially noteworthy is that changes in gene expression were associated with different disease etiology. It was further proposed that M. populorum gained the capacity to infect woody tissue via horizontal gene transfer and changes in gene dosage, which could have changed an innocuous, coevolved pathogen into a destructive pathogen of poplar plantations.
The genome of Grosmannia clavigera, an ascomycetous fungus associated with blue stain of conifers, was sequenced by combining data derived from different sequencing methods [25]. First insights into potential infection mechanisms of a pathogen associated with Armillaria root disease (A. mellea) was obtained through genomic and proteomic analysis [21]. This research found that A. mellea has a broad suite of carbohydrate-degrading enzymes, similar to both basidiomycete and ascomycete glycodegrative arsenals. The genome of Dothistroma septosporum (Mycosphaerella pini), the cause of red band needle blight of pines, was sequenced and compared to a closely related fungus to examine genes related to lifestyle adaptations and genes of common ancestry [23•, 42]. Genomic sequencing of the tip blight pathogen of pines (Diplodia pinea) provided the basis for a study of the MAT genes in pathogen populations [6]. Results suggested that D. pinea, which was previously considered exclusively asexual, may have a cryptic, heterothallic sexual cycle.
The myrtle rust pathogen (A. psidii) was found to contain sequences associated with transposable elements within ca. 27% of its genome, which may enhance its ability to generate genetic variability associated with adaptation to new hosts and environments [86]. The associated phylogenetic analyses of three DNA regions placed A. psidii within a separate branch with the Pucciniaceae lineage. Lastly, insights into the pathogenicity of the elm bark beetle-vectored Dutch elm disease pathogen were obtained by the annotation of the ascomycetous Ophiostoma novo-ulmi genome, which identified 1731 genes encoding proteins that were potentially involved in pathogenicity and diverse other genes related to carbohydrate utilization, electron transfer/detoxification, metabolism, growth/reproduction, signaling/plant defense relationships, etc. [22].
Transcriptome
The transcriptome of a forest fungus is the set of all expressed genes (transcribed as mRNA) associated with the fungal sample that reflects a specific time, tissue, developmental stage, abiotic/biotic environment, and other conditions under which the sample was collected. Brief examples of transcriptome sequencing of forest-associated fungi are presented below.
Root-Associated Fungi
Root disease pathogens are among the most actively investigated forest fungal pathogens using transcriptomic approach to examine diverse functions, such as pathogenicity and pathogen-host interactions. Transcribed genes in an active mycelial fan of Armillaria solidipes in association with grand fir (Abies grandis) were sequenced, which provided insights into putative signal peptides and genes with functions in pathogenesis, such as those encoding plant cell wall-degrading enzymes and responses to post-infection host environment, and other genes related to forest root/butt diseases (Fig. 3; [74]). Transcriptomes of invasive, vegetative, and reproductive developmental stages of A. ostoyae were compared, which provided detailed information on the regulation of pathogenicity-related genes, and evolutionary relationships of genes involved in wood-decay, morphogenesis, and complex multicellularity [80]. Gene expression studies of the laminated root-rot pathogen, Phellinus sulphurascens, revealed differential expression of genes encoding potential virulence factors, putatively secreted proteins, and many other enzymes [92]. Early studies on expressed genes of Heterobasidium annosum during infection of Scots pine (Pinus sylvestris) identified genes that were differentially expressed and genes with unknown function, which identified the need for genomic sequences of this and other forest fungi [45]. Genomic sequencing and transcript profiling of H. irregulare, a root/butt-rot pathogen of coniferous trees, was used to examine the differentially expressed genes associated with mechanisms for wood decay and parasitism [71]. Genes encoding putative ligninolytic enzymes in H. irregulare were shown to be differentially regulated in a substrate-specific manner [93]. Gene expression of Norway spruce (Picea abies) inoculated with H. annosum s.s. [62•] identified expression of several pathogen genes, including genes associated with virulence, in addition to a multitude of host genes.
Classification of sequences from de novo transcriptome assembly of Armillaria solidipes RNA1 based on predicted gene ontology (GO) terms. a Molecular function. b Biological process. c Cellular component. GO terms were determined using Blast2GO PRO V.2.6.4 with an e-value cutoff of 1e−3, filtered by fungal homology, and are sorted based on level 2 classifications. Reprinted with permission from Ross-Davis et al. ([74] Forest Pathology 43:468–477)
Wood-Associated Fungi
Analysis of the genome and transcriptome of a wood-rot fungus, Phlebiopsis gigantea, that is capable of colonizing freshly cut conifer stumps, revealed potential gene-based mechanisms that allow tolerance for resinous compounds while degrading complex polymers of wood [44]. Genome and transcriptome sequencing was performed on Grosmannia clavigera, the fungal symbiont of mountain pine beetle (Dendroctonus ponderosae) and causal agent of blue stain disease of lodgepole pine (Pinus contorta), which provided insights into how this pathogen tolerates conifer-defense chemicals during tree colonization [26].
