Abstract
This study evaluated the preventative effects of metformin (Met) on glucocorticoid (GC)-induced osteoporosis in a rat model, compared with alendronate (Aln). Twenty-eight 3-month-old female Sprague–Dawley rats were randomly assigned into four groups: normal control (Ctr), methylprednisolone (MP, 13 mg/kg/day, sc, 5 days per week), MP plus Aln orally (1 mg/kg/day), and MP plus Met orally (200 mg/kg/day). After 9 weeks, serum bone metabolic biochemistry, bone densitometry and histomorphometry were performed. The GC-induced osteoporosis model was characterized by decreased osteocalcin, increased tartrate-resistant acid phosphatase-5b (TRAP-5b), and decreased bone mineral density (BMD) in the femur and fifth lumbar vertebra (L5). Histomorphometrically, MP significantly decreased trabecular bone volume, decreased bone formation and increased bone resorption in proximal metaphysis, compared with the controls. Aln and Met increased the BMDs of femur (0.305 ± 0.011 vs. 0.280 ± 0.012, P < 0.05; 0.304 ± 0.019 vs. 0.280 ± 0.012, P < 0.05) and L5 (0.399 ± 0.029 vs. 0.358 ± 0.022, P < 0.05; 0.397 ± 0.022 vs. 0.358 ± 0.022, P < 0.05), compared with the model group. Met increased osteocalcin and decreased TRAP-5b, but Aln only decreased TRAP-5b, compared with model group. In histomorphometry of tibial proximal metaphysis, Aln and Met increased trabecular bone volume (39.21 ± 2.46 vs. 30.98 ± 5.83, P < 0.05; 38.97 ± 5.56 vs. 30.98 ± 5.83, P < 0.05), while Met increased the bone formation dynamic parameters and decreased bone resorption dynamic parameters, but Aln just decreased bone resorption dynamic parameters, compared with model group significantly. These findings suggest that metformin prevents GC-induced bone loss by suppressing bone resorption and stimulating bone formation in trabecular bone. The action mode of metformin was different from alendronate, which only suppressed bone resorption.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glucocorticoids (GCs) have been widely used in clinical practice for the treatment of various diseases, such as asthma, lupus and rheumatoid arthritis. However, these are often associated with serious side effects, especially after long-term administration, including disorder of glucose metabolism, abnormality of lipid metabolism, water–sodium retention and fat redistribution. GC-induced osteoporosis is one of the most serious problems for patients receiving long-term GC therapy. The long-term use of glucocorticoids can lead to a reduction in bone mineral density and bone mass, and thereby result in increased risk of fractures. The direct effects of GCs on bones include the early and transient increase in bone resorption, long-term suppression of bone formation, and the promotion of bone resorption at tissue and cellular levels. Therefore, it is important to identify medicines that could prevent GC-induced osteoporosis.
Bisphosphonate drugs, such as alendronate, have been commonly used for the prevention and treatment of osteoporosis [1]. However, the long-term administration of alendronate is associated with serious side effects, such as osteonecrosis of the mandible. In addition, it has been reported that long-term alendronate administration results in increased risks of atypical fractures, although bone mineral density (BMD) is not significantly decreased [2,3,4,5]. Therefore, there is an important need to develop new drugs for preventing GC-induced osteoporosis for GC users, to maintain the high quality of the bone.
Metformin is the first-line medication for the treatment of type-2 diabetes, especially in overweight patients [6] and patients with the disorder of lipid metabolism [7]. Recently, several studies have shown that metformin can prevent osteopenia [8,9,10,11]. A large-sample case–control study revealed that metformin reduces diabetes-related osteoporosis [11]. Several clinical studies have further found that metformin produces protective effects in reducing bone loss [8,9,10]. The protective effects of metformin in reducing bone loss were further supported in many animal models, such as ovariectomized rats [12, 13] and insulin-deficient diabetic osteopathy rats [14]. Furthermore, in vitro studies have found that metformin promotes the differentiation of bone marrow stromal cells to osteoblasts, increases the activity of osteoblasts, and inhibits the activity of osteoclasts [15,16,17]. However, some clinical and animal studies have revealed that metformin produced no protective effect on bone [18, 19]. Therefore, more pre-clinical and clinical studies are required to investigate the effects of metformin on bone. To our knowledge, it remains unclear whether metformin can prevent GC-induced osteoporosis.
In the present study, the effect of metformin on the bone structure and metabolism of rats treated with methylprednisolone (MP) in a rat model of glucocorticoid-induced osteoporosis were investigated, and compared with alendronate. The purpose of the present study was to explore whether metformin prevents GC-induced osteoporosis.
