Abstract
Objective
This study aimed to investigate the effects of liraglutide on bone metabolism markers in rat models with glucocorticoid-induced osteoporosis (GIOP), including the effects on bone mass, bone tissue microstructure, bone biomechanics, and bone turnover markers.
Method
Thirty male Sprague–Dawley rats aged 8 weeks were randomly divided into three groups: the control group (n = 10) was intramuscularly injected with an equal volume of 0.9% sodium chloride, the dexamethasone group (n = 10) was intramuscularly injected with dexamethasone at 1 mg/kg (twice a week) to induce GIOP, the dexamethasone plus liraglutide group (n = 10) was subcutaneously injected with liraglutide at 200 μg/kg daily, simultaneously. The bilateral femurs and the fifth lumbar vertebrae were collected after 12 weeks to perform micro-computed tomography and bone biomechanical examinations. Also, tartrate-resistant acid phosphatase (TRACP), cross-linked carboxy-terminal telopeptide of type I collagen (CTX-I), alkaline phosphatase (ALP), and osteocalcin (OC) were tested.
Results
The bone mineral density (BMD), bone microstructure, and bone biomechanical markers reduced significantly in the dexamethasone group compared with the control group. The bone resorption indicators (TRACP and CTX-I) increased, while the bone formation indicators (ALP and OC) decreased. After liraglutide treatment, BMD, bone microstructure, and bone biomechanical markers improved significantly. Moreover, TRACP and CTX-I decreased significantly, while ALP and OC increased compared with the dexamethasone group.
Conclusions
Liraglutide improved BMD, bone microstructure, and bone strength and reversed GIOP, primarily through the reduction of bone resorption and promotion of bone formation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is a metabolic bone disease characterized by decreased bone mass, impaired bone microstructure, increased bone fragility, and increased risk of bone fracture. The incidence rate of osteoporosis is increasing in the aging population worldwide. The incidence rate of bone fracture associated with osteoporosis has been estimated to be about 40% in older women and about 13% in men worldwide [1]. The pain and bone fracture induced by osteoporosis seriously affect the quality of life of patients. Osteoporosis imposes a great burden on families and the society and has become a worldwide problem. Glucocorticoids are widely used clinically due to their anti-inflammatory and immunosuppressive effects. Changes in the ratio of osteoprotegerin to receptor activator of nuclear factor-κB ligand during corticosteroid treatment lead to increased bone resorption during the first 3–6 months, resulting in particularly prominent adverse reactions for osteoporosis and increased risk of bone fracture [2]. Glucocorticoid-induced osteoporosis (GIOP) ranks first among secondary osteoporosis.
Glucocorticoids can also affect the process of glucose metabolism by antagonizing insulin. In recent years, studies have demonstrated a correlation between bone metabolism and glucose metabolism [3, 4]. A prospective study [5] has revealed that the risk of bone mineral loss in male patients with type 1 diabetes is similar to that in female patients with type 2 diabetes, which is higher than that in the relevant control groups, and may be associated with increased bone resorption or decreased bone formation [6]. Another study [7] has shown that GLP-1 receptor agonists have osteoprotective effects on postmenopausal osteoporosis. However, a few studies are available on GIOP. Therefore, this study aimed to discuss the role and mechanism of liraglutide as a novel hypoglycemic agent for bone metabolism in GIOP.
Materials and methods
Experimental animals and drug and reagent intervention
Thirty 8-week-old specific pathogen free, male Sprague–Dawley (SD) rats weighing about (220 + 10) g were purchased from the Anhui Experimental Animal Center, China. All procedures on the animals were approved by the Institutional Animal Care and Use Committee of The second Affiliated Hospital of Anhui Medical University. Dexamethasone (Shiyao Yinhu Pharmaceutical Co., Ltd., China; batch number: H14022567) and liraglutide (Novo Nordisk, Norway; batch number: J20160037) were used. Food for the rats was purchased from Xietong Organism, Jiangsu, China. The detection kits of tartrate-resistant acid phosphatase (TRACP), cross-linked carboxy-terminal telopeptide of type I collagen (CTX-I), and alkaline phosphatase (ALP), together with osteocalcin (OC) enzyme-linked immunosorbent assay (ELISA) kit, were purchased from Yuanye Biotechnology (Shanghai) Co., Ltd (China).