Foliage/Branch/Stem-Associated Fungi
Transcriptome analyses of the white pine blister rust pathogen predicted the secretome, candidate effectors, other pathogenicity determinants, and genes that were differentially regulated during different life-cycle stages [61]. Differential gene expression of Dothistroma septosporum, a needle endophyte and pathogen of radiata pine (Pinus radiata), was examined at different stages of infection [8••]. Genes encoding wall-modifying enzymes and signaling proteins appeared upregulated in the initial biotrophic stage and genes encoding enzymes associated with secondary metabolism were upregulated in later necrotrophic stages. Recent genomics and transcriptomics studies of Marssonina brunnea infection of poplar leaves examined changes in gene expression of the pathogen and host, which demonstrated differential regulation of pathogen virulence genes in association with differential regulation of host resistance genes, which exemplified the complex host-pathogen interactions that occur during the infection process [15, 94].
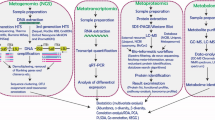
Metagenomics/Metatransciptomics
Metagenomic and metatranscriptomic studies elucidate the bacterial and fungal (and other) organisms within microbial communities and the function of those organisms within environmental samples through DNA and RNA sequencing. These approaches can determine the phytobiome (microbes associated with plants) within or associated with forest foliage, branches/stems, wood, roots, or rhizosphere using DNA barcoding, e.g., tagged amplicon sequencing of the ITS region, or shotgun (whole community) sequencing. The utility of metagenomic studies to help understand ecological interactions within forest ecosystems is greatly increased by the collection of environmental metadata (e.g., temperature, moisture, biotic environment, soil physical and chemical properties, and site history) and a well-designed study with suitable sampling [60••]. Such studies can determine the identity and role of microbes in forest ecosystems processes, such as disease, decomposition, and symbiosis. An early metagenomic study on forest soils found a high diversity of fungal species in six different forest soils, with the majority of species belonging to Ascomycota and Basidiomycota [10], and DNA barcoding was used to identify diverse fungal taxa associated with wood decay in tropical forests [91•]. Forest harvesting was associated with a reduction in genes associated with biomass decomposition [13]. Uroz et al. [90••] examined relationships among bacteria and fungi in temperate and boreal forests in association with nutrient recycling and other ecological functions. Štursová et al. [85••] showed that death of Norway spruce (Picea abies) forests caused by bark beetle (Ips typographus) resulted in profound changes in the fungal communities in the soil with decreased biomass and reduced fungal symbionts of tree roots, and relative increases of saprophytic taxa. Recently, Žifčáková et al. [95] used metagenomic and metatranscriptomics approaches to examine soil and litter microbes, including fungi, and ecological functions in coniferous forest soil, which were substantially different during summer and winter, especially in the soil. That study also showed reduced activities of ectomycorrhizal fungi in winter, an indication that photosynthetic output is a driver of changes in microbial function of soil microbes in coniferous forests. Metagenomic approaches have also been used to examine wood decomposition. For example, studies by Kubartová et al. [56] revealed different patterns in fungal communities within decaying logs. Such approaches are essential to understand intricate microbial interactions and successional processes in complex ecological activities in forests, such as decomposition and nutrient cycling.
The clear trend from research on microbial communities within forest ecosystems and in other systems as well is that ecological processes are much more complex and networked than was previously recognized. Metagenomics studies are radically transforming traditional forest pathology paradigms by demonstrating that ecological functions, such as root/canker diseases, wood rots, and symbioses, are the result of complex interactions among microbial communities, and not the direct result of a single microbial pathogen, decomposer, or symbiont interacting with a single host or substrate. Though in its infancy, the field of metagenomics shows great promise for helping researchers understand complex interactions among forest fungi (e.g., pathogens, symbionts, decomposers, and biocontrol agents), hosts, and associated microbial communities that will aid in the development of novel tools for forest management in the future.
Conclusions
This review is a brief summary with selected examples of widely used approaches in (1) DNA-based identification and detection; (2) phylogenetics; (3) population genetics/genomics; (4) genomics/transcriptomics; and (5) metagenomics/metatransciptomics. The selection of suitable genetic/genomic approaches for application to forest-associated fungi is situation specific and depends on various parameters (i.e., research objective, quality/quantity of available DNA, supporting genetic information for the fungus, cost considerations, available labor skills and equipment, etc.). Genetic and genomic technologies continue to become more powerful and affordable, which allows new and expanded applications to forest-associated fungi, and these applications will likely continue to grow in a manner that reflects advances in next-generation sequencing technologies, computational power, and bioinformatics. Such genetic and genomic approaches will expand our understanding of ecological processes in forest ecosystems by determining the diverse roles of microbial communities in processes such as pathogenesis, biological control, symbiosis, decomposition, or other critical processes in relation to forest-associated fungi and the environment. This highlights the significance of genetic and genomic approaches which could foster a paradigm shift away from single microbial pathogens, decomposers, or symbionts interacting with a single host or substrate and provide more holistic approaches toward understanding interactions among microbial communities that drive forest health processes. Diverse molecular genetic approaches offer the potential to better understand the ecological interactions of forest fungi with biotic/abiotic components within forest ecosystems, and this information can be used to foster forest health, productivity (carbon sequestration), sustainability, and resilience.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Akiba M, Ota Y, Tsai IJ, Hattori T, Sahashi N, Kikuchi T. Genetic differentiation and spatial structure of Phellinus noxius, the causal agent of brown root rot of woody plants in Japan. PLoS One. 2015;10:e0141792.