Materials and methods
Materials
MP was purchased from Pfizer Inc. (NY, USA). In the form of crude drug, metformin and alendronate were bought from Hubei Kangbaotai Fine Chemical Co., Ltd. (Wuhan, China). A compound feed containing 1.14% calcium, 0.98% phosphorus and 800 IU/kg of vitamin D3 was purchased from KeaoXieli Feed Co., Ltd. (Beijing, China). The Sprague–Dawley rats were purchased from Vital River Laboratories (Beijing, China).
Animal procedures
The experimental protocol was approved by the Institutional Authority for Laboratory Animal Care of Peking University Health Center, and all the animals received humane care according to the Institutional Authority for Laboratory Animal Care of Peking University Health Center. A total of 28 3-month-old female Sprague–Dawley rats, weighing 280 ± 14 g, were used in the present study. These rats were housed in room temperature (25 °C) with 60% humidity and a 12-h light/dark cycle in the Laboratory Animal Department of Peking University Health Center (four rats per cage). Rats were fed with standard rat chow and water ad libitum.
After adaptation for 2 weeks, these rats were randomly assigned into four groups (n = 7 per group): (1) control group, received saline subcutaneously for 5 days/week and by gavage for 7 days/week; (2) MP group, received MP (13 mg/kg/day) subcutaneously for 5 days/week and saline by gavage for 7 days/week [20]; (3) MP + Aln group, received MP (13 mg/kg/day) subcutaneously for 5 days/week and alendronate (1 mg/kg/day) by gavage for 7 days/week [21]; and (4) MP + Met group, received MP (13 mg/kg/day) subcutaneously for 5 days/week and metformin (200 mg/kg/day) by gavage for 7 days/week [22]. These animals were treated with the experimental protocol for 9 weeks. The body weights were determined at the initiation of the experiment, and monitored twice a week to adjust the dosage of the drug. At the beginning of the study and the fifth week, fasting blood was drawn from the retro-orbital plexus for biochemical tests under anesthesia. At killing, the animals were euthanized by exsanguination through cardiac puncture under anesthesia, and blood was collected for biochemical tests. The left femurs and fifth lumbar vertebrae (L5) were collected and stored at − 20 °C prior to the bone density tests, and the right tibias were collected for bone histomorphometry. For dynamic parameters, rats were subcutaneously injected with calcein (10 mg/kg) at 13, 12, 3, and 2 days before necropsy.
Serum biochemical assays
Serum glucose, triglyceride (TG), total cholesterol (CHO), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) at the beginning of the study, at the fifth week, and at killing were measured at the clinical laboratory of Inner Mongolia Medical University Affiliated Hospital using a HITACHI 7170S analyzer (Tokyo, Japan). The serum concentrations of bone turnover markers at killing, tartrate-resistant acid phosphatase-5b (TRAP-5b) and osteocalcin were measured using rat TRAP-5b and osteocalcin enzyme-linked immunosorbent assay (ELISA) kits (Rigorbio, Beijing, China), according to manufacturer’s instructions.
Bone densitometry
Before bone densimetic measurement, the vertebral arch of L5 was removed. Then, the inferior surface closest to the spinal cord was horizontally placed downwards. The femur was positioned with the long axis parallel to the horizontal plane. The left femurs and L5 were examined by dual-energy X-ray absorptiometry (DXA) using a QDR-Discovery densitometer (Hologic, Bedford, MA, USA) with a regional high-resolution mode. Then, the average BMDs of the femur and L5 vertebral body were measured. The region of interest was the total femur for femur densitometry and the total vertebral body for L5 densitometry. The BMD data were expressed in grams per square centimeter.
