Abstract
Specific, sensitive and non-invasive biomarkers are always needed in endocrine disorders. miRNAs are short, non-coding RNA molecules with well-known role in gene expression regulation. They are frequently dysregulated in metabolic and endocrine diseases. Recently it has been shown that they are secreted into biofluids by nearly all kind of cell types. As they can be taken up by other cells they may have a role in a new kind of paracrine, cell-to-cell communication. Circulating miRNAs are protected by RNA-binding proteins or microvesicles hence they can be attractive candidates as diagnostic or prognostic biomarkers. In this review, we summarize the characteristics of extracellular miRNA’s and our knowledge about their origin and potential roles in endocrine and metabolic diseases. Discussions about the technical challenges occurring during identification and measurement of extracellular miRNAs and future perspectives about their roles are also highlighted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
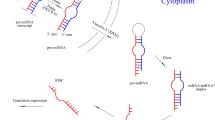
MicroRNAs (miRNAs) are small, protein non-coding RNAs that regulate gene expression post-transcriptionally, through RNA interference by targeting mRNAs at the 3′, 5′ untranslated regions or even the coding sequence [1–4]. The 60–70 nucleotide (nt) primary miRNAs are generated through transcription by RNA polymerase II, which then are cleaved to the shorter precursor miRNAs (pre-miRs) by Drosha. The pre-miRNA molecule is transported to the cytoplasm by Exportin-5 and processed by another RNase III enzyme (Dicer). The product is an approximately 21 nt miRNA:miRNA* duplex, one strand of which is incorporating into miRNA-induced silencing complex (miRISC) [5]. In the miRISC complex, through base pair alignment miRNAs cause translational repression, mRNA destabilization or mRNA cleavage. The other strand (passenger strand or miRNA*) is usually degraded [6]. It is thought that approximately 30–50 % of all protein-coding genes might be controlled by miRNAs [7, 8]. One miRNA potentially affects the expression of several proteins, and one protein is influenced by numerous miRNAs. Their role is considered to set the gene expression to the optimal level, or with other words to provide “fine tuning” and adaptive setting of gene expression [9].
Their roles have been demonstrated in the regulation of various physiological and pathophysiological cellular processes such as proliferation, differentiation, metabolism and apoptosis. Differential miRNA expression in endocrine disorders including malignancies has also been reported [10–13].
MiRNAs as biomarkers in extracellular fluids (“cell-free systems”) recently have been investigated and linked to diagnosis, prognosis and recurrence detection [14, 15]. Many reports showed correlations between miRNAs dysregulation in the peripheral blood and pathophysiological conditions. Related to endocrine diseases, dysregulated miRNAs have been described in diabetes mellitus [16], in disorders affecting reproductive tissues [17], vitamin D signaling and bone [18], thyroid [19, 20], adrenal [21] and pituitary gland [22]. miRNAs have also been implicated in developmental processes of pituitary through regulating Lef-1 transcription factor [23], in pancreas through HES-1 and neurogenin 3 [24, 25] and in the female reproductive system as well [26]. In addition, expression of mature miRNAs, genetic variations and polymorphisms of miRNAs encoding genes were associated with the prognosis and/or progression of diseases and drug responses [27–30].
A new chapter in miRNAs-related research has been starting by their identification outside from cells, in biofluids. These extracellular miRNAs have been considered as a novel type of signaling molecules and being secreted and taken up by various cells they may indeed function similarly to hormones or cytokines. In this review we present recent data about the origin and function of these extracellular miRNAs focusing on endocrine and metabolic diseases.
Extracellular (EC) miRNAs
Origin and function
Extracellular miRNAs have been detected in a wide variety of body fluids such as serum, plasma, urine, saliva, tears, peritoneal, pleural and cerebrospinal fluid, bronchoalveolar lavage, breast milk, amniotic and seminal fluid [31].
Unprotected “naked” miRNAs are sensitive to degradation mainly through RNAses present in large amount in these fluids. Protection of miRNAs against RNases is provided by association of miRNAs with Argonaute (AGO) proteins and by packing them in microvesicles [32, 33] (Fig. 1). AGO proteins have been demonstrated in microvesicles as well suggesting their essential role in miRNA’s protection [34]. Microvesicles are secreted by nearly all kinds of cell such as like neural, stem cells, epithelial cells, dendritic cells and lymphocytes [35–40].
The term microvesicle is often used generally for any type of vesicle regardless of the size or its origin. Exosomes are small (approximately 30–100 nm) membrane-limited secreted vesicles [41]. They are formed in the endosomal compartments of cell (multivesicular endosomes or MVEs). In this compartment, extracellular molecules are stored, released or degraded by fusing with lysosomes after endocytosis. Exosomes can be released by fusion of the multivesicular endosomes with the plasma membrane [41]. Cells can release other types of vesicles as well by directly budding or shedding off the plasma membrane. These vesicles are also referred as microvesicles, shed vesicles, or ectosomes but their size is more variable (typically between 50 and 1000 nm) than the exosomes. Both exosomes and other microvesicles contain various molecules including mRNA, miRNA, proteins, cytokines and different surface receptors specific for their cell origin [42].
Beside exosomes and microvesicles, several miRNAs were found in high-density lipoprotein (HDL) particles and, therefore, their extracellular uptake by the host cells is dependent on the presence of HDL receptors [34].
It is described that one part of the extracellular miRNAs is byproduct of cell death, and it is also shown that the amount of miRNAs correlates with cell death in vitro [34]. But, interestingly, not all mRNA molecules were found in exosomes, and literature data suggest that different RNA species can specifically be packaged by active sorting [35, 43, 44].
