Abstract
Purpose of Review
Dual-energy CT (DECT) is a recently introduced computed tomography (CT) technique with the ability to acquire data in multiple energies allowing energy-based material separation based on energy-dependent attenuation profiles of specific materials.
Recent Findings
There are several clinical applications of DECT that are relevant in pancreatic imaging which will be reviewed in this article. Pathologies of the pancreas can vary widely in their clinical significance and can be subtle and difficult to detect. In this article, we review the current literature on DECT in pancreatic imaging, specifically in the evaluation of benign and malignant pathologic conditions of the pancreas, including inflammatory, neoplastic, and vascular disorders, as well as in the assessment of traumatic injuries.
Summary
This paper will review the clinical implementation and application of DECT in pancreatic imaging, thereby allowing for improved detection and characterization of various pancreatic pathologies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The pancreas is an exocrine and endocrine retroperitoneal organ located in the anterior pararenal space. Due to its deep location, detailed evaluation of pancreatic pathologies is challenging with ultrasonography (US), especially in large patients. Cross-sectional imaging such as multi-detector computed tomography (MDCT) and magnetic resonance imaging (MRI) have become cornerstone in imaging evaluation of the pancreas. Advances in CT technology with recent implementation of dual-energy CT (DECT) in routine clinical practice enables increased lesion conspicuity and improved tissue characterization [1, 2•]. There are many other advantages of DECT including the reduction of metallic artifacts, radiation dose and contrast volume reduction and, in some situations, the avoidance of the acquisition of an unenhanced phase CT [3,4,5,6,7]. In most situations, radiation doses of DECT are comparable to or less than those of traditional single-energy CT [8].
Basic Principles and Physics of the DECT and Postprocessing Techniques
Conventional CT images represent a cross-sectional map of the X-ray attenuation of different tissues within a patient. The measured CT voxel depends on its linear attenuation coefficient which is not unique for any given material [2•, 9]. Two materials with similar attenuation coefficients (e.g., iodine and calcium) can appear similar and therefore are difficult to visualize separately on conventional single-energy CT [10•]. DECT provides the unique ability to differentiate materials that are similar in attenuation.
The fundamental principle of DECT is energy-dependent attenuation of materials when exposed to two different photon energy levels due to different attenuation mechanisms (Compton vs. Photoelectric effect) [11]. DECT uses two X-ray beams with different energy levels (usually 80 or 100 kVp and 140 kVp) over the same anatomic region. Acquisition of DECT can be done in multiple ways: Two distinct X-ray sources and matching detector pairs at a 90-degree offset, with each source operating at a different tube voltage (dual-source dual-energy); a single source-detector pair with rapid switching between low and high tube potential, and an X-ray source with constant tube voltage with a dual-layer detector capable of differentiating between low- and high-energy photons [2•].
DECT has a variety of postprocessing techniques. DECT allows material decomposition on the basis of energy-dependent attenuation profiles of specific materials. Two datasets concurrently acquired from high- and low-kVp X-ray spectra are processed to generate material-attenuation images, including virtual noncontrast (VNC), virtual monochromatic (VMC), and iodine-selective imaging [2•, 10•].
Iodine-Selective Imaging
Dual-source dual-energy CT uses a three-material decomposition algorithm based on the known X-ray absorption properties of three materials (iodine, soft tissue, and fat for most applications in the abdomen and pelvis) at low and high X-ray energies [10•, 12,13,14]. The estimated contribution of each material in a particular voxel is calculated on the basis of its attenuation profile at different energy levels. Iodine content can be color coded and visually displayed on images as an iodine map. Alternatively, an iodine overlay image can be generated which displays iodine content superimposed on the gray-scale VNC image for better anatomical visualization [15].
Virtual Noncontrast (VNC)
VNC images are generated by mathematical subtraction of the iodine from contrast-enhanced images. VNC images help to avoid a separate unenhanced acquisition while enabling differentiation of iodine (contrast enhancement, hemorrhage, active extravasation) from calcium or other dense material. Studies have compared the VNC and true noncontrast (TNC) images to calculate the potential dose reduction and image quality [14, 16]. In a study of 51 patients with known or suspected pancreatic masses, the authors have found no significant differences in image quality and image noise of VNC and TNC images while achieving significant mean radiation dose reduction by omitting acquisition of an unenhanced scan [17]. In another study of 44 patients with focal pancreatic disease, more than 90% of VNC cases were reported acceptable in replacing TNC images [18]. Some studies have found similar Hounsfield units in VNC compared to TNC images with a decrease in image noise and satisfactory edge sharpness with VNC images [19].