Alamouti SM, Wang V, DiGuistini S, Six DL, Bohlmann J, Hamelin RC, et al. Gene genealogies reveal cryptic species and host preferences for the pine fungal pathogen Grosmannia clavigera. Mol Ecol. 2011;20:2581–601.
Aylward J, Steenkamp ET, Dreyer LL, Roets F, Wingfield BD, Wingfield MJ. A plant pathology perspective of fungal genome sequencing. IMA Fungus. 2017;8(1):1–15. https://doi.org/10.5598/imafungus.2017.08.01.01.
Baysal-Gurel F, Cinar A. First report of Armillaria root rot caused by Armillaria mellea infecting Carrizo citrange and sour orange rootstocks in Turkey. Plant Dis. 2014;98:1439. https://doi.org/10.1094/PDIS-05-14-0463-PDN.
Beenken L. Austropuccinia: a new genus name for the myrtle rust Puccinia psidii placed within the redefined family Sphaerophragmiaceae (Pucciniales). Phytotaxa. 2017;297(1):053–61.
Bihon W, Wingfield MJ, Slippers B, Duong TA, Wingfield BD. MAT gene idiomorphs suggest a heterothallic sexual cycle in a predominantly asexual and important pine pathogen. Fungal Genet Biol. 2014;62:55–61.
Bini AP, Quecine MC, da Silva TM, Silva LD, Labate CA. Development of a quantitative real-time PCR assay using SYBR Green for early detection and quantification of Austropuccinia psidii in Eucalyptus grandis. Eur J Plant Pathol. 2017;150:735–46. https://doi.org/10.1007/s10658-017-1321-7.
•• Bradshaw RE, Guo Y, Sim AD, Kabir MS, Chettri P, Ozturk IK, et al. Genome-wide gene expression dynamics of the fungal pathogen Dothistroma septosporum throughout its infection cycle of the gymnosperm host Pinus radiata. Mol Plant Pathol. 2016;17(2):210–24. Genome-wide expression patterns were characterized as a time series during the infection cycle of Dothistroma septosporum on Pinus radiata. The time series highlights the early biotrophic phase that is characterized by genes encoding fungal cell wall-modifying enzymes and signaling proteins to later necrotrophic stages that is characterized by genes for secondary metabolism, oxidoreductases, transporters, and starch degradation.
• Brar S, Tsui CKM, Dhillon B, Bergeron M-J, Joly DL, Zambino PJ, et al. Colonization history, host distribution, anthropogenic influence and landscape features shape populations of white pine blister rust, an invasive alien tree pathogen. PLoS One. 2015;10(5):e0127916. https://doi.org/10.1371/journal.pone.0127916. This study highlights the genetic diversity of Cronarium ribicola , the causal agent of white pine blister rust, 100 years after its introduction into North America. Using SNP analysis of 1292 samples from 66 locations, the authors found two distinct populations, western and eastern. The eastern population is 2 to 5 times more diverse than the western populations, indicating more introductions have occurred in eastern North America compared to the western region.
Buée M, Reich M, Murat C, Morin E, Nilsson RH, Uroz S, et al. 454 pyrosequencing analyses of forest soils reveal an unexpectedly high fungal diversity. New Phytol. 2009;184:449–56.
Burns KS, Hanna JW, Klopfenstein NB, Kim M-S. First report of the Armillaria root disease pathogen, Armillaria sinapina, on subalpine fir (Abies lasiocarpa) and quaking aspen (Populus tremuloides) in Colorado. Plant Dis. 2016;100:217.
• Burokiene D, Prospero S, Jung E, Marciulyniene D, Moosbrugger K, Norkute G, et al. Genetic population structure of the invasive ash dieback pathogen Hymenoscyphus fraxineus in its expanding range. Biol Invasions. 2015;17:2743–56. https://doi.org/10.1007/s10530-015-0911-6. This research examined the genetic diversity of the exotic pathogen Hymenoscyphus fraxineus , causal agent of ash dieback at the epidemic disease front in Switzerland, compared to the post-epidemic phase in Lithuania. The authors found that diversity was comparable at both locations indicating weak founder effects and no reduction in diversity occurred at the epidemic disease phase, though more mating occurred at the post-epidemic phase.
Cardenas E, Kranabetter JM, Hope G, Maas KR, Hallam S, Mohn WW. Forest harvesting reduces the soil metagenomic potential for biomass decomposition. ISME J. 2015;9:2465–76.
Carnegie AJ, Lidbetter JR, Walker J, Horwood MA, Tesoriero L, Glen M, et al. Uredo rangelii, a taxon in the guava rust complex, newly recorded on Myrtaceae in Australia. Australas Plant Pathol. 2010;39:463–6.
Chen C, Yao Y, Zhang L, Xu M, Jiang J, Dou T, et al. A comprehensive analysis of the transcriptomes of Marssonina brunnea and infected poplar leaves to capture vital events in host-pathogen interactions. PLoS One. 2015;10(7):e0134246. https://doi.org/10.1371/journal.pone.0134246.