Bone histomorphometry
At killing, the right tibias were cleaned off with the soft tissue and fixed in 4% paraformaldehyde. After dehydration, each sample was cut into the proximal segment and middle-distal segment, which contained the proximal one-third and distal two-thirds of the tibia, respectively. Then, the connection of the tibia and fibia with the proximal 1.5-cm-long tibia shaft in the middle-distal segment was removed. The proximal segments and parts removed from the middle-distal segments were embedded in methyl methacrylate. After full polymerization, the frontal sections (4-μm thick) of the proximal segments were cut using a Leica RM2255 microtome (Bannockburn, IL, USA), and stained with Masson–Goldner Trichrome, to measure static parameters. The unstained sections (8-μm thick) were used to measure the dynamic parameters. To examine cortical bone geometry, 45-μm-thick cross-sections of the distal tibia shaft were made using a Leica SP1600 saw microtome (Nussloch, Germany). These sections were grounded and mounted onto slides for measurement of the cortical bone area and porosity area. All histomorphometric evaluations were based on the standardized nomenclature and formulae [23] using a semiautomatic image analysis system (Bioquant, Nashville, TN, USA). In the proximal tibial metaphysic (PTM), the measured static parameters included bone volume/tissue volume (BV/TV), trabecular number (Tb.N), trabecular thickness (Tb.Th), and trabecular separation (Tb.Sp). The bone formation dynamic parameters were as follows: percent labeled perimeter (%L.Pm), mineralization apposition rate (MAR), bone formation rate/bone surface referent (BFR/BS), bone formation rate/bone volume referent (BFR/BV), and bone formation rate/tissue volume referent (BFR/TV). Bone resorption dynamic parameters, including osteoclast number/bone surface (Oc.N/BS) and osteoclast perimeter/bone surface (Oc.Pm/BS), were measured. In the tibial diaphysis, the measured static parameters included the total tissue area (T.Ar), cortical area (Ct.Ar), marrow area (Ma.Ar), percent cortical area (%Ct.Ar), percent marrow area (%Ma.Ar) and cortical width (Ct.Wi). The periosteal dynamic parameters were as follows: percent periosteal-labeled perimeter (%P-L.Pm), periosteal-MAR (P-MAR), and periosteal-BFR/BS referent (P-BFR/BS). The endocortical dynamic parameters were as follows: percent endocortical-labeled perimeter (%E-L.Pm), endocortical-MAR (E-MAR), and endocortical-BFR/BS referent (E-BFR/BS).
Statistics
Statistics were performed using the SPSS 16.0 software. Data are expressed as mean ± standard deviation (SD). For parametric variables, one-way analysis of variance (ANOVA) was used to compare the differences among groups, followed by Tukey’s HSD post hoc test. For nonparametric variables, Kruskal–Wallis test was used to compare the differences among groups, followed by Mann–Whitney U test for multiple comparisons. ANOVA for repeated measurement was used to compare the differences in body weight, followed by Tukey’s HSD post hoc test. A P value < 0.05 was considered statistically significant.
Results
Body weight and serum glucose and lipid
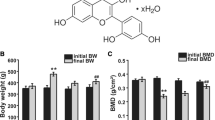
Body weight was examined during the 9-week experimental period among the four groups (Fig. 1). The body weight gradually increased during the experimental period in the control group. Furthermore, the body weight decreased in the first 6 weeks and gradually increased in the following 3 weeks in the MP group. Similar to the MP group, the body weight in the MP + Aln and MP + Met groups decreased in the first 6 weeks and slowly increased. The body weight in the MP + Aln and MP + Met groups was not significantly different from that in the MP group (P > 0.05). None of the rats died, and no serious adverse event occurred.
Figure 2 shows the serum concentration of glucose, TG, CHO, HDL-C, and LDL-C in the control, MP, MP + Aln, and MP + Met groups. At the end of the 9th week, serum glucose was significantly higher in the MP group than in the control group (5.06 ± 0.97 vs. 3.75 ± 0.69 mmol/L, P < 0.05), but no statistical difference was found when comparing the MP group with the MP + Aln group or MP + Met group. At the end of the 9th week, serum TG was significantly higher in the MP group than in the control group (1.09 ± 0.16 vs. 0.66 ± 0.22 mmol/L, P < 0.05), but no difference in serum TG was found between the MP group and MP + Aln group, or between the MP group and MP + Met group. Furthermore, there were no significant differences in the serum concentrations of CHO, HDL-C and LDL-C among the four groups (Fig. 2).
The serum concentration of glucose (a), triglyceride (b), total cholesterol (c), high-density lipoprotein cholesterol (d), and low-density lipoprotein cholesterol (e) in the control, MP, MP + Aln and MP + Met groups. Data are expressed as mean ± standard deviation (SD), and n = 7. a At the end of the 9th week, #P < 0.05 in the MP group vs. the control group; ##P < 0.01, in the Aln and Met groups vs. the control group. b At the end of the 9th week, #P < 0.05 in the MP and Aln group vs. the control group
Serum concentrations of TRAP-5b and osteocalcin
Figure 3 shows the serum concentrations of TRAP-5b and osteocalcin in the four groups at killing. Serum osteocalcin concentration was significantly lower in the MP group than in the control group (0.77 ± 0.24 vs. 1.48 ± 0.42 ng/mL, P < 0.05). However, there was no significant difference in serum osteocalcin concentration between the MP and MP + Aln groups (0.72 ± 0.31 vs. 0.77 ± 0.24 ng/mL, P > 0.05). Furthermore, serum osteocalcin concentration was significantly higher in the MP + Met group, when compared with the MP group (1.26 ± 0.54 vs. 0.77 ± 0.24 ng/mL, P < 0.05).