Animal grouping and modeling
All male SD rats were adaptively fed for 1 week, and then fasting blood glucose (FBG) was measured. The rats were randomly divided into three groups with ten rats in each group: (1) The control group (Con group) (n = 10) was intramuscularly injected with 0.1 mL of saline. (2) The dexamethasone group (DEX group) (n = 10) was intramuscularly injected with 0.1 mL of dexamethasone solution at a dose of 1 mg/kg (twice a week). (3) The dexamethasone plus liraglutide group (DEX + Lir group) (n = 10) was injected with 0.1 mL of dexamethasone solution twice a week. Simultaneously, a subcutaneous injection of liraglutide at 200 μg/kg was administered daily. Rat models of GIOP were established by continuous intervention with dexamethasone for 3 months. Meanwhile, liraglutide was subcutaneously injected until the end of the experiment. Two rats died in the DEX + Lir group due to a significant reduction in their weight. Before being killed, the FBG of the rats was measured. The peripheral serum, bilateral femurs, and the fifth lumbar vertebrae were collected. The bilateral femurs and the fifth lumbar vertebrae were wrapped in saline gauze and placed in the refrigerator at a temperature of − 20 °C.
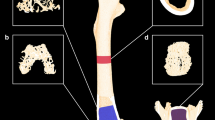
Evaluation of bone density, bone tissue microstructure, and reconstruction images using micro-CT
The left femur and lumbar vertebrae were thawed at room temperature and fixed in a sample holder for micro-computed tomography (micro-CT) scanning using SCANCO uCT80. The scanner was set at a voltage of 50 kVp, current of 200 μA, and resolution of 15 μm/pixel. After scanning, the tissues and structures were reconstructed to obtain reconstructed images. Then, the parameters of bone microstructure, including bone mineral density (BMD), tissue BMD (TMD), number of trabecular bone (Tb.N), trabecular thickness (Tb.Th), bone volume/total volume (BV/TV), the relative bone surface (BS/TV), trabecular separation (Tb.Sp), and connectivity density (Conn.D), were determined by quantitative analysis using a built-in software.
Biomechanical testing of femur
After the right femur was thawed at room temperature, a three-point bending test was performed. Corresponding fixtures were made according to different sizes of bone specimens. When performing the test, the femur was horizontally placed on the surface of the machine and the probe was slowly lowered. The loading direction was perpendicular to the test platform, acting on the central axis of the bone with a loading speed of 2 mm/min. The load–displacement curve was automatically recorded by the software specific to the computer connected to the test machine; the maximum load along with the elastic modulus was obtained.
Detection of serum bone turnover metabolic markers
The serum TRACP, CTX-I, ALP, and OC levels of rats were detected using kits, and the operations were strictly performed according to the manufacturer’s instructions on the kit. Blood harvested from the inferior vena cava was centrifuged at 3000 rpm for 10 min to extract the supernatant. The levels of serum TRACP, CTX-I, ALP, and OC were detected using the ELISA kit. The optical density value was measured on a microplate after the following steps: application of samples, discarding the fluid, addition of enzyme conjugate working solution, discarding the fluid, addition of substrate solution, and finally addition of the stop buffer.
Statistical analysis
Statistical analysis was performed using SPSS 17.0. Normal distribution was presented by (\(\bar{x} \pm s\)). Group-wise comparison was performed using the least significant difference test, and multiple groups were compared using the one-way analysis of variance. The continuous data of nonnormal distribution and heterogeneity of variance were analyzed using the rank-sum test.