The function of microvesicles is still not yet clear; however, more and more studies proved their significant role in cell–cell communication in immunology and tumor biology. Exosomes secreted by dendritic cells carry antigens and are able to induce immune response [39]. They can mediate paracrine signals of cancer cells as well influencing tumor microenvironment by exosome secretion in promoting growth by inhibiting antitumor immune response and by facilitating angiogenesis, cell migration and metastasis [45–49]. Exosomal miR-21 and miR-29a were described to be internalized by immune cells surrounding cancer cells where they bind as ligands to the Toll-like receptor (TLR) family (TLR7 in murine and TLR8 in human) triggering a TLR-mediated prometastatic inflammatory response that ultimately may lead to tumor growth and metastasis [50]. Interestingly, tumor cells were found to exhibit self-promoting effect by secreting microvesicles in glioblastoma and renal cancer cell lines [49, 51]. On the other hand, it was also described that metastatic gastric cancer cells can eliminate tumor suppressor miRNAs by exosome secretion [52]. Taking these data together it is reasonable to hypothesize that in a malignant tumor overexpressed, oncogenic and downregulated tumor suppressor miRNAs can be exploited as potential biomarkers. As miRNAs are also tissue specific they may be unique identifiers of certain tumor types [27], and if they secreted they could be specific biomarkers to a certain diseases.
Extracellular miRNAs as biomarkers
MiRNAs are found to be very stable in extracellular fluids and miRNA levels were demonstrated to be reproducible across individuals, robust against enzymatic cleavage, thawing–freezing cycles or pH changes [53–55].
The major source of extracellular miRNA in blood is obviously the blood and endothelial cells; however, tissue-specific miRNAs, i.e., miR-122 from the liver, miR-124 from the brain or miR-208b from the heart [56–58] are also detectable in the blood.
Tissue-specific miRNAs as biomarkers have been linked to tissue injuries, as miR-499 and miR-208b specific for myocardial infarction or mir-122 for the drug-induced liver injury [57, 58]. Thus in malignant diseases, tumor-associated miRNAs were found in serum of patients with hematological diseases and in patients with solid tumors as well. For instance, the serum levels of miR-141, a miRNA expressed in prostate cancer, could distinguish prostate cancer patients from healthy individuals [55]. However, it is still not clear if extracellular miRNAs are derived from the tumor directly or derived from non-malignant cells as a response to cancer for instance from white blood cells or tumor-reactive immune cells [34].
The level and composition of extracellular miRNAs have been shown to correlate well with disease progression, i.e., miR-221 in renal cell carcinoma, let-7f and miR-30e-3p in breast cancer [15, 59] or to predict biochemical recurrence in prostate cancer patients [60] or response to chemotherapy in esophageal cancer [61].
Serum biomarkers in endocrine diseases
Microvesicles containing miRNAs can mediate autocrine, paracrine signaling and also can be transported substantially longer distances via the circulatory system, hence they can be considered as a new kind of hormone-like entities. In this part, we summarized the data about circulating miRNAs and their associations found in endocrine and metabolic diseases.
In diabetes and metabolic disorders, Guay and Regazzi excellently reviewed the role of circulating miRNAs [62]. In brief, in diabetes mellitus type 2 (T2DM), Zampetaki and colleagues found that reduced miR-15a, miR-29b, miR-126, miR-223, and elevated miR-28-3p levels preceded the manifestation of disease [63]. Another study performed by Kong et al. showed that 7 serum miRNAs (miR-9, miR-29a, miR-30d, miR34a, miR-124a, miR146a and miR-375) were significantly upregulated in patients with T2DM compared with patients having normal glucose tolerance (NGT) and five (miR-9, miR-29a, miR-34a, miR-146a, miR-375) were significantly upregulated in patients with T2DM compared with patients having pre-diabetes [64]. However, the authors could not show difference between NGT and pre-diabetes groups regarding these miRNAs [64]. Karolina et al. reported increased expression of miR-150, miR-192, miR-27a, miR-320a, and miR-375 in T2DM and metabolic syndrome compared to healthy individuals [65]. Expression of miR-27a and miR-320a displayed a strong positive correlation with fasting glucose level, thus highlighting their potential as key players in early-phase hyperglycemia, which could eventually result in the development of diabetes over time. Beside these miRNAs, miR-17 was found to be downregulated in T2DM, and the authors suggest a stronger implication of miR-17 in fully manifested diabetes, instead of early-phase dysglycemia as seen in patients having metabolic syndrome [65]. They found miR-144 to be upregulated in T2D, but metabolic syndrome [65]. Zhang et al. found that the expression of miR-126 was significantly reduced in patients with T2DM and pre-diabetic individuals with fasting glucose between 6.1 and 6.9 mmol/L compared to healthy controls [66]. They proposed that miR-126 in circulation could serve as a potential biomarker for early identification of susceptible individuals to T2DM.
In type 1 diabetes mellitus (T1DM), Nielsen and colleagues identified twelve upregulated miRNAs (miR-152, miR-30a-5p, miR-181a, miR-24, miR-148a, miR-210, miR-27a, miR-29a, miR-26a, miR-27b, miR-25, miR-200a) in sera of children with newly diagnosed disease [67]. They also found miR-25 as being negatively associated with residual beta cell function, and positively associated with glycaemic control (HbA1c) 3 months after onset. The authors suggest that miR-25 might be a “tissue-specific” miRNA for glycaemic control.
However, not in serum but in blood lymphocytes, miR-326 was described to be overexpressed in T1DM subjects with ongoing islet autoimmunity compared to controls by Sebastiani et al. [68]. Similarly, not in cell-free serum, but in peripheral blood mononuclear cells miR-21a and miR-93 were shown to be downregulated in T1DM patients [69]. Based on in vitro and in vivo experiments, circulating miR-375 was identified as a biomarker of β cell death and diabetes in mice by Erener et al. [70]. Increased miR-375 level was detected after high-dose streptozotocin (STZ) administration, prior to the onset of hyperglycemia both in vitro and in vivo mice experiments.