Serum TRAP-5b concentration was significantly higher in the MP group than in the control group (7.74 ± 0.61 vs. 6.94 ± 0.23 U/L, P < 0.05). Furthermore, serum TRAP-5b concentrations were significantly lower in the MP + Aln and MP + Met groups, when compared to the MP group (6.75 ± 0.73 vs. 7.74 ± 0.6 1 U/L, P < 0.05; 6.73 ± 0.50 vs. 7.74 ± 0.61 U/L, P < 0.05).
Areal BMD
Figure 4 presents the BMD values of the total left femur and L5 vertebral body in the four groups. The BMD values of the femur were significantly lower in the MP group than in the control group (0.280 ± 0.012 vs. 0.311 ± 0.016 g/cm2, P < 0.01). Compared with the MP group, the BMD values of the femur significantly increased in the MP + Aln and MP + Met groups (0.305 ± 0.011 vs. 0.280 ± 0.012 g/cm2, P < 0.05; 0.304 ± 0.019 vs. 0.280 ± 0.012 g/cm2, P < 0.05).
Areal BMD values of the total left femur and L5 vertebral body in the control, MP, MP + Aln and MP + Met groups. BMD, bone mineral density. The region of interest is the total femur for femur densitometry and the total vertebral body for L5 densitometry. Data were expressed as mean ± standard deviation (SD); n = 7; #P < 0.05 vs. the control group; ##P < 0.01 vs. the control group; *P < 0.05 vs. the MP group
The BMD value of the L5 vertebral body was significantly lower in the MP group than in the control group (0.358 ± 0.022 vs. 0.407 ± 0.033 g/cm2, P < 0.05). Compared with the MP group, the BMD values of the L5 vertebral body were significantly higher in the MP + Aln and MP + Met groups (0.399 ± 0.029 vs. 0.358 ± 0.022 g/cm2, P < 0.05; 0.397 ± 0.022 vs. 0.358 ± 0.022 g/cm2, P < 0.05).
Bone histomorphometric analysis
Static and dynamic bone histomorphometric parameters were measured in the PTM at the end of the 9th week (Table 1 and Fig. 5). MP administration significantly decreased the values of BV/TV, Tb.Th and Tb.N, and significantly increased the value of Tb.Sp. For the dynamic parameters, MP treatment significantly reduced bone formation parameter values, including %L.Pm, MAR, BFR/BV, BFR/TV, and BFR/BS, and significantly increased bone resorption parameter values, including Oc.N/BS and Oc.Pm/BS (Table 1). Compared with the MP group, the value of BV/TV in the MP + Aln group significantly increased by 26.57% (39.21 ± 2.46 vs. 30.98 ± 5.83, P < 0.05). Furthermore, the values of Tb.Th and Tb.N exhibited an increasing tendency, while the value of Tb.Sp exhibited a decreasing tendency in the MP + Aln group, when compared with the MP group, but no statistical significance was found. In addition, bone reabsorption parameter values (Oc.N/BS and Oc.Pm/BS) were significantly lower in the MP + Aln group than in the MP group. There was no significant difference in bone formation parameter values (%L.Pm, MAR, BFR/BV, BFR/TV and BFR/BS) between the MP and MP + Aln groups. Compared with the MP group, the value of BV/TV in the MP + Met group significantly increased by 25.79% (38.97 ± 5.56 vs. 30.98 ± 5.83, P < 0.05). Furthermore, the values of Tb.Th and Tb.N exhibited an increasing tendency, while the value of Tb.Sp exhibited a decreasing tendency in the MP + Met group, when compared with the MP group, but no statistical significance was found. In addition, bone formation parameter values (%L.Pm, MAR, BFR/BV, BFR/TV, and BFR/BS) were significantly higher in the MP + Met group, when compared with the MP group. Furthermore, bone reabsorption parameter values (Oc.N/BS and Oc.Pm/BS) were significantly lower in the MP + Met group, when compared with the MP group. In addition, it was found that bone formation parameters (%L.Pm, MAR, BFR/BV, BFR/TV, and BFR/BS) in the MP + Met group were significantly higher, when compared to the MP + Aln group (Table 1).