Results
Growth status of rats and FBG changes
After 12 weeks of intervention, the body weight of rats in the DEX group was significantly lower than that in the Con group (P < 0.05). However, no significant difference in the body weight was observed between the DEX + Lir and DEX groups. No statistical differences in FBG were observed among the three groups (Table 1).
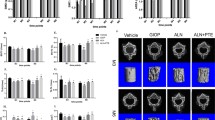
Micro-CT 3D image, 2D sectional images, and bone microparameters
A three-dimensional reconstruction of the region of interest was performed using micro-CT (Fig. 1). The 2D sectional images of the femur and the lumbar vertebrae are shown in Fig. 2. The microparameters of the lumbar vertebrae are provided in Table 2 and those of the femur in Table 3. Compared with the Con group, BMD, TMD, Conn.D., Tb.Th, and Tb.Sp of the lumbar vertebrae decreased significantly in the DEX group (P < 0.05). BMD, TMD, Conn.D, Tb.N, Tb.Th, and Tb.Sp were significantly increased in the DEX + Lir group compared to the DEX group (P < 0.05). Likewise, the left femur of DEX group showed a significant decrease of BMD, TMD, Conn.D, Tb.N, Tb.Th, Tb.Sp, and BS/TV compared with the Con group (P < 0.05). BMD, Conn.D, and Tb.Th of the left femur were significantly higher in the DEX + Lir group compared with the DEX group (P < 0.05).
Biomechanical changes in femur
The three-point bending test of the femur showed that the maximum load and elastic modulus in the DEX + Lir group were higher than those in the DEX group, with statistically significant difference between the groups (P < 0.05) (Table 4).
Changes in serum ALP, OC, TRACP, and CTX-I
The indicators of serum bone formation, including ALP and OC, decreased significantly (P < 0.05), while the bone resorption indicators, including TRACP and CTX-I, increased significantly (P < 0.05) in the DEX group compared with the Con group. The indicators of serum bone formation, including ALP and OC, increased significantly (P < 0.05), while bone resorption indicators, including TRACP and CTX-I, decreased significantly (P < 0.05) in the DEX + Lir group compared with the DEX group (Table 5).
Discussion
Osteoporosis is a bone metabolic disease with a high incidence rate. GIOP has leapt to the first place in secondary osteoporosis. The prevalence of GIOP was obviously higher in rheumatoid arthritis with glucocorticoid group (41.6%), compared with rheumatoid arthritis without glucocorticoid group (29.4%) in China [8]. Vertebral fractures are the most common fractures associated with glucocorticoids. Data from published studies indicate that the risk of vertebral fracture increases 3 months after the initiation of treatment and peaks at 12 months. The risk of fracture in patients who continuously received GCs is strongly associated with daily dose of oral GCs [9]. Therefore, osteoporosis has gained increasing attention of researchers. The current treatment is mainly anti-bone resorption drugs, such as bisphosphonates, denosumab [10], and calcitonin. However, few drugs promote bone formation as well. Therefore, new therapeutic drugs for GIOP need to be explored.
Our study showed that no significant differences were observed between control and DEX group with regard to FBS in 8-week-old rats. This finding was similar to the results of the previous study [11] that FBS levels were not modified by the DEX treatment in 3-month-old rats, whereas older rats developed a marked hyperglycemia. On the other hand, DEX treatment rapidly induced a twofold increase of plasma insulin levels in young rats, and a 4 ~ 5-fold increase in older rats. A moderate hyperinsulinemia is sufficient to inhibit the increase of plasma glucose levels in young rats, whereas older rats may be at risk for the development of age-related failure of the adaptive mechanisms regulating glucose homeostasis. Therefore, the impacts of GCs on the FBS in rats are dependent on the age of rats [11] and dosing regimen of GC administration [12]. Next, we found that the body weight of rats in the DEX group was significantly lower than that in the Con group. Our findings were similar to the results of the previous studies [12, 13] that treating adult male rats (approximately 3 months old) with dexamethasone is known to induce a significant reduction in both body mass and food intake during treatment. This reduced body mass in the GC-treated rats is partially explained by their hypophagic behavior. GC-induced hypophagy may be a result of anorexigenic insulin and leptin effects on the hypothalamus, as the concentration of both hormones is elevated in adult dexamethasone-treated rats [14].