Choi J, Lee G-W, Kim K-T, Jeon J, Détry N, Kuo H-C, et al. Comparative analysis of genome sequences of the conifer tree pathogen, Heterobasidion annosum s.s. Genomics Data. 2017;14:106–13.
Chung C-L, Huang S-Y, Huang Y-C, Tzean S-S, Ann P-J, Tsai J-N, et al. The genetic structure of Phellinus noxius and dissemination pattern of brown root rot disease in Taiwan. PLoS One. 2015;10(10):e0139445. https://doi.org/10.1371/journal.pone.0139445.
• Chung C-L, Lee TJ, Akiba M, Lee H-H, Kuo T-H, Liu D, et al. Comparative and population genomics landscape of Phellinus noxius: a hypervariable fungus causing root rot in trees. Mol Ecol. 2017. early view. https://doi.org/10.1111/mec.14359. The authors found an expanded 1,3-beta-glucan synthase gene family in Phellinus noxius and suggested that this may be linked to fast-growing abilities of P. noxius . Further, the authors found extreme genetic variability.
Coetzee MPA, Wingfield BD, Harrington TC, Steimel J, Coutinho TA, Wingfield MJ. The root rot fungus Armillaria mellea introduced into South Africa by early Dutch settlers. Mol Ecol. 2001;10:387–96.
Coetzee MPA, Wingfield BD, Roux J, Crous PW, Denman S, Wingfield MJ. Discovery of two northern hemisphere Armillaria species on Proteaceae in South Africa. Plant Pathol. 2003;52:604–12.
Collins C, Keane TM, Turner DJ, O’Keeffe G, Fitzpatrick DA, Doyle S. Genomic and proteomic dissection of the ubiquitous plant pathogen, Armillaria mellea: toward a new infection model system. J Proteome Res. 2013;12:2552−2570.
Comeau AM, Dufour J, Bouvet GF, Jacobi V, Nigg M, Henrissat B, et al. Functional annotation of the Ophiostoma novo-ulmi genome: insights into the phytopathogenicity of the fungal agent of Dutch elm disease. Genome Biol Evol. 2014;7(2):410–30.
• de Wit PJGM, van der Burgt A, Ökmen B, Stergiopoulos I, Abd-Elsalam KA, Aerts AL, et al. The genomes of the fungal plant pathogens Cladosporium fulvum and Dothistroma septosporum reveal adaptation to different hosts and lifestyles but also signatures of common ancestry. PLoS Genet. 2012;11(12):e1005775. https://doi.org/10.1371/journal.pgen.1005775. This research compares and contrasts two phylogenetically related species that have different lifestyles and hosts. The authors found genes that were unique to Cladosporium fulvum for detoxification of tomatine. Each genome had genes involved in the production of the toxin dothistrormin; however, these genes were only expressed in Dothistroma septosporum , suggesting a recent adaption in their lifestyle.
•• Dhillon B, Feau N, Aerts AL, Beauseigle S, Bernier L, Copeland A, et al. Horizontal gene transfer and gene dosage drives adaptation to wood colonization in a tree pathogen. PNAS. 2015;112:3451–6. A genomics approach was used to compare Mycosphaerella populorum (poplar canker pathogen) with M. populicola (poplar leaf pathogen) and found signatures of horizontal gene transfer for several carbohydrate degradation genes associated with wood decay suggesting that adaption in the canker pathogen was the result of these horizontally acquired genes.
DiGuistini S, Liao NY, Platt D, Robertson G, Seidel M, Chan SK, et al. De novo genome sequence assembly of a filamentous fungus using Sanger, 454 and Illumina sequence data. Genome Biol. 2009;10:R94. https://doi.org/10.1186/gb-2009-10-9-r94.
DiGuistini S, Wang Y, Liao NY, Taylor G, Tanguay P, Feau N, et al. Genome and transcriptome analyses of the mountain pine beetle-fungal symbiont Grosmannia clavigera, a lodgepole pine pathogen. PNAS. 2011;108:2504–9.
du Plessis E, McTaggart AR, Granados GM, Wingfield MJ, Roux J, Ali MIM, et al. First report of myrtle rust caused by Austropuccinia psidii on Rhodomyrtus tomentosa (Myrtaceae) from Singapore. Plant Dis. 2017;101:1676.
Dumroese RK, Kim M-S, James RL. Fusarium oxysporum protects Douglas-fir (Pseudotsuga menziesii) seedlings from root disease caused by Fusarium commune. Plant Pathol J. 2012;28(3):311–6.
Duplessis S, Cuomo CA, Lin YC, Aerts A, Tisserant E, Veneault-Fourrey C, et al. Obligate biotrophy features unraveled by the genomic analysis of rust fungi. Proc Natl Acad Sci U S A. 2011;108(22):9166–71. https://doi.org/10.1073/pnas.1019315108.
• Eaton DAR, Ree RH. Inferring phylogeny and introgression using RADseq data: an example from flowering plants (Pedicularis: Orobanchaceae). Syst Biol. 2013;62:689–706. https://doi.org/10.1093/sysbio/syt032. The authors were able to assess phylogeny and confirm introgression in broomrape plant family using 40,000 restriction-site associated DNA markers. The research highlights the importance of geographic isolation in the emergence of new species.