The bone structure and mineralized bone formation in the proximal tibial metaphysis (PTM) in the control, MP, MP + Aln and MP + Met groups. The upper panel shows the representative micrographs of PTM with Goldner’s Trichrome stain in the control, MP, MP + Aln and MP + Met groups. The green stripes represent the bone trabeculae (indicated by blank arrows). Compared with the control group, the trabeculae in the PTM become sparse in the MP group. Compared with the MP group, the trabeculae in the PTM become dense in the MP + Aln group and MP + Met group. The lower panel shows the representative fluorescence images of undecalcified sections. The fluorescence shows the mineralized bone formation in the PTM trabeculae (the representative fluorescence is indicated by white arrows), which was labeled by the subcutaneous injection of calcein. Compared with the control group, the dim fluorescence indicates the depressed mineralized bone formation in the PTM trabeculae in the MP group and MP + Aln group. Compared with the MP group, the bright fluorescence indicates the active mineralized bone formation in the PTM trabeculae in the MP + Met group. Original magnification × 20
Static and dynamic bone histomorphometric parameters were also measured in the tibial diaphysis at the end of the 9th week (Table 2 and Fig. 6). For the static parameters, there were no statistic differences among the four groups. Compared with the control group, periosteal bone formation parameter values (%P-L.Pm, P-MAR, and P-BFR/BS) and endocortical bone formation parameter values (%E-L.Pm and E-BFR/BS) were significantly lower in the MP group. Furthermore, there was no significant difference in dynamic parameters between the MP + Aln and MP groups. Compared with the MP group, periosteal bone formation parameter values (%P-L.Pm, P-MAR, and P-BFR/BS) were significantly higher in the MP + Met group (P < 0.01). Furthermore, the value of the endocortical bone formation parameter %E-L.Pm was significantly higher in the MP + Met group than in the MP group (P < 0.05). In addition, periosteal dynamic parameters %P-L.Pm and P-BFR/BS, and the endocortical dynamic parameter %E-L.Pm were significantly higher in the MP + Met group than in the MP + Aln group (Table 2).
Mineralized bone formation in the tibial shaft (TS) in the control, MP, MP + Aln, and MP + Met groups. Representative fluorescence micrographs of undecalcified sections from the TS in the control (a), MP (b), MP + Aln (c) and MP + Met (d) groups are shown. The fluorescence (indicated by white arrows) shows the mineralized bone formation in the cortical bone of the tibial shaft, which was labeled by the subcutaneous injection of calcein. Compared with the control group, the dim fluorescence indicates the depressed mineralized bone formation in the TS endocortical and pericortical bone in the MP group and MP + Aln group. Compared with the MP group, the bright fluorescence indicates the active mineralized bone formation in the TS pericortical bone in the MP + Met group. Original magnification × 20
Discussion
To our knowledge, the present study was the first to investigate the preventative effects of metformin on GC-induced osteoporosis in a rat model, and compared these with alendronate. It was found that metformin prevented GC-induced bone loss by suppressing bone resorption and stimulating bone formation in the trabecular bone. In contrast, alendronate reduced GC-induced bone loss merely by suppressing bone resorption. The present study suggests that metformin may be used to prevent GC-induced osteoporosis.
GCs mainly induce osteoporosis by suppressing osteoblast activity and inhibiting osteoclast apoptosis, thereby resulting in a decrease in bone formation and an increase in bone resorption [24]. In the present study, a rat model of GC-induced osteoporosis was established by subcutaneously injecting MP. The success of the model was demonstrated by a reduction in trabecular bone volume and BMDs in the femur and L5 vertebra. In addition, as previously reported [20, 25], the biomarkers of bone metabolism and bone histomorphometric dynamic parameters revealed that MP inhibited bone formation and promoted bone resorption. This further suggests that the animal model mimics the clinical condition of GC-induced osteoporosis.
Bisphosphonates are the most commonly used agents for preventing and treating GC-induced osteoporosis [26,27,28]. Bisphosphonates reduces osteoporosis mainly by inhibiting osteoclasts, thereby leading to the reduction in bone resorption and turnover, and an increase in bone mass [29]. Similarly, it was found that alendronate produced bone protective effects by increasing BMD in the femur and L5, and increasing trabecular bone volume. In addition, the present data also suggest that alendronate inhibited osteoclasts, as evidenced by the decrease in serum concentration of bone resorption biomarker TRAP-5b and dynamic bone resorption parameters, such as Oc.N/BS and Oc.Pm/BS. However, long-term bisphosphonate usage during GC administration can lead to very low bone remodeling and bone formation activity [30]. This might lead to low-quality bones, as manifested by fragile bones, bones with critically deteriorated micro-architecture, or low bone strength, and bones with more susceptibility to fractures, such as atypical fractures in the femur [3]. Some case reports support the idea that GC users developed atypical fractures after the use of bisphosphonates [2, 4, 5]. Large long-term clinical trials should be performed to investigate the effect of bisphosphonates on bone metabolism in GC-induced osteoporosis.