Liraglutide is a synthetic glucagon-like peptide-1 receptor agonist and is currently clinically used as a novel type 2 diabetes hypoglycemic drug. Under hyperglycemic conditions in humans, GLP-1 stimulates insulin secretion and normalizes blood glucose levels, whereas GLP-1 does not stimulate insulin secretion at normal glucose levels [15]. Therefore, glucose lowering effect with liraglutide treatment was absent in our study. A recent meta-analysis of 16 RCTs showed that liraglutide, as a hypoglycemic agent, could reduce the risk of bone fracture in patients with diabetes (odds ratio = 0.38) [16]. It also indicated that liraglutide might have a positive effect on both bone and glucose metabolism. In a randomized controlled study of 37 obese women with body mass more than 34 kg/m2, Eva et al. [17] showed that bone formation increased by 16% and bone loss due to the low-calorie diet could be effectively avoided after 52 weeks of continuous subcutaneous injection of liraglutide. Besides, receptor knockout animal experiments demonstrated that GLP-1 was essential for bone metabolism. Male mice with GLP-1R knockout underwent three-point bending test, quantitative X-ray micrography, and micro-CT. The results revealed that the maximum load, fracture load, and stiffness of the animals lacking GLP-1R decreased significantly. Moreover, the bone cortex, outer diameter of the bone, and bone strength decreased. Further, in a mouse ovariectomy model of osteoporosis, intervention using GLP-1 receptor agonists, including liraglutide and exenatide, was performed for 4 weeks. The results suggested that liraglutide and exenatide could significantly improve the volume, thickness, and quantity of trabecular bones and increase the connectivity, which was especially significant for liraglutide, compared with the castrated mice [7]. The aforementioned studies suggested that the GLP-1 receptor agonists were closely related to bone metabolism.
An assessment of bone metabolism includes two main aspects: bone mass and bone quality. The most common indicator of bone mass is BMD. BMD is also a necessary condition for the diagnosis of osteoporosis. In the present study, the bone density of femur and lumbar vertebrae of rats in the DEX group was found to decline significantly using micro-CT, suggesting that the modeling was successful. However, liraglutide increased the BMD in rats, which had consistent performances in the lumbar vertebrae and femur. The bone quality included the material composition of bone at the molecular level, bone microarchitecture at the tissue level, and integrity of bone microstructure. A micro-CT examination of diabetic mice modeled by Sity Aishah et al. [18] using streptozotocin, which were killed after 21 days of intervention with liraglutide, revealed a decreasing trend of BV/TB and Tb.N without statistical significance. Moreover, no significant change was noted after intervention with liraglutide, which might be because the action time of drug was too short to affect the bone microparameters. Micro-CT examinations in previous studies also demonstrated that after 16 weeks of intervention with exenatide-4 in rat ovariectomy models of osteoporosis, BV/TV, Tb.N, Tb.Th, Tb.Sp, and Conn.D in the femur and lumbar vertebrae increased significantly compared with the dexamethasone group [19], consistent with the results of the present study. After the treatment of GIOP rats with liraglutide, BV/TV, Tb.N, Tb.Th, and Conn.D showed an increasing trend compared with the DEX group, indicating that the microstructure of trabecular bones was improved.
The bone structure mechanical index test in bone biomechanics is the most direct method to understand the risk of osteoporotic fracture. It is also the comprehensive manifestation of bone strength, bone structure, and bone mass. However, observation of bone histomorphology and measurement of bone density alone cannot fully reflect the bone quality. Evaluation of the efficacy of interventions using mechanical properties is irreplaceable by other methods in treating osteoporosis. In the present study, a three-point bending test suggested that the maximum load and elastic modulus of the DEX group were significantly lower than those of the control group, and liraglutide treatment could significantly improve the fracture resistance of bone tissue in rats. These findings were similar to the results of the previous studies that bone biomechanical indicators in rats lacking GLP-1 receptors decreased significantly [20].