Elías-Román RD, Guzmán-Plazola RA, Klopfenstein NB, Alvarado-Rosales D, Calderón-Zavala G, García-Espinosa R, et al. Incidence and phylogenetic analyses of Armillaria spp. associated with root disease in peach orchards in the State of Mexico, Mexico. For Pathol. 2013;43:390–401.
Elías-Román RD, Medel R, Klopfenstein NB, Hanna JW, Kim M-S, Alvarado D. Armillaria mexicana (Agaricales, Physalacriaceae), a newly described species from Mexico. Mycologia. 2018. https://doi.org/10.1080/00275514.2017.1419031.
•• Fitz-Gibbon S, Hipp AL, Pham KK, Manos PS, Sork VL. Phylogenomic inferences from reference-mapped and de novo assembled short-read sequence data using RADseq sequencing of California white oaks (Quercus section Quercus). Genome. 2017;60:743–55. https://doi.org/10.1139/gen-2016-0202. This research compares and contrasts bioinformatics pipelines, de novo assembly versus reference-mapped assemblies to produce phylogenies for California white oaks. The research shows that both methodologies yield similar phylogenies, but that downstream uses of RAD-seq data varied.
Floudas D, Binder M, Riley R, Barry K, Blanchette RA, Henrissat B, et al. The Paleozoic origin of enzymatic lignin decomposition reconstructed from 31 fungal genomes. Science. 2012;336:1715–9.
Garbelotto M, Guglielmo F, Mascheretti S, Croucher PJP, Gonthier P. Population genetic analyses provide insights on the introduction pathway and spread patterns of the North American forest pathogen Heterobasidion irregulare in Italy. Mol Ecol. 2013;22:4855–69.
Gonthier P, Garbelotto M. Amplified fragment length polymorphism and sequence analyses reveal massive gene introgression from the European fungal pathogen Heterobasidion annosum into its introduced congener H. irregulare. Mol Ecol. 2011;20:2756–70.
Gonthier P, Warner R, Nicolotti G, Mazzaglia A, Garbelotto M. Pathogen introduction as a collateral effect of military activity. Mycol Res. 2004;108:468–70.
Graça RN, Ross-Davis AL, Klopfenstein NB, Kim M-S, Peever TL, Cannon PG, et al. Rust disease of eucalypts, caused by Puccinia psidii, did not originate via host jump from guava in Brazil. Mol Ecol. 2013;22(24):6033–47.
Hacquard S, Joly DL, Lin Y-C, Tisserant E, Feau N, Delaruelle C, et al. A comprehensive analysis of genes encoding small secreted proteins identifies candidate effectors in Melampsora larici-populina (poplar leaf rust). MPMI. 2012;25:279–93. https://doi.org/10.1094/MPMI-09-11-0238.
Hamelin RC, Grigoriev IV, Szabo LJ, Martin F. Obligate biotrophy features unraveled by the genomic analysis of rust fungi. PNAS. 2011;108:9166–71.
Hanna JW, Klopfenstein NB, Kim M-S. First report of the root-rot pathogen, Armillaria nabsnona, from Hawaii. Plant Dis. 2007;91:634.
de Wit PJGM, van der Burgt A, Ökmen B, Stergiopoulos I, Abd-Elsalam K, et al. The genomes of the fungal plant pathogens Cladosporium fulvum and Dothistroma septosporum reveal adaptation to different hosts and lifestyles but also signatures of common ancestry. PLoS Genet. 2012;8(11):e1003088. https://doi.org/10.1371/journal.pgen.1003088.
Hipp AL, Eaton DAR, Cavender-Bares J, Fitzek E, Nipper R, Manos PS, et al. A framework phylogeny of the American oak clade based on sequenced RAD data. PLoS One. 2014;9:e93975. https://doi.org/10.1371/journal.pone.0093975.
Hori C, Ishida T, Igarashi K, Samejima M, Suzuki H, Master E, et al. Analysis of the Phlebiopsis gigantea genome, transcriptome and secretome provides insight into its pioneer colonization strategies of wood. PLoS Genet. 2014;10(12):e1004759. https://doi.org/10.1371/journal.pgen.1004759.
Karlsson M, Olson A, Stenlid J. Expressed sequences from the basidiomycetous tree pathogen Heterobasidion annosum during early infection of Scots pine. Fungal Genet Biol. 2003;39:51–9.
• Kaul S, Sharma T, Dhar MK. “Omics” tools for better understanding the plant–endophyte interactions. Front Plant Sci. 2016;7:955. https://doi.org/10.3389/fpls.2016.00955. This review paper discusses methodologies and importance of understanding endophytic roles and complex interactions associated with plant systems.
Kawanishi T, Uemastu S, Kakishima M, Kagiwada S, Hamamoto H, Horie H, et al. First report of rust disease on ohia and the causal fungus, Puccinia psidii, in Japan. J Gen Plant Pathol. 2009;75:428–31.