Virtual Monochromatic (VMC)
VMC imaging simulates CT acquisitions at different simulated X-ray energies (keV). With the attenuation characteristics of tissues at two different polychromatic energy spectra, simulated VMC images can be created as if they were acquired using an X-ray beam composed of a single energy value [20]. Images created with energies around 70 keV are similar to those commonly acquired with single-energy CT at 120 kVp. VMC images provide improved image quality with less image noise. Significant accentuation of contrast enhancement can be achieved by choosing an energy level close to the k-edge of iodine. Different studies have demonstrated higher contrast of pancreatic adenocarcinoma with surrounding parenchyma at low X-ray energies around 50–60 keV resulting in increased tumor conspicuity, which allows early detection of smaller tumors [21, 22].
CT Imaging Protocol
Conventional pancreatic imaging CT protocol includes both an arterial or pancreatic phase (35–40 s delay) and a portal venous phase (60–70 s delay) [23]. Optimal enhancement of the pancreas occurs at 35–40 s due to the blood supply from the celiac artery branches and the pancreatic phase has the highest contrast-to-noise ratio showing optimal contrast between normal pancreatic tissue and lesions. Arterial or pancreatic phase is also added for pre-surgical vascular evaluation and for investigation of neuroendocrine tumors. A commonly utilized DECT pancreatic imaging protocol includes the dual-energy acquisition of the arterial or pancreatic phase, without a true unenhanced phase acquisition; however, some institutions acquire both arterial or pancreatic and portal venous phases with DECT [23, 24].
DECT Applications in Pancreatic Imaging
Pancreatitis
Imaging is generally considered unnecessary in cases of mild acute pancreatitis. Imaging is usually needed to detect causes of pancreatitis such as gallstones. Cross-sectional imaging, in particular CT, is useful in the setting of complications such as necrosis, pseudocysts, hemorrhage, vascular complications, and infectious complications like gangrenous pancreatitis. DECT VNC imaging can help in increasing the conspicuity of pancreatic and peripancreatic hemorrhage and differentiating it from normal pancreatic parenchyma (Fig. 1). DECT with VMC is useful to increase conspicuity of noncalcified gallstones compared with conventional CT imaging [25, 26]. By enhancing contrast enhancement, DECT low-energy VMC imaging improves CNR between the necrotic area and normal pancreas and helps in improved subjective assessment of acute necrotizing pancreatitis [27, 28] (Fig. 2).
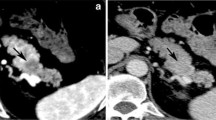
60-year-old man with pancreatitis. a Axial DE CT “mixed” image (combination of low- and high-kilovolt peak images and simulates a traditional 120-kVp image) showing a fluid density lesion in the neck of the pancreas with no iodine uptake on an iodine overlay image (b) consistent with a small pancreatic pseudocyst (arrows). Patient again presents to the emergency department after 2 weeks with complaints of sudden onset of abdominal pain. c Axial DE CT “mixed” image (combination of low- and high-kilovolt peak images and simulates a traditional 120-kVp image) showing hyperdense lesion in the neck of pancreas that could represent enhancement or hematoma (arrow). d, e This lesion remains hyperattenuating on an axial VNC image (d) and contains no iodine content on an iodine overlay image (e), which confirms that it represents a hematoma (hemorrhage into the pseudocyst)
Dual-energy imaging in pancreatitis. a, b 46-year-old man with necrotizing pancreatitis. Coronal arterial phase DE CT “mixed” image (a) and iodine color map (b) showing edematous pancreas with peripancreatic stranding (arrowheads in a). There is hypoattenuated area in pancreatic neck and proximal body (arrow in a) with lack of iodine on color map (arrow in b), consistent with pancreatic necrosis. c, d 45-year-old man with pancreatitis. Coronal arterial phase DE CT “mixed” image (a) and iodine color map (b) showing bulky pancreas with peripancreatic edema consistent with pancreatitis (arrows in a). As compared to the above case, there is relative preservation of pancreatic parenchymal enhancement without definite areas of necrosis
Trauma
Blunt pancreatic trauma is an uncommon injury with high morbidity and mortality. Peak pancreatic enhancement occurs earlier than typical portal venous phase imaging used in most trauma protocols posing a particular challenge in diagnosing pancreatic injury [15]. Management of pancreatic injury is largely dependent on determination of the pancreatic ductal involvement, which usually requires surgical intervention. Recent studies suggest that MDCT has high sensitivity (more than 90%) in diagnosing pancreatic ductal injury [29, 30]. To the best of our knowledge, there is no literature directly comparing DECT versus conventional CT in pancreatic trauma; however, the ability of DECT to better visualize hypodense pancreatic lesions with increased CNR can be applied to detecting pancreatic lacerations (lake of iodine content on an iodine overlay image) (Fig. 3). By analyzing VNC and iodine overlay images side-by-side, DECT allows differentiation of calcium from enhancement (vascular leak or pseudoaneurysm in cases of trauma) without the need of a true noncontrast phase. Unlike iodine, calcium remains visible on both VNC and iodine-selective images. Also DECT helps in the detection and characterization of active vascular extravasation and pseudoaneurysms in traumatic injuries.