Serum CTX-I is a degradation product of type I collagen in bone. Its specific structure protects it from kidney degradation. Moreover, it is stable in serum. Detection of serum CTX-I content can specifically reflect the absorption activity of osteoclasts. TRACP is mainly produced by noncollagen proteins released by osteoclasts, which are secreted outside the cells together with collagen metabolites. Therefore, TRACP and CTX-1 levels were positively correlated with bone resorption. A previous study demonstrated that 8 weeks of intervention with liraglutide could significantly reduce serum CTX-I and osteoclast number in ovariectomized rats with streptozotocin-induced diabetes [21], which was consistent with the results of the present study, suggesting that liraglutide could inhibit osteoclast activity and protect bone tissue by reducing bone loss. Osteoblasts can secrete bone-specific ALP, which is an important component of total ALP and can reflect the state of bone formation. OC is the most abundant marker in the bone matrix, which is released by the osteoblasts to the outside of cells, reflecting the state of bone formation. A previous study demonstrated that GLP-1 receptors were expressed in osteoblasts, increased type 1 collagen expression and ALP activity, and promoted bone anabolism [22]. In addition, a study by Sun et al. [23] revealed that after 4 weeks of subcutaneous injection of liraglutide in nonobese spontaneously hyperglycemic GK rats in the early stage of life, the bone density of rats improved. Moreover, the bone formation markers, including the expression of OC, ALP, and collagen 1, also increased significantly. The results of the present study were consistent with the aforementioned findings, suggesting that liraglutide might promote osteoblast activity and protect bone tissue by promoting bone formation in rats with GIOP.
In summary, liraglutide improved BMD, bone microstructure, and bone strength and reversed GIOP, primarily through the reduction of bone resorption and promotion of bone formation. Liraglutide may become a new drug for treating osteoporosis. Further studies should investigate the mechanism underlying the effect of liraglutide on bone metabolism.
References
Drake MT, Clarke BL, Lewiecki EM (2015) The pathophysiology and treatment of osteoporosis. Clin Ther 37:1837–1850
Weinstein RS (2011) Clinical practice. glucocorticoid-induced bone disease. N Engl J Med 365:62–70
Kanazawa I (2017) Interaction between bone and glucose metabolism [Review]. Endocr J 64:1043–1053
DeShields SC, Cunningham TD (2018) Comparison of osteoporosis in US adults with type 1 and type 2 diabetes mellitus. J Endocrinol Invest 41:1051–1060
Hamilton EJ, Rakic V, Davis WA, Paul Chubb SA, Kamber N, Prince RL, Davis TM (2012) A five-year prospective study of bone mineral density in men and women with diabetes: the fremantle diabetes study. Acta Diabetol 49:153–158
Rubin MR (2015) Bone cells and bone turnover in diabetes mellitus. Curr Osteoporos Rep 13:186–191
Pereira M, Jeyabalan J, Jorgensen CS, Hopkinson M, Al-Jazzar A, Roux JP, Chavassieux P, Orriss IR, Cleasby ME, Chenu C (2015) Chronic administration of glucagon-like peptide-1 receptor agonists improves trabecular bone mass and architecture in ovariectomised mice. Bone 81:459–467
Ma CC, Xu SQ, Gong X, Wu Y, Qi S, Liu W, Xu JH (2017) Prevalence and risk factors associated with glucocorticoid-induced osteoporosis in Chinese patients with rheumatoid arthritis. Arch Osteoporos 12:33