Alendronate is one of the most widely used bisphosphonates [31, 32], and is commonly used as a positive control drug in studies of anti-osteoporosis drugs. Clinically, alendronate is commonly given orally [31, 32]. Therefore, in the present study, the oral administration of alendronate was used, which mimics the clinical condition, although this administration route may reduce the effect of alendronate on the bone.
Metformin is an insulin-sensitizing biguanide that has been widely used for the treatment of type-2 diabetes, with some advantages, including limited side effects and additional beneficial effects, such as weight control [6] and reduced macrovascular complications [33]. In a Danish population-based study, Vestergaard et al. reported that metformin administration significantly decreased the risk of fractures in patients with type-1 and type-2 diabetes [11]. Furthermore, several studies have demonstrated that metformin produces a protective effect on the bone [8,9,10]. Metformin promotes the differentiation of bone marrow stromal cells into osteoblasts by increasing osteoblast-specific transcription factor Runx2, decreasing PPAR-γ expression [34], and activating adenosine 5′-monophosphate-activated protein kinase (AMPK) [15, 35]. In addition, metformin increases the bone-forming activity of osteoblasts through the transactivation of Runx2 via the AMPK/USF-1/SHP regulatory cascade [36], and by inhibiting the deleterious effects of advanced glycation end-products on osteoblasts [37]. In addition, metformin inhibits the bone-resorbing activity of osteoclasts in vitro by increasing osteoprotegerin (OPG) and reducing the receptor activator for nuclear factor-κ b ligand (RANKL) mRNA and protein expression [12]. The beneficial effects of metformin on bone metabolism have been further supported by animal studies. For example, metformin has been reported to prevent the deleterious effects of rosiglitazone on bone metabolism in rodents [35, 38]. It has also been reported that metformin prevents ovariectomy-induced bone loss in ovariectomized rats [12, 13]. In the present study, it was revealed that metformin reduced GC-induced bone loss in the femur and L5 vertebrae.
In the present study, it was found that metformin prevented GC-induced bone loss in the trabecular bone, but had little effects on the cortical bone (Tables 1 and 2). Furthermore, the histomorphometric results revealed that metformin prevented GC-induced bone loss by promoting bone formation and suppressing bone resorption. Moreover, metformin increased the serum concentration of TRAP-5b and osteocalcin, further suggesting that metformin produces a promoting effect on osteoblasts and inhibiting effect on osteoclasts. The beneficial effects of metformin on trabecular bone volume in the GC-induced osteoporosis rat model were similar to that in the ovariectomized rat model [12, 13]. However, it was found that metformin did not produce significant effects on GC-induced bone loss in the cortical bone. Since the remodeling time in the cortical bone is longer than in the trabecular bone [39], the minimal effect of metformin on the cortical bone is likely due to the short action duration of metformin (9 weeks) in the present study. Although it was found that metformin did not produce any significant effect on cortical bone volume and endosteal bone formation activity, metformin enhanced the bone formation activity in the periosteum, as evidenced by the increased periosteal bone formation parameters. Further studies should be performed to investigate the long-term effect of metformin on cortical bone volume in the rat model of GC-induced osteoporosis.
In the present study, serum glucose level was used at the beginning of the study, at the 5th week, and at killing to evaluate the effects of MP and metformin on rat glucose metabolism. However, the serum glucose level at these three time points may not fully reflect the glucose metabolism profile. The daily determination of urinary glucose concentration may be more adequate to evaluate the glucose metabolism in rats with GC-induced osteoporosis.
In conclusion, the present study demonstrated that metformin prevented GC-induced bone loss by suppressing bone resorption and stimulating bone formation in the trabecular bone. The action of metformin was different from alendronate, which acted only by suppressing bone resorption. Metformin may be used as a potential drug for the treatment of GC-induced osteoporosis.