Kim M-S, Hanna JW, Klopfenstein NB. First report of an Armillaria root disease pathogen, Armillaria gallica, associated with several new hosts in Hawaii. Plant Dis. 2010a;94:1510. https://doi.org/10.1094/PDIS-04-10-0266.
Kim MS, Klopfenstein NB, Hanna JW, Cannon P, Medel R, López A. First report of Armillaria root disease caused by Armillaria tabescens on Araucaria araucana in Veracruz, Mexico. Plant Dis. 2010b;94(6):784.
• Kim M-S, Hohenlohe P, Kim KH, Seo S-T, Klopfenstein NB. Genetic diversity and population structure of Raffaelea quercus-mongolicae, a fungus associated with oak mortality in South Korea. For Pathol. 2016;46:164–7. The authors used restriction-site-association DNA sequencing to highlight the low genetic diversity and no apparent population structure among populations of Raffaelea quercus-mongolicae , a fungus associated with oak mortality in South Korea. These results support the hypothesis that this pathogen was recently introduced to South Korea.
Kim M-S, Fonseca NR, Hauff RD, Cannon PG, Hanna JW, Klopfenstein NB. First report of the root-rot pathogen, Armillaria gallica, on koa (Acacia koa) and ‘ōhi‘a lehua (Metrosideros polymorpha) on the island of Kaua‘i, Hawai‘i. Plant Dis. 2017;101:255. https://doi.org/10.1094/PDIS-07-16-1043-PDN.
Klopfenstein NB, Lundquist JE, Hanna JW, Kim M-S, McDonald GI. First report of Armillaria sinapina, a cause of Armillaria root disease, associated with a variety of tree hosts on sites with diverse climates in Alaska. Plant Dis. 2009;93:111.
Klopfenstein NB, Hanna JW, Cannon PG, Medel-Ortiz R, Alvarado-Rosales D, Lorea-Hernández F, et al. First report of the Armillaria root-disease pathogen, Armillaria gallica, associated with several woody hosts in three states of Mexico. Plant Dis. 2014;98:1280.
• Klopfenstein NB, Stewart JE, Ota Y, Hanna JW, Richardson BA, Ross-Davis AL, et al. Insights into the phylogeny of Northern Hemisphere Armillaria: neighbor-net and Bayesian analyses of translation elongation factor 1-α gene sequences. Mycologia. 2017;109:75–91. This research highlights the existence of four superclades within the genus of Armillaria and the utility of using the translation elongation factor 1-alpha as a species barcode for this genus.
•• Koch RA, Wilson AW, Séné O, Henkel TW, Aime MC. Resolved phylogeny and biogeography of the root pathogen Armillaria and its gasteroid relative, Guyanagaster. BMC Evol Biol. 2017;17:33. https://doi.org/10.1186/s12862-017-0877-3. This research used morphological and genetic data to determine the evolution of armillarioid fungi. Results suggest that the armillarioid lineage evolved in Eurasia around 51 million years ago and Armillaria species evolved in association with climate shift from warm/tropical to cool/arid during the late Eocene. Melanized rhizomorphs, which are unique to Armillaria species, could represent an adaption to harsh environments.
Kubartová A, Ottosson E, Dahlberg A, Stenlid J. Patterns of fungal communities among and within decaying logs, revealed by 454 sequencing. Mol Ecol. 2012;21:4514–32.
• Lamarche J, Potvin A, Pelletier G, Stewart D, Feau N, Alayon DIO, et al. Molecular detection of 10 of the most unwanted alien Forest pathogens in Canada using real-time PCR. PLoS One. 2015;10(8):e0134265. https://doi.org/10.1371/journal.pone.0134265. This research developed molecular detection tools for 10 of the most unwanted forest pathogens in Canada. These tools could help detection/eradication of unwanted pathogens before they get established.
Lee SK, Seo S-T. First report of Armillaria root disease caused by Armillaria tabescens on Carpinus tschonoskii in South Korea. Plant Dis. 2016;100:213. https://doi.org/10.1094/PDIS-06-15-0651-PDN.
Lee SK, Seo S-T, Park J-H, Lee SK. First report of Armillaria root disease caused by Armillaria ostoyae on Japanese larch (Larix kaempferi) and eastern white pine (Pinus strobus) in South Korea. Plant Dis. 2016;100:528. https://doi.org/10.1094/PDIS-07-15-0744-PDN.
•• Lindahl BD, Nilsson RH, Tedersoo L, Abarenkov K, Carlsen T, Kjøller R, et al. Fungal community analysis by high-throughput sequencing of amplified markers – a user’s guide. New Phytol. 2013;199:288–99. The article discusses methodologies for conducting metagenomics for fungal community analyses.
Liu J-J, Sturrock RN, Sniezko RA, Williams H, Benton R, Zamany A. Transcriptome analysis of the white pine blister rust pathogen Cronartium ribicola: de novo assembly, expression profiling, and identification of candidate effectors. BMC Genomics. 2015;16:678. https://doi.org/10.1186/s12864-015-1861-1.