Pancreatic laceration in a 19-year-old man after a motor vehicle collision. a Coronal DE CT mixed image shows edema involving neck of the pancreas and ill-defined peripancreatic fluid (arrow). b There is complete lack of contrast in the region of the neck of the pancreas on corresponding coronal iodine overlay image (arrow) consistent with pancreatic transection
Pancreatic Cancer
Majority of the focal pancreatic lesions are mildly hypodense to isodense to the surrounding pancreatic parenchyma on CT, limiting early detection of smaller lesions. Pancreatic ductal adenocarcinoma (PDAC) is characterized by relatively late onset of clinical symptoms and rapid growth pattern making early diagnosis extremely important for improving survival rates. PDAC is mostly hypovascular, associated with desmoplastic reaction and causes pancreatic duct obstruction with upstream ductal dilatation and parenchymal atrophy. As the pancreas lacks a true capsule, PDAC can extensively infiltrate the adjacent retroperitoneal structures with propensity for vascular invasion making majority of the cases surgically inoperable at the time of diagnosis.
DECT iodine-selective imaging and low-kVp VMC imaging increase the conspicuity of iodine content which enables easier detection and better characterization of pancreatic lesions which are often subtle and difficult to detect on conventional CT [31]. For pancreatic mass evaluation, use of a single image-based VMC reconstruction (around 45–55 keV) has shown improved objective image quality and reader preference compared to routine images with significantly higher contrast-to-noise ratio [31, 32] (Fig. 4). Another approach is to use a non-linear blending technique (low HU values derived mostly from the high kVp dataset and the high HU values derived mostly from the low-kVp dataset) to increase tumor to the pancreas CNR [33]. DECT is also helpful in the assessment of vascular invasion by augmenting iodine enhancement on low-keV VM images and iodine maps.
Pancreatic ductal adenocarcinoma in a 65-year-old man presented with left-sided abdominal pain. a Axial DE CT “mixed” image showing fat stranding and edema near tail of pancreas (arrow) and pancreatic ductal dilatation (arrowheads). b, c Axial postprocessed monoenergetic 50-keV CT image shows improved conspicuity of lesion relative to background parenchyma (arrow in b), compared with the postprocessed monoenergetic 120-keV CT image (arrow in c). d Coronal T2-weighted fat-suppressed 3D magnetic resonance cholangiopancreatography (MRCP) image showing abrupt cut-off of the pancreatic duct (arrow). e Axial gadolinium-enhanced T1-weighted MR image showing the ill-defined enhancing mass in the neck of the pancreas. Surgical resection was performed and histopathologic analysis revealed adenocarcinoma
Improved contrast resolution enables better delineation of the lesion margins and assessment of vascular invasion, peripancreatic spread, and regional and distant metastases. Studies have demonstrated increased lesion conspicuity for small PDAC using DECT [32, 34, 35]. In a retrospective study of 46 patients with small (< 3 cm) PDACs, the authors found very high reader interpretive agreement with DECT simulated monoenergetic and iodine material density (MD) images [34]. DECT has shown potential in improving detection and characterization of benign and malignant hepatic lesions including metastases [36] (Fig. 5).