Related to patients having metabolic syndrome, several circulating miRNAs were also identified in serum. miR-197, miR-23a, and miR-509-5p appeared as potential contributors of dyslipidemia in metabolic syndrome and miR-130a and miR-195 as contributors of hypertension (expression of these miRNAs correlated with blood pressure; p = 0.019 and 0.045, respectively) [65]. Decreased miR-221 and miR-28-3p and increased concentrations in plasma of miR-486-5p, miR-486-3p, miR-142-3p, miR-130b, and miR-423-5p were reported in childhood obesity [71]. Another study also found elevated miR-130b in a mouse model of obesity as well as in obese individuals. The authors showed that miR-130b was secreted during adipogenesis and was able to target muscle cells and reduce the expression of its direct target gene, PGC-1α (also known as PPARGC1A), which plays a key role in lipid oxidation in muscle. It is claimed that circulating miR-130b reflects the degree of obesity and could function as a metabolic mediator for adipose–muscle crosstalk [72].
Altered circulating miRNA expression was linked to non-alcoholic fatty liver disease (NAFLD). It was shown that serum levels of miR-122, miR-34a and miR-16 were significantly higher in patients with NAFLD compared to controls and positively correlated with disease severity [73]. The expression of these two miRNAs also correlated with liver enzymes levels, serum lipids, fibrosis stage and inflammation activity in NAFLD patients [73]. Vickers et al. reported that human HDL-miRNA profile of normal subjects is significantly different from that of individuals with familial hypercholesterolemia [74]. HDL containing miRNAs (miR-223 and miR-375) from atherosclerotic subjects induced differential gene expression with decrease of RhoB and Ephrin A1 in cultured hepatocytes [74]. Based on these results miRNAs embedded in HDL molecules can be delivered to the liver and mediate gene expression regulation.
Endocrine tumors
Compared to other tumor types in endocrine tumors, there is still a lot of opportunity to investigate extracellular miRNAs. There are several publications showing miRNA expression data in neuroendocrine tumors (NET) [75, 76], but there is a lack of information regarding, e.g., pituitary adenomas and pheochromocytomas. Serum miR-1290 was found to be overexpressed but statistically not significantly in pancreatic NET compared to normal controls (Table 1); however, it was able to discriminate pNETs from pancreatic cancer with 0.80 (95 % CI 0.67–0.93) AUC characteristic [77].
Regarding thyroid neoplasms, Yu et al. published that the expression of serum let-7e, miR-151-5p and miR-222 was increased in papillary thyroid cancer (PTC) compared to benign lesions and healthy controls [78]. The ROC analysis of the combination of these three miRNAs in discriminating PTC from benign nodules and healthy controls showed 0.917 and 0.897 AUC values, respectively. Moreover, the serum let-7e, miR-151-5p and miR-222 expression correlated well with nodal status, tumor size, multifocal status and TNM stage. Increased expression of miR-151-5p and miR-222 was detected in the tumor tissues as well, and in serum the level of the two miRs decreased after tumor excision [78].
In another study, Lee et al. also published that miR-222, beside miR-146b, was overexpressed in PTC compared to healthy individuals [79]. Also, these two miRs’ expressions in the plasma were higher in recurrent PTCs compared to non-recurrent disease. It was also described that miR-222 and miR-146b levels dropped in plasma following total thyroidectomy [79]. Another publication of this group showed that miR-146b and miR-155 were expressed at a higher level in plasma of patients having PTC compared to benign thyroid nodules [80]. They also found that miR-146b, miR-155 and miR-222 were slightly higher in patients with lymph node metastasis than in patients without lymph node involvement [80]. The third study identified miR-579, miR-95, miR-29b as being downregulated and miR-190 upregulated in serum of PTC patients compared to patients with nodular goiter (NG) and healthy controls [81]. By combining expression data of miR-95 and miR-190, the authors developed a multivariate risk model which was able to differentiate PTC samples from NGs with 0.99 AUC [81]. In addition, miR-579 and miR-95 were significantly downregulated in PTC compared with NG, while miR-190 was upregulated in PTC both at tissue level and in serum samples.
Regarding adrenal neoplasms, several studies showed that adrenocortical cancer-specific miRNAs miR-34a and miR-483-5p were able to differentiate adrenocortical carcinoma (ACC) from adrenocortical adenomas (ACA) [82]. Moreover, they observed miR-34a and miR-483-5p secretion by ACC cells to cell culture media. Szabó and colleagues reported that the difference between expression of miR-210 and miR-181 [(dCT(miR-210)-dCT(miR-181b)] and the ratio of dCT(miR-100)/dCt(miR181b) could be used with high diagnostic accuracy in differentiating ACC from ACA [83]. In a third publication, Chabre et al. showed that miR-195 and miR-335 levels were decreased in both tumor tissues and serum samples compared to ACA patients or healthy controls [84]. They also found that miR-483-5p, which was markedly upregulated in ACC patients compared to ACA, was detectable only in the serum obtained from patients with aggressive ACC [84]. They also showed that high miR-483-5p and low miR-195 circulating levels were associated with shorter recurrence-free and overall survival, hence the authors claimed that these miRNAs could be used as a prognostic biomarker for clinical outcome of ACC patients. In all three studies, miR-483-5p was found to be upregulated in ACC patients; therefore, it is probably a very useful, non-invasive biomarker candidate for this very aggressive cancer.
In pituitary tumors, several targets have been validated at tissue level. For instance PTEN, BMI1, E2F1 in growth hormone-producing adenomas, SMAD3 in non-functioning adenomas and PRKCD in ACTH-secreting tumors have been proved to be targeted by several miRNAs [85–88]. Also, there are genes like HMGA1-2, WEE1, AIP, CCNA2 that are controlled by miRNAs in more than one pituitary adenoma type as well [89–92]. However, several miRNAs were identified correlating with clinical features such as tumor size [87, 93], disease recurrence [94] or treatment [95–97], there is no study yet profiling circulating miRNAs (or exosomes) as biomarkers in pituitary adenoma patients based on literature search [98]. However, Wang et al. investigated the plasma levels of three miRNAs (miR-21, miR-128 and miR-342-3p) in 10 pituitary adenoma specimens as control for identifying biomarkers for glioma, but those were proved to be specific only in glioma patients [99].