References
Iglesias JE, Salum FG, Figueiredo MA, Cherubini K (2015) Important aspects concerning alendronate-related osteonecrosis of the jaws: a literature review. Gerodontology 32:169–178
Saita Y, Ishijima M, Mogami A, Kubota M, Baba T et al (2015) The incidence of and risk factors for developing atypical femoral fractures in Japan. J Bone Miner Metab 33:311–318
Shane E, Burr D, Abrahamsen B, Adler RA, Brown TD et al (2014) Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 29:1–23
Armamento-Villareal R, Napoli N, Diemer K, Watkins M, Civitelli R, Teitelbaum S, Novack D (2009) Bone turnover in bone biopsies of patients with low-energy cortical fractures receiving bisphosphonates: a case series. Calcif Tissue Int 85:37–44
Odvina CV, Zerwekh JE, Rao DS, Maalouf N, Gottschalk FA, Pak CY (2005) Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab 90:1294–1301
Tamura Y, Watada H, Sato F, Kumashiro N, Sakurai Y, Hirose T, Tanaka Y, Kawamori R (2008) Effects of metformin on peripheral insulin sensitivity and intracellular lipid contents in muscle and liver of overweight Japanese subjects. Diabetes Obes Metab 10:733–738
Giugliano D, De Rosa N, Di Maro G, Marfella R, Acampora R, Buoninconti R, D’Onofrio F (1993) Metformin improves glucose, lipid metabolism, and reduces blood pressure in hypertensive, obese women. Diabetes Care 16:1387–1390
Pradeep AR, Nagpal K, Karvekar S, Patnaik K, Naik SB, Guruprasad CN (2015) Platelet-rich fibrin with 1% metformin for the treatment of intrabony defects in chronic periodontitis: a randomized controlled clinical trial. J Periodontol 86:729–737
Borges JL, Bilezikian JP, Jones-Leone AR, Acusta AP, Ambery PD, Nino AJ, Grosse M, Fitzpatrick LA, Cobitz AR (2011) A randomized, parallel group, double-blind, multicentre study comparing the efficacy and safety of Avandamet (rosiglitazone/metformin) and metformin on long-term glycaemic control and bone mineral density after 80 weeks of treatment in drug-naive type 2 diabetes mellitus patients. Diabetes Obes Metab 13:1036–1046
Melton LJ 3rd, Leibson CL, Achenbach SJ, Therneau TM, Khosla S (2008) Fracture risk in type 2 diabetes: update of a population-based study. J Bone Miner Res 23:1334–1342
Vestergaard P, Rejnmark L, Mosekilde L (2005) Relative fracture risk in patients with diabetes mellitus, and the impact of insulin and oral antidiabetic medication on relative fracture risk. Diabetologia 48:1292–1299
Mai QG, Zhang ZM, Xu S, Lu M, Zhou RP, Zhao L, Jia CH, Wen ZH, Jin DD, Bai XC (2011) Metformin stimulates osteoprotegerin and reduces RANKL expression in osteoblasts and ovariectomized rats. J Cell Biochem 112:2902–2909
Gao Y, Li Y, Xue J, Jia Y, Hu J (2010) Effect of the anti-diabetic drug metformin on bone mass in ovariectomized rats. Eur J Pharmacol 635:231–236
Tolosa MJ, Chuguransky SR, Sedlinsky C, Schurman L, McCarthy AD, Molinuevo MS, Cortizo AM (2013) Insulin-deficient diabetes-induced bone microarchitecture alterations are associated with a decrease in the osteogenic potential of bone marrow progenitor cells: preventive effects of metformin. Diabetes Res Clin Pract 101:177–186
Molinuevo MS, Schurman L, McCarthy AD, Cortizo AM, Tolosa MJ, Gangoiti MV, Arnol V, Sedlinsky C (2010) Effect of metformin on bone marrow progenitor cell differentiation: in vivo and in vitro studies. J Bone Miner Res 25:211–221
Kanazawa I, Yamaguchi T, Yano S, Yamauchi M, Sugimoto T (2008) Metformin enhances the differentiation and mineralization of osteoblastic MC3T3-E1 cells via AMP kinase activation as well as eNOS and BMP-2 expression. Biochem Biophys Res Commun 375:414–419
Cortizo AM, Sedlinsky C, McCarthy AD, Blanco A, Schurman L (2006) Osteogenic actions of the anti-diabetic drug metformin on osteoblasts in culture. Eur J Pharmacol 536:38–46
Nordklint AK, Almdal TP, Vestergaard P, Lundby-Christensen L, Boesgaard TW et al (2018) The effect of metformin versus placebo in combination with insulin analogues on bone mineral density and trabecular bone score in patients with type 2 diabetes mellitus: a randomized placebo-controlled trial. Osteoporos Int 29:2517–2526
Jeyabalan J, Viollet B, Smitham P, Ellis SA, Zaman G, Bardin C, Goodship A, Roux JP, Pierre M, Chenu C (2013) The anti-diabetic drug metformin does not affect bone mass in vivo or fracture healing. Osteoporos Int 24:2659–2670
Ortoft G, Andreassen TT, Oxlund H (2005) Growth hormone can reverse glucocorticoid-induced low bone turnover on cortical but not on cancellous bone surfaces in adult Wistar rats. Bone 36:123–133