Pancreatic neuroendocrine tumor in a 62-year-old man with hepatic metastases. a Coronal arterial phase DE CT “mixed” image showing well-defined solid-enhancing mass in the head of pancreas and subtle arterial enhancing lesion in the left lobe of the liver (arrow) concerning for metastasis. The same lesion is more conspicuous on the coronal iodine overlay image (b)
One of the newer promising applications of DECT in pancreatic imaging is CT perfusion. Recent studies have demonstrated potential of DECT perfusion in improving delineation of PDAC [37,38,39]. However, it is important to note that available data on DECT perfusion is still at preliminary stages and further studies and technical improvements are needed to establish its role in routine clinical practice.
Cystic Pancreatic Lesions
DECT is helpful in determining the complexity of cystic masses [18]. In a study of 44 patients with focal pancreatic disease, the authors found usefulness of the iodine maps in the determination of the cystic or solid nature of a lesion and improved lesion conspicuity and assessment of the pancreatic duct to adjacent vascular structures in 50% of cases. Also high level of acceptance (90.9%) of the VNC images in replacing TNC images was observed with significantly lower image noise with VNC images [18]. Studies have demonstrated the role of DECT in differentiating between serous oligocystic adenomas and mucinous cystic neoplasms [40, 41]. VNC and iodine maps helps in identifying the hemorrhage within the tumor (Fig. 6).
Pancreatic solid pseudopapillary epithelial neoplasm in a 21-year-old woman. a Axial DE CT “mixed” image showing a well-defined heterogeneous mass lesion in the tail of the pancreas (arrow). b Iodine overlay images demonstrate hemorrhage within the lesion with significant iodine uptake. c, d Axial unenhanced T1-weighted MR image (c) showing well-defined heterogeneous hyperintense mass with corresponding areas of hypointensity on unenhanced axial fat-suppressed T2-weighted image (d) confirming hemorrhage within the lesion
Other Pancreatic Masses and Tumor Mimics
DECT is useful in differentiating an intrapancreatic accessory spleen from hypervascular malignancies. Other potential applications of DECT include the evaluation of enhancement in neuroendocrine tumors [42, 43] (Fig. 5) and differentiation between chronic mass-forming pancreatitis and pancreatic adenocarcinoma. Chronic mass-forming pancreatitis presents as focal pancreatic enlargement and mimics PDAC on CT and even on histopathology. DECT with Iodine images have shown promise in this setting with a retrospective study of 35 patients with either chronic mass-forming pancreatitis or PDAC, showing a significantly higher iodine concentration in chronic mass-forming pancreatitis compared with PDAC [44].
Radiation and Contrast Dose
A common misconception with DECT is that there is increased radiation dose due to both low- and high-energy image acquisition. However, studies have shown that dual-energy acquisition does not increase the radiation dose in abdominal DSCT, while the third-generation DSCT shows improved dose efficiency compared to second-generation DSCT [4]. With the elimination of true noncontrast phase, DECT enables dose reduction by avoiding multiple scan phases and also at the same time decreasing contrast usage. In a study of 60 patients who were imaged for suspected or known pancreatic lesions comparing three-phase MDCT acquisition to a single portal venous phase DECT acquisition, the authors have observed up to 57% radiation dose reduction by replacing a TNC with a VNC [45].
Limitations of DECT and Artifacts
Field of view (FOV) of the first and the second-generation dual-source CT scanners is limited to 26 and 33 cm in diameter, respectively, resulting in truncation of the dual-energy acquisition at the periphery of the abdomen, thereby limiting its role in larger patients. However, due to further increase in FOV in the third-generation dual-source CT scanners up to 50 cm, FOV limitation is generally not an issue [23].
Another limitation of single-source DECT is suboptimal image quality in large patients or in regions of metallic hardware such as a hip prosthesis because of poor penetration of the low-energy X-rays. Low-tube-voltage (80 kVp) DECT images are associated with increased image noise in larger patients. Workflow requirements of dual-energy CT postprocessing also remains a significant challenge to routine clinical implementation.