In patients with pheochromocytoma miR-483-5p, miR-183, and miR-101 were proved to be diagnostic markers for distinguishing malignant from benign pheochromocytomas at tissue level [100]. Though these miRNAs were detectable in patients’ serum as well, their expression level was too low to obtain significant difference [100]. This study draws our attention to summarize those technical considerations which have to be considered during studying extracellular miRNAs.
Technical considerations
In general, an ideal marker should be easily measured by non-invasive sample collection. Therefore, blood and urine are the most commonly used matrixes used in laboratory testing. The marker should be specific to the pathologic condition bearing clinically acceptable (high) specificity and sensitivity and relatively inexpensive to quantify. Biomarkers that can be used to stratify disease and assess therapy response or disease progression are also valuable.
Compared to messenger RNAs, miRNAs are more stable and, therefore, they are attractive potential biomarker candidates. However, there are some inconsistencies among the results of different studies which may be related to different sampling, different detection techniques and/or selection of internal controls.
There is no clear recommendation whether serum or plasma is more suitable for investigating extracellular miRNAs. However, a higher miRNA concentration was observed in serum compared to the corresponding plasma samples. The difference between serum and plasma miRNA concentrations showed some associations with miRNA originated from platelets suggesting that the coagulation process may affect the spectrum of extracellular miRNA in blood [101]. Because a large amount of miRNAs are present in blood cells, it is important to avoid cell contamination and disruption, such as hemolysis. In this latter case, miRNAs characteristic to red blood cells can appear in the sample and may bias the miRNA expression profile [102].
To date, there are no standardized protocols for miRNA extraction from biofluids or quality/quantity assessment of the purified RNA either. Different, even commercially available extraction kits are available for cell-free total RNA extraction from biofluids. According to microvesicles, the most common used methods are differential centrifugation, combining ultracentrifugation and nanomembrane ultrafiltration. Extraction from cell-free body fluids usually yields very low RNA concentrations especially in case of urine or cerebrospinal fluid. Nanospectrophotometry is greatly affected by RNA concentration, and these low-concentration samples often do not pass through the conventional RNA quality criteria (A260/A230 and A260/A280). Even so, they typically perform well in real-time quantitative PCR (RT-qPCR) experiments.
Recent years resulted in several high-throughput techniques such as miRNA microarrays, PCR arrays or next generation sequencing which may be useful for an initial screening for identification of the miRNome expression profile and for selection of the specific biomarker candidates. However, regarding cell-free biofluids, they require a large volume of starting material. The analysis of miRNA expression by sequencing is challenging because the sequences are short, and there is a high homology among members of miRNA families (sometimes only one base difference), hence it is hard to achieve high sensitivity and specificity. The most widely accepted “gold standard” method is a two-step approach using looped miRNA-specific reverse transcription primers and TaqMan probes.
Another key factor in miRNA expression measurements is the reference standard. A proper endogenous control should be the same biotype molecule, stable, abundant and expressed irrespective of biological variance and medical conditions. A valid normalization could help to minimize the technical differences and remove systemic bias among different studies. Different small nuclear RNA (RNU) molecules or miR-16 are often used as endogenous control; however, lacking a valid housekeeping normalization by input volume is also applied using different spike-in controls [77, 103–105]. It is worth noticing that the same starting volume does not guarantee equal amount of RNA/miRNA content. For instance, the purified RNA yield can differ among samples from different individuals which make the normalization even more complicated.
Conclusion and future perspectives
Specific, sensitive, non-invasive and cheaply detectable biomarkers are always needed in diagnosis of diseases. Circulating miRNAs can be actively secreted and delivered to distant cell types. In this context, they can be considered as a new type of paracrine regulators, and a miRNA-mediated cell–cell communication can be hypothesized. Extracellular miRNAs are detectable in malignant diseases hence they can be diagnostic or prognostic biomarkers. To date, only limited data are available about the true diagnostic power of extracellular miRNAs. Harmonization of extraction and quantification methods is essential in identification of clinically significant miRNAs. In the future, there is still a need to improve simple standard assays for quantification, establish the specificity and sensitivity by comparing miRNA expressions among different types of cancer and healthy or pathological conditions.