• Lundén K, Danielsson M, Durling MB, Ihrmark K, Gorriz MN, Stenlid J, et al. Transcriptional responses associated with virulence and defense in the interaction between Heterobasidion annosum s.s. and Norway spruce. PLoS One. 2015;10(7):e0131182. https://doi.org/10.1371/journal.pone.0131182. This research highlights the transcriptional responses in a Heterobasidion -Norway spruce as a time series analysis. This research identified novel transcripts with expression patterns that suggest roles in defense.
Machado PS, Alfenas AC, Alfenas RF, Mohammed CL, Glen M. Microsatellite analysis indicates that Puccinia psidii in Australia is mutating but not recombining. Australas Plant Pathol. 2015;44:455–62.
Martin F, Aerts A, Ahrén D, Brun A, Danchin EGJ, Duchaussoy F, et al. The genome of Laccaria bicolor provides insights into mycorrhizal symbiosis. Nature. 2008;452:88–92.
Martinez D, Larrondo LF, Putnam N, Gelpke MDS, Huang K, Chapman J, et al. Genome sequence of the lignocellulose degrading fungus Phanerochaete chrysosporium strain RP78. Nat Biotechnol. 2004;22:695–700.
McDonald GI, Richardson BA, Zambino PJ, Klopfenstein NB, Kim M-S. Pedicularis and Castilleja are natural hosts of Cronartium ribicola in North America: a first report. For Pathol. 2006;36:73–82.
McTaggart AR, Roux J, Granados GM, Gafur A, Tarrigan M, Santhakumar P, et al. Rust (Puccinia psidii) recorded in Indonesia poses a threat to forests and forestry in South-East Asia. Australasian Plant Pathol. 2016;45:83–9.
Mmbaga MT, Klopfenstein NB, Kim M-S, Mmbaga NC. PCR-based identification of Erysiphe pulchra and Phyllactinia guttata from Cornus florida using ITS-specific primers. For Pathol. 2004;34:321–8.
Nelson EV, Fairweather ML, Ashiglar SM, Hanna JW, Klopfenstein NB. First report of the Armillaria root disease pathogen, Armillaria gallica, on Douglas-fir (Pseudotsuga menziesii) in Arizona. Plant Dis. 2013;97:1658.
Ohm RA, Riley R, Salamov A, Min B, Choi I-G, Grigoriev IV. Genomics of wood-degrading fungi. Fungal Genet Biol. 2014;72:82–90.
Olson A, Aerts A, Asiegbu F, Belbahri L, Bouzid O, Broberg A, et al. Insight into trade-off between wood decay and parasitism from the genome of a fungal forest pathogen. New Phytol. 2012;194:1001–13.
Ota Y, Tokuda S, Buchanan PK, Hattori T. Phylogenetic relationships of Japanese species of Heterobasidion—H. annosum sensu lato and an undetermined Heterobasidion sp. Mycologia. 2006;98:717–25.
Richardson BA, Klopfenstein NB, Zambino PJ, McDonald GI, Geils BW, Carris LM. The influence of host resistance on the genetic structure of the white pine blister rust fungus, Cronartium ribicola, in western North America. Phytopathology. 2008;98:413–20.
Ross-Davis AL, Stewart JE, Hanna JW, Kim M-S, Knaus B, Cronn R, et al. Transcriptome of an Armillaria root disease pathogen reveals candidate genes involved in substrate utilization at the host-pathogen interface. For Pathol. 2013;43:468–77.
Roux J, Greyling I, Coutinho TA, Verleur M, Wingfield MJ. The Myrtle rust pathogen, Puccinia psidii, discovered in Africa. IMA Fungus. 2013;4:155–9.
Roux J, Granados GM, Shuey L, Barnes I, Wingfield MJ, McTaggart AR. A unique genotype of the rust pathogen, Puccinia psidii, on Myrtaceae in South Africa. Australas Plant Pathol. 2016;45:645–52. https://doi.org/10.1007/s13313-016-0447-y.
Sandhu KS, Karaoglu H, Zhang P, Park RF. Simple sequence repeat markers support the presence of a single genotype of Puccinia psidii in Australia. Plant Pathol. 2016;65:1084–94.
Schnabel G, Bryson PK, Williamson MA. First report of Armillaria tabescens causing Armillaria root rot of pindo palm in South Carolina. Plant Dis. 2006;90:1106.
Schoch CL, Seifert KA, Huhndorf S, Robert V, Spouge JL, Leveseque CA, et al. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. PNAS. 2012;109:6241–6.
Sipos G, Prasanna AN, Walter MC, O’Connor E, Bálint B, Krizsán K, et al. Genome expansion and lineage-specific genetic innovations in the forest pathogenic fungi Armillaria. Nat Ecol Evol. 2017;1:1931–41.
Skovgaard K, Rosendahl S, Nirenberg HI. Fusarium commune is a new species identified by morphological and molecular phylogenetic data. Mycologia. 2003;95:630–6.
Stewart JE, Kim M-S, James RL, Dumroese RK, Klopfenstein NB. Molecular characterization of Fusarium oxysporum and Fusarium commune isolates from a conifer nursery. Phytopathology. 2006;96:1124–33.
Stewart JE, Abdo Z, Dumroese RK, Klopfenstein NB, Kim M-S. Virulence of Fusarium oxysporum and F. commune to Douglas-fir (Pseudotsuga menziesii) seedlings. For Pathol. 2012;42:220–8.