Conclusion
Recent advances in DECT have expanded the potential for assessment of various pancreatic pathologies. DECT offers many advantages over conventional CT in pancreatic imaging with ongoing technical advances improving on some limitations of earlier generation DECT scanners. Low-KeV monochromatic images and iodine maps improve tissue contrast with increased conspicuity of focal pancreatic lesions and aid in the detection of smaller pancreatic tumors. By eliminating the need of a true noncontrast phase, DECT VNC enables reduction in the radiation dose to the patient. DECT is helpful to reduce contrast media dose by selecting low-energy VMC.
References
Recently published papers of particular interest have been highlighted as: • Of importance
Heye T, Nelson RC, Ho LM, Marin D, Boll DT. Dual-energy CT applications in the abdomen. AJR Am J Roentgenol. 2012;199:S64–70.
•McCollough CH, Leng S, Yu L, Fletcher JG. Dual- and multi-energy CT: principles, technical approaches, and clinical applications. Radiology. 2015;276:637–653. This article succinctly reviews the physical principles of dual energy CT which are imperative to understand in order to apply this technology to various clinical situations.
Pessis E, Sverzut JM, Campagna R, Guerini H, Feydy A, Drape JL. Reduction of metal artifact with dual-energy CT: virtual monospectral imaging with fast kilovoltage switching and metal artifact reduction software. Semin Musculoskelet Radiol. 2015;19:446–55.
Wichmann JL, Hardie AD, Schoepf UJ, Felmly LM, Perry JD, Varga-Szemes A, Mangold S, Caruso D, Canstein C, Vogl TJ, De Cecco CN. Single- and dual-energy CT of the abdomen: comparison of radiation dose and image quality of 2nd and 3rd generation dual-source CT. Eur Radiol. 2017;27:642–50.
Takrouri HS, Alnassar MM, Amirabadi A, Babyn PS, Moineddin R, Padfield NL, BenDavid G, Doria AS. Metal artifact reduction: added value of rapid-kilovoltage-switching dual-energy CT in Relation to Single-energy CT in a piglet animal model. AJR Am J Roentgenol. 2015;205:W352–9.
De Cecco CN, Muscogiuri G, Schoepf UJ, Caruso D, Wichmann JL, Cannao PM, Canstein C, Fuller SR, Snider L, Varga-Szemes A, Hardie AD. Virtual unenhanced imaging of the liver with third-generation dual-source dual-energy CT and advanced modeled iterative reconstruction. Eur J Radiol. 2016;85:1257–64.
Wortman JR, Bunch PM, Fulwadhva UP, Bonci GA, Sodickson AD. Dual-energy CT of incidental findings in the abdomen: can we reduce the need for follow-up imaging? AJR Am J Roentgenol. 2016;207:W1–11.
Henzler T, Fink C, Schoenberg SO, Schoepf UJ. Dual-energy CT: radiation dose aspects. AJR Am J Roentgenol. 2012;199:S16–25.
Hounsfield GN. Computerized transverse axial scanning (tomography). 1. Description of system. Br J Radiol. 1973;46:1016–22.
•Patino M, Prochowski A, Agrawal MD, Simeone FJ, Gupta R, Hahn PF, Sahani DV. Material separation using dual-energy CT: current and emerging applications. Radiographics. 2016;36:1087–1105. This review article discusses the various current and emerging applications of dual energy CT and the benefits of generating material-specific images, especially the ability to assess iodine content and distribution in images. DECT has the benefit of generating energy-specific images as well as virtual monochromatic images.
Millner MR, McDavid WD, Waggener RG, Dennis MJ, Payne WH, Sank VJ. Extraction of information from CT scans at different energies. Med Phys. 1979;6:70–1.
Liu X, Yu L, Primak AN, McCollough CH. Quantitative imaging of element composition and mass fraction using dual-energy CT: three-material decomposition. Med Phys. 2009;36:1602–9.
Kaza RK, Platt JF, Cohan RH, Caoili EM, Al-Hawary MM, Wasnik A. Dual-energy CT with single- and dual-source scanners: current applications in evaluating the genitourinary tract. Radiographics. 2012;32:353–69.
Agrawal MD, Pinho DF, Kulkarni NM, Hahn PF, Guimaraes AR, Sahani DV. Oncologic applications of dual-energy CT in the abdomen. Radiographics: a review publication of the Radiological Society of North America, Inc. 2014;34:589-612.