References
Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T (2001) Identification of novel genes coding for small expressed RNAs. Science 294:853–858
Place RF, Li LC, Pookot D, Noonan EJ, Dahiya R (2008) MicroRNA-373 induces expression of genes with complementary promoter sequences. Proc Natl Acad Sci USA 105:1608–1613
Orom UA, Nielsen FC, Lund AH (2008) MicroRNA-10a binds the 5′UTR of ribosomal protein mRNAs and enhances their translation. Mol Cell 30:460–471
Zhang J, Guo H, Qian G, Ge S, Ji H, Hu X, Chen W (2010) MiR-145, a new regulator of the DNA fragmentation factor-45 (DFF45)-mediated apoptotic network. Mol Cancer 9:211
Chendrimada TP, Gregory RI, Kumaraswamy E, Norman J, Cooch N, Nishikura K, Shiekhattar R (2005) TRBP recruits the Dicer complex to Ago2 for microRNA processing and gene silencing. Nature 436:740–744
Gregory RI, Chendrimada TP, Cooch N, Shiekhattar R (2005) Human RISC couples microRNA biogenesis and posttranscriptional gene silencing. Cell 123:631–640
Chen K, Rajewsky N (2006) Natural selection on human microRNA binding sites inferred from SNP data. Nat Genet 38:1452–1456
Lewis BP, Burge CB, Bartel DP (2005) Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120:15–20
Mattick JS, Makunin IV (2005) Small regulatory RNAs in mammals. Hum Mol Genet. 14 Spec No 1:R121–R132
Szabó PM, Butz H, Igaz P, Rácz K, Hunyady L, Patócs A (2013) Minireview: miRomics in endocrinology: a novel approach for modeling endocrine diseases. Mol Endocrinol 27:573–585
Farazi TA, Hoell JI, Morozov P, Tuschl T (2013) MicroRNAs in human cancer. Adv Exp Med Biol 774:1–20
Rottiers V, Naar AM (2012) MicroRNAs in metabolism and metabolic disorders. Nat Rev Mol Cell Biol 13:239–250
Sivapragasam M, Rotondo F, Lloyd RV, Scheithauer BW, Cusimano M, Syro LV, Kovacs K (2011) MicroRNAs in the human pituitary. Endocr Pathol 22:134–143
Lawrie CH, Gal S, Dunlop HM, Pushkaran B, Liggins AP, Pulford K, Banham AH, Pezzella F, Boultwood J, Wainscoat JS, Hatton CS, Harris AL (2008) Detection of elevated levels of tumour-associated microRNAs in serum of patients with diffuse large B-cell lymphoma. Br J Haematol 141:672–675
Silva J, García V, Zaballos Á, Provencio M, Lombardía L, Almonacid L, García JM, Domínguez G, Peña C, Diaz R, Herrera M, Varela A, Bonilla F (2011) Vesicle-related microRNAs in plasma of nonsmall cell lung cancer patients and correlation with survival. Eur Respir J 37:617–623
Li X (2014) MiR-375, a microRNA related to diabetes. Gene 533:1–4
Hossain MM, Sohel MM, Schellander K, Tesfaye D (2012) Characterization and importance of microRNAs in mammalian gonadal functions. Cell Tissue Res 349:679–690
Lisse TS, Adams JS, Hewison M (2013) Vitamin D and microRNAs in bone. Crit Rev Eukaryot Gene Expr 23:195–214
Pallante P, Battista S, Pierantoni GM, Fusco A (2014) Deregulation of microRNA expression in thyroid neoplasias. Nat Rev Endocrinol 10:88–101
Leone V, D’Angelo D, Ferraro A, Pallante P, Rubio I, Santoro M, Croce CM, Fusco A (2011) A TSH-CREB1-microRNA loop is required for thyroid cell growth. Mol Endocrinol 25:1819–1830
Singh P, Soon PS, Feige JJ, Chabre O, Zhao JT, Cherradi N, Lalli E, Sidhu SB (2012) Dysregulation of microRNAs in adrenocortical tumors. Mol Cell Endocrinol 351:118–128
Gadelha MR, Kasuki L, Dénes J, Trivellin G, Korbonits M (2013) MicroRNAs: suggested role in pituitary adenoma pathogenesis. J Endocrinol Invest 36:889–895
Zhang Z, Florez S, Gutierrez-Hartmann A, Martin JF, Amendt BA (2010) MicroRNAs regulate pituitary development, and microRNA 26b specifically targets lymphoid enhancer factor 1 (Lef-1), which modulates pituitary transcription factor 1 (Pit-1) expression. J Biol Chem 285:34718–34728
Lynn FC, Skewes-Cox P, Kosaka Y, McManus MT, Harfe BD, German MS (2007) MicroRNA expression is required for pancreatic islet cell genesis in the mouse. Diabetes 56:2938–2945
Correa-Medina M, Bravo-Egana V, Rosero S, Ricordi C, Edlund H, Diez J, Pastori RL (2009) MicroRNA miR-7 is preferentially expressed in endocrine cells of the developing and adult human pancreas. Gene Expr Patterns 9:193–199
Hong X, Luense LJ, McGinnis LK, Nothnick WB, Christenson LK (2008) Dicer1 is essential for female fertility and normal development of the female reproductive system. Endocrinology 149:6207–6212
Mishra PJ, Merlino G (2009) MicroRNA reexpression as differentiation therapy in cancer. J Clin Invest 119:2119–2123
Mishra PJ, Humeniuk R, Mishra PJ, Longo-Sorbello GS, Banerjee D, Bertino JR (2007) A miR-24 microRNA binding-site polymorphism in dihydrofolate reductase gene leads to methotrexate resistance. Proc Natl Acad Sci USA 104:13513–13518
Mishra PJ, Bertino JR (2009) MicroRNA polymorphisms: the future of pharmacogenomics, molecular epidemiology and individualized medicine. Pharmacogenomics 10:399–416
Mishra PJ, Song B, Mishra PJ, Wang Y, Humeniuk R, Banerjee D, Merlino G, Ju J, Bertino JR (2009) MiR-24 tumor suppressor activity is regulated independent of p53 and through a target site polymorphism. PLoS One 4:e8445
Weber JA, Baxter DH, Zhang S, Huang DY, Huang KH, Lee MJ, Galas DJ, Wang K (2010) The microRNA spectrum in 12 body fluids. Clin Chem 56:1733–1741
Turchinovich A, Weiz L, Langheinz A, Burwinkel B (2011) Characterization of extracellular circulating microRNA. Nucleic Acids Res 39:7223–7233
Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, Mitchell PS, Bennett CF, Pogosova-Agadjanyan EL, Stirewalt DL, Tait JF, Tewari M (2011) Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci USA 108:5003–5008
Turchinovich A, Weiz L, Burwinkel B (2012) Extracellular miRNAs: the mystery of their origin and function. Trends Biochem Sci 37:460–465
Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO (2007) Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9:654–659
Krämer-Albers EM, Bretz N, Tenzer S, Winterstein C, Möbius W, Berger H, Nave KA, Schild H, Trotter J (2007) Oligodendrocytes secrete exosomes containing major myelin and stress-protective proteins: trophic support for axons? Proteomics Clin Appl 1:1446–1461
Quesenberry PJ, Aliotta JM (2008) The paradoxical dynamism of marrow stem cells: considerations of stem cells, niches, and microvesicles. Stem Cell Rev 4:137–147
Mallegol J, van NG, Heyman M (2005) Phenotypic and functional characterization of intestinal epithelial exosomes. Blood Cells Mol Dis 35:11–16
Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S (1998) Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med 4:594–600
Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ (1996) B lymphocytes secrete antigen-presenting vesicles. J Exp Med 183:1161–1172
Thery C (2011) Exosomes: secreted vesicles and intercellular communications. F1000 Biol Rep 3:15
Thery C, Zitvogel L, Amigorena S (2002) Exosomes: composition, biogenesis and function. Nat Rev Immunol 2:569–579
Pigati L, Yaddanapudi SC, Iyengar R, Kim DJ, Hearn SA, Danforth D, Hastings ML, Duelli DM (2010) Selective release of microRNA species from normal and malignant mammary epithelial cells. PLoS One 5:e13515
Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brügger B, Simons M (2008) Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319:1244–1247
Poutsiaka DD, Schroder EW, Taylor DD, Levy EM, Black PH (1985) Membrane vesicles shed by murine melanoma cells selectively inhibit the expression of Ia antigen by macrophages. J Immunol 134:138–144
Kucharzewska P, Belting M (2013) Emerging roles of extracellular vesicles in the adaptive response of tumour cells to microenvironmental stress. J Extracell Vesicles 2:20304
Kucharzewska P, Christianson HC, Welch JE, Svensson KJ, Fredlund E, Ringnér M, Mörgelin M, Bourseau-Guilmain E, Bengzon J, Belting M (2013) Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc Natl Acad Sci USA 110:7312–7317
Peinado H, Lavotshkin S, Lyden D (2011) The secreted factors responsible for pre-metastatic niche formation: old sayings and new thoughts. Semin Cancer Biol 21:139–146
Skog J, Würdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT Jr, Carter BS, Krichevsky AM, Breakefield XO (2008) Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol 10:1470–1476
Fabbri M, Paone A, Calore F, Galli R, Gaudio E, Santhanam R, Lovat F, Fadda P, Mao C, Nuovo GJ, Zanesi N, Crawford M, Ozer GH, Wernicke D, Alder H, Caligiuri MA, Nana-Sinkam P, Perrotti D, Croce CM (2012) MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci USA 109:E2110–E2116
Yang L, Wu X, Luo C, He Y, Zhang Y, Chen X, Zhang L, Chen L (2012) Effects of renal carcinoma cell line ACHN-derived exosomes on ACHN cell proliferation and apoptosis. Nan Fang Yi Ke Da Xue Xue Bao 32:1498–1502
Ohshima K, Inoue K, Fujiwara A, Hatakeyama K, Kanto K, Watanabe Y, Muramatsu K, Fukuda Y, Ogura S, Yamaguchi K, Mochizuki T (2010) Let-7 microRNA family is selectively secreted into the extracellular environment via exosomes in a metastatic gastric cancer cell line. PLoS One 5:e13247
Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, Guo J, Zhang Y, Chen J, Guo X, Li Q, Li X, Wang W, Zhang Y, Wang J, Jiang X, Xiang Y, Xu C, Zheng P, Zhang J, Li R, Zhang H, Shang X, Gong T, Ning G, Wang J, Zen K, Zhang J, Zhang CY (2008) Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res 18:997–1006
Kroh EM, Parkin RK, Mitchell PS, Tewari M (2010) Analysis of circulating microRNA biomarkers in plasma and serum using quantitative reverse transcription-PCR (qRT-PCR). Methods 50:298–301
Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O’Briant KC, Allen A, Lin DW, Urban N, Drescher CW, Knudsen BS, Stirewalt DL, Gentleman R, Vessella RL, Nelson PS, Martin DB, Tewari M (2008) Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA 105:10513–10518
Laterza OF, Scott MG, Garrett-Engele PW, Korenblat KM, Lockwood CM (2013) Circulating miR-122 as a potential biomarker of liver disease. Biomark Med 7:205–210
Weng H, Shen C, Hirokawa G, Ji X, Takahashi R, Shimada K, Kishimoto C, Iwai N (2011) Plasma miR-124 as a biomarker for cerebral infarction. Biomed Res 32:135–141
Corsten MF, Dennert R, Jochems S, Kuznetsova T, Devaux Y, Hofstra L, Wagner DR, Staessen JA, Heymans S, Schroen B (2010) Circulating MicroRNA-208b and MicroRNA-499 reflect myocardial damage in cardiovascular disease. Circ Cardiovasc Genet 3:499–506
Teixeira AL, Ferreira M, Silva J, Gomes M, Dias F, Santos JI, Maurício J, Lobo F, Medeiros R (2014) Higher circulating expression levels of miR-221 associated with poor overall survival in renal cell carcinoma patients. Tumour Biol 35:4057–4066
Selth LA, Townley SL, Bert AG, Stricker PD, Sutherland PD, Horvath LG, Goodall GJ, Butler LM, Tilley WD (2013) Circulating microRNAs predict biochemical recurrence in prostate cancer patients. Br J Cancer 109:641–650