• Stewart JE, Ross-Davis AL, Graҫa RN, Alfenas AC, Peever TL, Hanna JW, et al. Insights into the genetic diversity of the myrtle rust pathogen (Austropuccinia psidii) in the Americas and Hawaii: global implications for invasive threat assessments. For Pathol. 2018;48:e12378. https://doi.org/10.1111/efp.12378/epdf. This research identifies three distinct lineages of the myrtle rust pathogen, Austropuccinia psidii, with different suitable climatic conditions. This research suggests that each lineage may behave differently as introduced pathogens.
•• Štursová M, Šnajdr J, Cajthaml T, Bárta J, Šantrůčková H, Baldrian P. When the forest dies: the response of forest soil fungi to a bark beetle-induced tree dieback. The ISME Journal. 2014;8:1920–31. This article highlights the drastic changes in fungal communities and their functions in response to bark beetle-induced decline in spruce forests. The overall fungal biomass was reduced two-fold with a concomitant decrease in mycorrhizal fungi and slight increase in saprotrophic taxa.
Tan M-K, Collins D, Chen Z, Englezou A, Wilkins MR. A brief overview of the size and composition of the myrtle rust genome and its taxonomic status. Mycology. 2014;5:52–63. https://doi.org/10.1080/21501203.2014.919967.
Tokuda S, Hattori T, Dai Y-C, Ota Y, Buchanan PK. Three species of Heterobasidion (Basidiomycota, Hericiales), H. parviporum, H. orientale sp. nov. and H. ecrustosum sp. nov. from East Asia. Mycoscience. 2009;50:190–202.
Tzean Y, Shu P-Y, Liou R-F, Tzean S-S. Development of oligonucleotide microarrays for simultaneous multi-species identification of Phellinus tree-pathogenic fungi. Microb Biotechnol. 2016;9:235–44.
Uchida J, Zhong S, Kilgore E. First report of a rust disease on 'ōhi'a caused by Puccinia psidii in Hawaii. Plant Dis. 2006;90:524.
•• Uroz S, Buée M, Deveau A, Mieszkin S, Martin F. Ecology of the forest microbiome: Highlights of temperate and boreal ecosystems. Soil Biol Biochem. 2016;103:471–88. This study highlights differences in microbial communities associated with tree species, soil types and properties, seasons, and forestry practices.
• Vaz ABM, Fonseca PLC, Leite LR, Badotti F, Salim ACM, Araujo FMG, et al. Using Next-Generation Sequencing (NGS) to uncover diversity of wood-decaying fungi in neotropical Atlantic forests. Phytotaxa. 2017;295(1):001–21. This study utilizes ITS1 and ITS2 amplified metagenomic sequencing methodologies to characterize the diversity of fungi associated with wood decay in neotropical forests of Brazil.
Williams HL, Sturrock RN, Islam MA, Hammett C, Ekramoddoullah AKM, Leal I. Gene expression profiling of candidate virulence factors in the laminated root rot pathogen Phellinus sulphurascens. BMC Genomics. 2014;15:603. http://www.biomedcentral.com/1471-2164/15/603
Yakovlev IA, Hietala AM, Courty P-E, Lundell T, Solheim H, Fossdal CG. Genes associated with lignin degradation in the polyphagous white-rot pathogen Heterobasidion irregulare show substrate-specific regulation. Fungal Genet Biol. 2013;56:17–24.
Zhu S, Cao Y-Z, Jiang C, Tan B-Y, Wang Z, Feng S, et al. Sequencing the genome of Marssonina brunnea reveals fungus-poplar co-evolution. BMC Genomics. 2012;13:382. http://www.biomedcentral.com/1471-2164/13/382
Žifčáková L, Větrovský T, Howe A, Baldrian P. Microbial activity in forest soil reflects the changes in ecosystem properties between summer and winter. Environ Microbiol. 2016;18(1):288–301.
Otrosina WJ, Garbelotto M. Heterobasidion occidentale sp. nov. and Heterobasidion irregulare nom. nov.: A disposition of north American Heterobasidion biological species. Fungal Biol. 2010;114:16–25.
Acknowledgments
The authors extend thanks to the researchers who facilitated the research described herein.
Funding
Funding provided for this manuscript includes grants from the USDA Forest Service, Forest Health Protection, Special Technology Development Program, Evaluation Monitoring Program, the Western Wildland Environmental Threat Assessment Center, the Western Forest Conservation Association, and Colorado State University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Stewart, Dr. Kim, and Dr. Klopfenstein have no conflicts of interests to declare.
Human and Animal Rights and Informed Consent
This article contains no studies with human and animal subjects performed by the authors.
Additional information
This article is part of the Topical Collection on Forest Pathology
Rights and permissions
About this article
Cite this article
Stewart, J.E., Kim, MS. & Klopfenstein, N.B. Molecular Genetic Approaches Toward Understanding Forest-Associated Fungi and Their Interactive Roles Within Forest Ecosystems. Curr Forestry Rep 4, 72–84 (2018). https://doi.org/10.1007/s40725-018-0076-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40725-018-0076-5