Wortman JR, Uyeda JW, Fulwadhva UP, Sodickson AD. Dual-energy CT for abdominal and pelvic trauma. Radiographics. 2018;38:586–602.
Mahmood U, Horvat N, Horvat JV, Ryan D, Gao Y, Carollo G, DeOcampo R, Do RK, Katz S, Gerst S, Schmidtlein CR, Dauer L, Erdi Y, Mannelli L. Rapid switching kVp dual energy CT: value of reconstructed dual energy CT images and organ dose assessment in multiphasic liver CT exams. Eur J Radiol. 2018;102:102–8.
Mileto A, Mazziotti S, Gaeta M, Bottari A, Zimbaro F, Giardina C, Ascenti G. Pancreatic dual-source dual-energy CT: is it time to discard unenhanced imaging? Clin Radiol. 2012;67:334–9.
Chu AJ, Lee JM, Lee YJ, Moon SK, Han JK, Choi BI. Dual-source, dual-energy multidetector CT for the evaluation of pancreatic tumours. Br J Radiol. 2012;85:e891–8.
Kaufmann S, Sauter A, Spira D, Gatidis S, Ketelsen D, Heuschmid M, Claussen CD, Thomas C. Tin-filter enhanced dual-energy-CT: image quality and accuracy of CT numbers in virtual noncontrast imaging. Acad Radiol. 2013;20:596–603.
Yu L, Christner JA, Leng S, Wang J, Fletcher JG, McCollough CH. Virtual monochromatic imaging in dual-source dual-energy CT: radiation dose and image quality. Med Phys. 2011;38:6371–9.
Holm J, Loizou L, Albiin N, Kartalis N, Leidner B, Sundin A. Low tube voltage CT for improved detection of pancreatic cancer: detection threshold for small, simulated lesions. BMC Med Imaging. 2012;12:20.
Zamboni GA, Ambrosetti MC, Guariglia S, Cavedon C, Pozzi Mucelli R. Single-energy low-voltage arterial phase MDCT scanning increases conspicuity of adenocarcinoma of the pancreas. Eur J Radiol. 2014;83:e113–7.
Almeida RR, Lo GC, Patino M, Bizzo B, Canellas R, Sahani DV. Advances in pancreatic CT imaging. AJR Am J Roentgenol. 2018;211:1–15.
Patel BN, Alexander L, Allen B, Berland L, Borhani A, Mileto A, Moreno C, Morgan D, Sahani D, Shuman W, Tamm E, Tublin M, Yeh B, Marin D. Dual-energy CT workflow: multi-institutional consensus on standardization of abdominopelvic MDCT protocols. Abdom Radiol. 2017;42:676–87.
Uyeda JW, Richardson IJ, Sodickson AD. Making the invisible visible: improving conspicuity of noncalcified gallstones using dual-energy CT. Abdom Radiol. 2017;42:2933–9.
Yang CB, Zhang S, Jia YJ, Duan HF, Ma GM, Zhang XR, Yu Y, He TP. Clinical application of dual-energy spectral computed tomography in detecting cholesterol gallstones from surrounding bile. Acad Radiol. 2017;24:478–82.
Yuan Y, Huang ZX, Li ZL, Song B. Deng LP [The value of dual-source dual-energy CT with iodine overlay in the diagnosis of acute necrotizing pancreatitis]. Sichuan da xue xue bao Yi xue ban. 2012;43:597–600.
Yuan Y, Huang ZX, Li ZL, Bin S, Deng LP. Dual-source dual-energy computed tomography imaging of acute necrotizing pancreatitis–preliminary study. Sichuan da xue xue bao Yi xue ban. 2011;42:691–4.
Panda A, Kumar A, Gamanagatti S, Bhalla AS, Sharma R, Kumar S, Mishra B. Evaluation of diagnostic utility of multidetector computed tomography and magnetic resonance imaging in blunt pancreatic trauma: a prospective study. Acta Radiol. 2015;56:387–96.
Gordon RW, Anderson SW, Ozonoff A, Rekhi S, Soto JA. Blunt pancreatic trauma: evaluation with MDCT technology. Emerg Radiol. 2013;20:259–66.
Hardie AD, Picard MM, Camp ER, Perry JD, Suranyi P, De Cecco CN, Schoepf UJ, Wichmann JL. Application of an advanced image-based virtual monoenergetic reconstruction of dual source dual-energy CT data at low keV increases image quality for routine pancreas imaging. J Comput Assist Tomogr. 2015;39:716–20.