Tanaka K, Miyata H, Yamasaki M, Sugimura K, Takahashi T, Kurokawa Y, Nakajima K, Takiguchi S, Mori M, Doki Y (2013) Circulating miR-200c levels significantly predict response to chemotherapy and prognosis of patients undergoing neoadjuvant chemotherapy for esophageal cancer. Ann Surg Oncol 20(Suppl 3):S607–S615
Guay C, Regazzi R (2013) Circulating microRNAs as novel biomarkers for diabetes mellitus. Nat Rev Endocrinol 9:513–521
Zampetaki A, Kiechl S, Drozdov I, Willeit P, Mayr U, Prokopi M, Mayr A, Weger S, Oberhollenzer F, Bonora E, Shah A, Willeit J, Mayr M (2010) Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ Res 107:810–817
Kong L, Zhu J, Han W, Jiang X, Xu M, Zhao Y, Dong Q, Pang Z, Guan Q, Gao L, Zhao J, Zhao L (2011) Significance of serum microRNAs in pre-diabetes and newly diagnosed type 2 diabetes: a clinical study. Acta Diabetol 48:61–69
Karolina DS, Tavintharan S, Armugam A, Sepramaniam S, Pek SL, Wong MT, Lim SC, Sum CF, Jeyaseelan K (2012) Circulating miRNA profiles in patients with metabolic syndrome. J Clin Endocrinol Metab 97:E2271–E2276
Zhang T, Lv C, Li L, Chen S, Liu S, Wang C, Su B (2013) Plasma miR-126 is a potential biomarker for early prediction of type 2 diabetes mellitus in susceptible individuals. Biomed Res Int 2013:761617
Nielsen LB, Wang C, Sørensen K, Bang-Berthelsen CH, Hansen L, Andersen ML, Hougaard P, Juul A, Zhang CY, Pociot F, Mortensen HB (2012) Circulating levels of microRNA from children with newly diagnosed type 1 diabetes and healthy controls: evidence that miR-25 associates to residual beta-cell function and glycaemic control during disease progression. Exp Diabetes Res 2012:896362
Sebastiani G, Grieco FA, Spagnuolo I, Galleri L, Cataldo D, Dotta F (2011) Increased expression of microRNA miR-326 in type 1 diabetic patients with ongoing islet autoimmunity. Diabetes Metab Res Rev 27:862–866
Salas-Pérez F, Codner E, Valencia E, Pizarro C, Carrasco E, Pérez-Bravo F (2013) MicroRNAs miR-21a and miR-93 are down regulated in peripheral blood mononuclear cells (PBMCs) from patients with type 1 diabetes. Immunobiology 218:733–737
Erener S, Mojibian M, Fox JK, Denroche HC, Kieffer TJ (2013) Circulating miR-375 as a biomarker of beta-cell death and diabetes in mice. Endocrinology 154:603–608
Prats-Puig A, Ortega FJ, Mercader JM, Moreno-Navarrete JM, Moreno M, Bonet N, Ricart W, López-Bermejo A, Fernández-Real JM (2013) Changes in circulating microRNAs are associated with childhood obesity. J Clin Endocrinol Metab 98:E1655–E1660
Wang YC, Li Y, Wang XY, Zhang D, Zhang H, Wu Q, He YQ, Wang JY, Zhang L, Xia H, Yan J, Li X, Ying H (2013) Circulating miR-130b mediates metabolic crosstalk between fat and muscle in overweight/obesity. Diabetologia 56:2275–2285
Cermelli S, Ruggieri A, Marrero JA, Ioannou GN, Beretta L (2011) Circulating microRNAs in patients with chronic hepatitis C and non-alcoholic fatty liver disease. PLoS One 6:e23937
Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT (2011) MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol 13:423–433
Roldo C, Missiaglia E, Hagan JP, Falconi M, Capelli P, Bersani S, Calin GA, Volinia S, Liu CG, Scarpa A, Croce CM (2006) MicroRNA expression abnormalities in pancreatic endocrine and acinar tumors are associated with distinctive pathologic features and clinical behavior. J Clin Oncol 24:4677–4684
Li SC, Essaghir A, Martijn C, Lloyd RV, Demoulin JB, Oberg K, Giandomenico V (2013) Global microRNA profiling of well-differentiated small intestinal neuroendocrine tumors. Mod Pathol 26:685–696
Li A, Yu J, Kim H, Wolfgang CL, Canto MI, Hruban RH, Goggins M (2013) MicroRNA array analysis finds elevated serum miR-1290 accurately distinguishes patients with low-stage pancreatic cancer from healthy and disease controls. Clin Cancer Res 19:3600–3610
Yu S, Liu Y, Wang J, Guo Z, Zhang Q, Yu F, Zhang Y, Huang K, Li Y, Song E, Zheng XL, Xiao H (2012) Circulating microRNA profiles as potential biomarkers for diagnosis of papillary thyroid carcinoma. J Clin Endocrinol Metab 97:2084–2092
Lee JC, Zhao JT, Clifton-Bligh RJ, Gill A, Gundara JS, Ip JC, Glover A, Sywak MS, Delbridge LW, Robinson BG, Sidhu SB (2013) MicroRNA-222 and microRNA-146b are tissue and circulating biomarkers of recurrent papillary thyroid cancer. Cancer 119:4358–4365
Lee YS, Lim YS, Lee JC, Wang SG, Park HY, Kim SY, Lee BJ (2015) Differential expression levels of plasma-derived miR-146b and miR-155 in papillary thyroid cancer. Oral Oncol 51:77–83
Cantara S, Pilli T, Sebastiani G, Cevenini G, Busonero G, Cardinale S, Dotta F, Pacini F (2014) Circulating miRNA95 and miRNA190 are sensitive markers for the differential diagnosis of thyroid nodules in a Caucasian population. J Clin Endocrinol Metab 99:4190–4198
Patel D, Boufraqech M, Jain M, Zhang L, He M, Gesuwan K, Gulati N, Nilubol N, Fojo T, Kebebew E (2013) MiR-34a and miR-483-5p are candidate serum biomarkers for adrenocortical tumors. Surgery 154:1224–1228
Szabó DR, Luconi M, Szabó PM, Tóth M, Szücs N, Horányi J, Nagy Z, Mannelli M, Patócs A, Rácz K, Igaz P (2014) Analysis of circulating microRNAs in adrenocortical tumors. Lab Invest 94:331–339
Chabre O, Libé R, Assie G, Barreau O, Bertherat J, Bertagna X, Feige JJ, Cherradi N (2013) Serum miR-483-5p and miR-195 are predictive of recurrence risk in adrenocortical cancer patients. Endocr Relat Cancer 20:579–594