Mosekilde L, Thomsen JS, Mackey MS, Phipps RJ (2000) Treatment with risedronate or alendronate prevents hind-limb immobilization-induced loss of bone density and strength in adult female rats. Bone 27:639–645
Quaile MP, Melich DH, Jordan HL, Nold JB, Chism JP, Polli JW, Smith GA, Rhodes MC (2010) Toxicity and toxicokinetics of metformin in rats. Toxicol Appl Pharmacol 243:340–347
Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR (1987) Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 2:595–610
Seibel MJ, Cooper MS, Zhou H (2013) Glucocorticoid-induced osteoporosis: mechanisms, management, and future perspectives. Lancet Diabetes Endocrinol 1:59–70
Hulley PA, Conradie MM, Langeveldt CR, Hough FS (2002) Glucocorticoid-induced osteoporosis in the rat is prevented by the tyrosine phosphatase inhibitor, sodium orthovanadate. Bone 31:220–229
Duru N, van der Goes MC, Jacobs JW, Andrews T, Boers M, Buttgereit F, Caeyers N, Cutolo M, Halliday S, Da Silva JA, Kirwan JR, Ray D, Rovensky J, Severijns G, Westhovens R, Bijlsma JW (2013) EULAR evidence-based and consensus-based recommendations on the management of medium to high-dose glucocorticoid therapy in rheumatic diseases. Ann Rheum Dis 72:1905–1913
Lekamwasam S, Adachi JD, Agnusdei D, Bilezikian J, Boonen S et al (2012) A framework for the development of guidelines for the management of glucocorticoid-induced osteoporosis. Osteoporos Int 23:2257–2276
Grossman JM, Gordon R, Ranganath VK, Deal C, Caplan L, Chen W, Curtis JR, Furst DE, McMahon M, Patkar NM, Volkmann E, Saag KG (2010) American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res (Hoboken) 62:1515–1526
Reszka AA, Rodan GA (2003) Bisphosphonate mechanism of action. Curr Rheumatol Rep 5:65–74
Teitelbaum SL, Seton MP, Saag KG (2011) Should bisphosphonates be used for long-term treatment of glucocorticoid-induced osteoporosis? Arthritis Rheum 63:325–328
Buckley L, Guyatt G, Fink HA, Cannon M, Grossman J et al (2017) 2017 American College of Rheumatology guideline for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res (Hoboken) 69:1095–1110
Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, Lindsay R (2014) Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int 25:2359–2381
UK Prospective Diabetes Study (UKPDS) Group (1998) Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 352:854–865
Gao Y, Xue J, Li X, Jia Y, Hu J (2008) Metformin regulates osteoblast and adipocyte differentiation of rat mesenchymal stem cells. J Pharm Pharmacol 60:1695–1700
Sedlinsky C, Molinuevo MS, Cortizo AM, Tolosa MJ, Felice JI, Sbaraglini ML, Schurman L, McCarthy AD (2011) Metformin prevents anti-osteogenic in vivo and ex vivo effects of rosiglitazone in rats. Eur J Pharmacol 668:477–485
Jang WG, Kim EJ, Bae IH, Lee KN, Kim YD, Kim DK, Kim SH, Lee CH, Franceschi RT, Choi HS, Koh JT (2011) Metformin induces osteoblast differentiation via orphan nuclear receptor SHP-mediated transactivation of Runx2. Bone 48:885–893
Schurman L, McCarthy AD, Sedlinsky C, Gangoiti MV, Arnol V, Bruzzone L, Cortizo AM (2008) Metformin reverts deleterious effects of advanced glycation end-products (AGEs) on osteoblastic cells. Exp Clin Endocrinol Diabetes 116:333–340
Wang C, Li H, Chen SG, He JW, Sheng CJ, Cheng XY, Qu S, Wang KS, Lu ML, Yu YC (2012) The skeletal effects of thiazolidinedione and metformin on insulin-resistant mice. J Bone Miner Metab 30:630–637
Hadjidakis DJ, Androulakis II (2006) Bone remodeling. Ann NY Acad Sci 1092:385–396
Acknowledgements
We thank Dr. Ping Sun for her kind help in facilitating the cooperation among the facilities. This work was supported by the Natural Science Foundation of Inner Mongolia Autonomous Region, China (No. 2014BS0807), the Program for Young Talents of Science and Technology in Universities of Inner Mongolia Autonomous Region, the Beijing Natural Science Foundation (No. 7162193), the Innovative Talents Group Foundation of Nephrology Department, Affiliated Hospital, Inner Mongolia Medical University of the Fifth Inner Mongolia ‘Prairie Meritocrat’ Project, and the Doctorial Scientific Research Starting Foundation of the Affiliated Hospital, Inner Mongolia Medical University (No. NYFYBS2015013).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All the authors have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Zhao, J., Li, Y., Zhang, H. et al. Preventative effects of metformin on glucocorticoid-induced osteoporosis in rats. J Bone Miner Metab 37, 805–814 (2019). https://doi.org/10.1007/s00774-019-00989-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-019-00989-y