De Vries F, Bracke M, Leufkens HG, Lammers JW, Cooper C, Van Staa TP (2007) Fracture risk with intermittent high-dose oral glucocorticoid therapy. Arthritis Rheum 56:208–214
Cairoli E, Palmieri S, Goggi G, Roggero L, Arosio M, Chiodini I, Eller-Vainicher C (2018) Denosumab or oral bisphosphonates in primary osteoporosis: a “real-life” study. J Endocrinol Invest 41:1005–1013
Novelli M, De Tata V, Bombara M, Lorenzini A, Masini M, Pollera M, Bergamini E, Masiello P (1999) Insufficient adaptive capability of pancreatic endocrine function in dexamethasone-treated ageing rats. J Endocrinol 162:425–432
Caperuto LC, Anhe GF, Amanso AM, Ribeiro LM, Medina MC, Souza LC, Carvalho OM, Bordin S, Saad MJ, Carvalho CR (2006) Distinct regulation of IRS proteins in adipose tissue from obese aged and dexamethasone-treated rats. Endocrine 29:391–398
dos Santos C, Ferreira FB, Goncalves-Neto LM, Taboga SR, Boschero AC, Rafacho A (2014) Age- and gender-related changes in glucose homeostasis in glucocorticoid-treated rats. Can J Physiol Pharmacol 92:867–878
Chimin P, Farias Tda S, Torres-Leal FL, Bolsoni-Lopes A, Campana AB, Andreotti S, Lima FB (2014) Chronic glucocorticoid treatment enhances lipogenic activity in visceral adipocytes of male Wistar rats. Acta Physiol (Oxf) 211:409–420
Gallwitz B (2005) Glucagon-like peptide-1-based therapies for the treatment of type 2 diabetes mellitus. Treat Endocrinol 4:361–370
Su B, Sheng H, Zhang M et al (2015) Risk of bone fractures associated with glucagon-like peptide-1 receptor agonists’ treatment: a meta-analysis of randomized controlled trials. Endocrine 48:107–115
Iepsen EW, Lundgren JR, Hartmann B, Pedersen O, Hansen T, Jorgensen NR, Jensen JE, Holst JJ, Madsbad S, Torekov SS (2015) GLP-1 receptor agonist treatment increases bone formation and prevents bone loss in weight-reduced obese women. J Clin Endocrinol Metab 100:2909–2917
Mansur SA, Mieczkowska A, Bouvard B, Flatt PR, Chappard D, Irwin N, Mabilleau G (2015) Stable incretin mimetics counter rapid deterioration of bone quality in type 1 Diabetes Mellitus. J Cell Physiol 230:3009–3018
Ma X, Meng J, Jia M, Bi L, Zhou Y, Wang Y, Hu J, He G, Luo X (2013) Exendin-4, a glucagon-like peptide-1 receptor agonist, prevents osteopenia by promoting bone formation and suppressing bone resorption in aged ovariectomized rats. J Bone Miner Res 28:1641–1652
Yamada C, Yamada Y, Tsukiyama K, Yamada K, Udagawa N, Takahashi N, Tanaka K, Drucker DJ, Seino Y, Inagaki N (2008) The murine glucagon-like peptide-1 receptor is essential for control of bone resorption. Endocrinology 149:574–579
Wen B, Zhao L, Zhao H, Wang X (2018) Liraglutide exerts a bone-protective effect in ovariectomized rats with streptozotocin-induced diabetes by inhibiting osteoclastogenesis. Exp Ther Med 15:5077–5083
Montagnani A, Gonnelli S (2013) Antidiabetic therapy effects on bone metabolism and fracture risk. Diabetes Obes Metab 15:784–791
Sun HX, Lu N, Luo X, Zhao L, Liu JM (2015) Liraglutide, the glucagon-like peptide-1 receptor agonist, has anabolic bone effects in diabetic Goto-Kakizaki rats. J Diabetes 7:584–588
Acknowledgements
This study was supported by the university natural science research fund of Anhui province (KJ2017A174).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, L., Yang, J., Pan, T. et al. Liraglutide increases bone formation and inhibits bone resorption in rats with glucocorticoid-induced osteoporosis. J Endocrinol Invest 42, 1125–1131 (2019). https://doi.org/10.1007/s40618-019-01034-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-019-01034-5