Palumbo T, Faucz FR, Azevedo M, Xekouki P, Iliopoulos D, Stratakis CA (2013) Functional screen analysis reveals miR-26b and miR-128 as central regulators of pituitary somatomammotrophic tumor growth through activation of the PTEN–AKT pathway. Oncogene 32:1651–1659
D’Angelo D, Palmieri D, Mussnich P, Roche M, Wierinckx A, Raverot G, Fedele M, Croce CM, Trouillas J, Fusco A (2012) Altered microRNA expression profile in human pituitary GH adenomas: down-regulation of miRNA targeting HMGA1, HMGA2, and E2F1. J Clin Endocrinol Metab 97:E1128–E1138
Butz H, Likó I, Czirják S, Igaz P, Korbonits M, Rácz K, Patócs A (2011) MicroRNA profile indicates downregulation of the TGFβ pathway in sporadic non-functioning pituitary adenomas. Pituitary 14:112–124
Gentilin E, Tagliati F, Filieri C, Molè D, Minoia M, Rosaria Ambrosio M, Degli Uberti EC, Zatelli MC (2013) miR-26a plays an important role in cell cycle regulation in ACTH-secreting pituitary adenomas by modulating protein kinase Cδ. Endocrinology 154:1690–1700
Qian ZR, Asa SL, Siomi H, Siomi MC, Yoshimoto K, Yamada S, Wang EL, Rahman MM, Inoue H, Itakura M, Kudo E, Sano T (2009) Overexpression of HMGA2 relates to reduction of the let-7 and its relationship to clinicopathological features in pituitary adenomas. Mod Pathol 22:431–441
Leone V, Langella C, D’Angelo D, Mussnich P, Wierinckx A, Terracciano L, Raverot G, Lachuer J, Rotondi S, Jaffrain-Rea ML, Trouillas J, Fusco A (2014) Mir-23b and miR-130b expression is downregulated in pituitary adenomas. Mol Cell Endocrinol 390:1–7
Butz H, Likó I, Czirják S, Igaz P, Khan MM, Zivkovic V, Bálint K, Korbonits M, Rácz K, Patócs A (2010) Down-regulation of Wee1 kinase by a specific subset of microRNA in human sporadic pituitary adenomas. J Clin Endocrinol Metab 95:E181–E191
Trivellin G, Butz H, Delhove J, Igreja S, Chahal HS, Zivkovic V, McKay T, Patócs A, Grossman AB, Korbonits M (2012) MicroRNA miR-107 is overexpressed in pituitary adenomas and inhibits the expression of aryl hydrocarbon receptor-interacting protein in vitro. Am J Physiol Endocrinol Metab 303:E708–E719
Bottoni A, Piccin D, Tagliati F, Luchin A, Zatelli MC, Degli Uberti EC (2005) miR-15a and miR-16-1 down-regulation in pituitary adenomas. J Cell Physiol 204:280–285
Amaral FC, Torres N, Saggioro F, Neder L, Machado HR, Silva WA Jr, Moreira AC, Castro M (2009) MicroRNAs differentially expressed in ACTH-secreting pituitary tumors. J Clin Endocrinol Metab 94:320–323
Bottoni A, Zatelli MC, Ferracin M, Tagliati F, Piccin D, Vignali C, Calin GA, Negrini M, Croce CM, Degli Uberti EC (2007) Identification of differentially expressed microRNAs by microarray: a possible role for microRNA genes in pituitary adenomas. J Cell Physiol 210:370–377
Mao ZG, He DS, Zhou J, Yao B, Xiao WW, Chen CH, Zhu YH, Wang HJ (2010) Differential expression of microRNAs in GH-secreting pituitary adenomas. Diagn Pathol 5:79
Wang C, Su Z, Sanai N, Xue X, Lu L, Chen Y, Wu J, Zheng W, Zhuge Q, Wu ZB (2012) microRNA expression profile and differentially-expressed genes in prolactinomas following bromocriptine treatment. Oncol Rep 27:1312–1320
Di Ieva A, Butz H, Niamah M, Rotondo F, De Rosa S, Sav A, Yousef GM, Kovacs K, Cusimano MD (2014) MicroRNAs as biomarkers in pituitary tumors. Neurosurgery. 75:181–189
Wang Q, Li P, Li A, Jiang W, Wang H, Wang J, Xie K (2012) Plasma specific miRNAs as predictive biomarkers for diagnosis and prognosis of glioma. J Exp Clin Cancer Res 31:97
Patterson E, Webb R, Weisbrod A, Bian B, He M, Zhang L, Holloway AK, Krishna R, Nilubol N, Pacak K, Kebebew E (2012) The microRNA expression changes associated with malignancy and SDHB mutation in pheochromocytoma. Endocr Relat Cancer 19:157–166
Wang K, Yuan Y, Cho JH, McClarty S, Baxter D, Galas DJ (2012) Comparing the MicroRNA spectrum between serum and plasma. PLoS One 7:e41561
Pritchard CC, Kroh E, Wood B, Arroyo JD, Dougherty KJ, Miyaji MM, Tait JF, Tewari M (2012) Blood cell origin of circulating microRNAs: a cautionary note for cancer biomarker studies. Cancer Prev Res (Phila) 5:492–497
Redova M, Poprach A, Nekvindova J, Iliev R, Radova L, Lakomy R, Svoboda M, Vyzula R, Slaby O (2012) Circulating miR-378 and miR-451 in serum are potential biomarkers for renal cell carcinoma. J Transl Med 10:55
Sanders I, Holdenrieder S, Walgenbach-Brünagel G, von Ruecker A, Kristiansen G, Müller SC, Ellinger J (2012) Evaluation of reference genes for the analysis of serum miRNA in patients with prostate cancer, bladder cancer and renal cell carcinoma. Int J Urol 19:1017–1025
Zen K, Zhang CY (2012) Circulating microRNAs: a novel class of biomarkers to diagnose and monitor human cancers. Med Res Rev 32:326–348
Acknowledgments
This study has been funded by “Lendület” grant provided to Attila Patocs by Hungarian Academy of Sciences.
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical approval
All procedures performing in studies involving human participants were in accordance with the ethical standards of institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent of the present retrospective study was waived.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Butz, H., Kinga, N., Racz, K. et al. Circulating miRNAs as biomarkers for endocrine disorders. J Endocrinol Invest 39, 1–10 (2016). https://doi.org/10.1007/s40618-015-0316-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-015-0316-5