Patel BN, Thomas JV, Lockhart ME, Berland LL, Morgan DE. Single-source dual-energy spectral multidetector CT of pancreatic adenocarcinoma: optimization of energy level viewing significantly increases lesion contrast. Clin Radiol. 2013;68:148–54.
He YL, Zhang DM, Xue HD, Jin ZY. Clinical value of dual-energy CT in detection of pancreatic adenocarcinoma: investigation of the best pancreatic tumor contrast to noise ratio. Chin Med Sci J. 2013;27:207–12.
McNamara MM, Little MD, Alexander LF, Carroll LV, Beasley TM, Morgan DE. Multireader evaluation of lesion conspicuity in small pancreatic adenocarcinomas: complimentary value of iodine material density and low keV simulated monoenergetic images using multiphasic rapid kVp-switching dual energy CT. Abdom Imaging. 2015;40:1230–40.
Li HO, Guo J, Sun C, Li X, Qi YD, Wang XM, Xu ZD, Chen JH, Liu C. Assessment of pancreatic adenocarcinoma: use of low-dose whole pancreatic CT perfusion and individualized dual-energy CT scanning. J Med Imaging Radiat Oncol. 2015;59:590–8.
Wang Q, Shi G, Qi X, Fan X, Wang L. Quantitative analysis of the dual-energy CT virtual spectral curve for focal liver lesions characterization. Eur J Radiol. 2014;83:1759–64.
Klauss M, Stiller W, Pahn G, Fritz F, Kieser M, Werner J, Kauczor HU, Grenacher L. Dual-energy perfusion-CT of pancreatic adenocarcinoma. Eur J Radiol. 2013;82:208–14.
Fritz F, Skornitzke S, Hackert T, Kauczor HU, Stiller W, Grenacher L, Klauss M. Dual-energy perfusion-CT in recurrent pancreatic cancer-preliminary results. RoFo. 2016;188:559–65.
Skornitzke S, Fritz F, Mayer P, Koell M, Hansen J, Pahn G, Hackert T, Kauczor HU, Stiller W. Dual-energy CT iodine maps as an alternative quantitative imaging biomarker to abdominal CT perfusion: determination of appropriate trigger delays for acquisition using bolus tracking. Br J Radiol. 2018;91:20170351.
Li C, Lin X, Hui C, Lam KM, Zhang S. Computer-aided diagnosis for distinguishing pancreatic mucinous cystic neoplasms from serous oligocystic adenomas in spectral CT images. Technol Cancer Res Treat. 2016;15:44–54.
Lin XZ, Wu ZY, Li WX, Zhang J, Xu XQ, Chen KM, Yan FH. Differential diagnosis of pancreatic serous oligocystic adenoma and mucinous cystic neoplasm with spectral CT imaging: initial results. Clin Radiol. 2014;69:1004–10.
Kartalis N, Mucelli RM, Sundin A. Recent developments in imaging of pancreatic neuroendocrine tumors. Ann Gastroenterol. 2015;28:193–202.
Tamm EP, Bhosale P, Lee JH, Rohren EM. State-of-the-art imaging of pancreatic neuroendocrine tumors. Surg Oncol Clin N Am. 2016;25:375–400.
Yin Q, Zou X, Zai X, Wu Z, Wu Q, Jiang X, Chen H, Miao F. Pancreatic ductal adenocarcinoma and chronic mass-forming pancreatitis: differentiation with dual-energy MDCT in spectral imaging mode. Eur J Radiol. 2015;84:2470–6.
Quiney B, Harris A, McLaughlin P, Nicolaou S. Dual-energy CT increases reader confidence in the detection and diagnosis of hypoattenuating pancreatic lesions. Abdom Imaging. 2015;40:859–64.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Abhishek Keraliya and Jennifer W. Uyeda each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical collection on Computed Tomography.
Rights and permissions
About this article
Cite this article
Keraliya, A., Uyeda, J.W. Dual-Energy Imaging of the Pancreas. Curr Radiol Rep 6, 49 (2018). https://doi.org/10.1007/s40134-018-0308-2
Published:
DOI: https://doi.org/10.1007/s40134-018-0308-2