Abstract
Purpose
To prospectively compare detection and reader confidence of pancreatic lesions using a standard multi-detector computed tomography (MDCT) imaging protocol to a dual-energy computed tomography (DECT) imaging protocol with additional virtual non-contrast series.
Methods
60 subjects imaged for suspected or known pancreatic lesions were included. Subjects underwent pancreatic MDCT including non-contrast, pancreatic phase, and portal venous phase (PVP). The PVP was performed in dual energy mode. Virtual non-contrast and blended 120 kVp weighted images were created from the DECT data. Overall noise and absolute attenuation differences of pancreatic lesions and normal pancreatic tissue were measured. Images were read by two staff radiologists blinded to the underlying diagnosis. MDCT and DECT scans were reviewed separately to evaluate image quality and level of confidence in diagnosis of a pancreatic lesion.
Results
Image quality was ranked excellent for 90 % and 95 % of the 120 kVp studies and 93 % and 95 % of the 100 kVp studies by readers 1 and 2, respectively. VNC was ranked sufficient quality or better by both readers. Average attenuation difference was 74 HU (120 kVp) and 71 HU (100 kVp). Average noise was 11.31 HU (120 kVp) and 15.89 HU (100 kVp). No lesions were missed by either approach. There was increased confidence in diagnostic interpretation in 14 % (± 9 % [95 % CI]) and 9 % (± 7 % [95 % CI]) of DECT scans compared to MDCT.
Conclusions
DECT compared to MDCT pancreatic imaging leads to increased reader confidence with identical diagnostic sensitivity for pathologically proven cases. This approach could be implemented as a single phase acquisition study with calculated VNC leading to a significant dose savings to the patient.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Pancreatic cancer is the 10th most common gastrointestinal malignancy and the 4th leading cause of cancer-related death [1]. Presentation is often at a late and incurable stage due to limited signs and symptoms during the early course of the disease [2]. Imaging plays an important role in lesion detection and characterization as early surgical intervention gives the only possible means of life prolonging treatment [3]. Multidetector computed tomography (MDCT) has become the gold standard first-line imaging test in the diagnosis and staging of pancreatic lesions due to its rapid availability and high sensitivity and specificity [4–6]. Modalities such as positron emission tomography (PET/CT), magnetic resonance imaging (MRI), and endoscopic ultrasound (EUS) are complementary with MRI excelling in sensitivity for suspected lesions which may be missed on CT imaging [5, 7].
Current MDCT protocols involve a multiphase approach with non-contrast, pancreatic parenchymal phase (PPP), and portal venous phase (PVP) imaging [8]. Pancreatic adenocarcinoma causes a localized desmoplastic reaction which results in decreased enhancement and increased lesion conspicuity. This effect is optimized during the PPP with a low attenuating tumor appearing more conspicuous against a background of maximal pancreatic enhancement [9, 10]. Despite the optimization of current MDCT techniques, early detection is achieved in only 20 % of cases with the majority of patients having only a 5 % five-year survival rate [2].
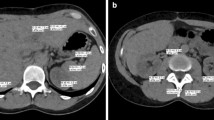
Dual-energy computed tomography (DECT) is a novel technique which is gaining utility in the abdomen [11–18]. This technology utilizes differing low (80–100 kVp) and high (140 kVp) energy spectra with the lower kVp (80–100) source being closer to the k-edge of iodine [19]. This results in greater photon absorption and an inherent increase in Hounsfield measurements in tissues that have taken up iodinated contrast compared to the surrounding non-enhancing tissues (Fig. 1) [19]. In the setting of pancreatic neoplasms or cystic pancreatic lesions, this could theoretically result in increased conspicuity and detection of subtle lesions [10]. DECT also allows for the creation of virtual non-contrast (VNC) images and color-coded iodine overlay maps at no extra dose to the patient [14].
48-year-old male for follow-up of a known pancreatic lesion. Axial DECT blended 120 kVp (A) image and 100 kVp (B) image at the same position and identical windowing performed in the portal venous phase, demonstrating increased conspicuity of the hypoattenuating lesion in the body of the pancreas (red arrow).
Our hypothesis is that using a DECT pancreatic imaging protocol in the setting of suspected or known pancreatic hypoattenuating lesion will result in increased reader confidence. The potential application of DECT in the pancreas has already been shown by other research groups [10, 13, 20], however, to our knowledge this approach has not been formally validated for reader confidence or sensitivity in detection and diagnosis.
Methods and materials
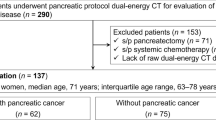
This study was approved by our local institutional ethics review board. Informed consent was waived based on ethics review board approval. Inclusion criteria involved all patients referred for CT scan of known or suspected pancreatic lesions between the dates of October 2011–December 2012. Patients were excluded if they met any of the following criteria: known pregnancy, renal failure with GFR less than 40 ml/min/1.73 cm2, known allergy to iodinated contrast. Subjects were enrolled in a prospective fashion, with decision to proceed with the DECT protocol at the time of requisition review by the supervising staff radiologist or fellow. A total of 60 subjects were included in this study [30 male, 30 female; mean age ± standard deviation 64.08 ± 13.06 years, range 38–90 years].
Scan protocol
Images were acquired on the SOMATOM second generation 128 slice dual-source CT scanner (SOMATOM DEFINITION Dual Source; Siemens Medical Solutions, Forchheim, Germany). Our standard departmental pancreatic mass protocol involves a conventional non-contrast (CNC) phase followed by post-contrast images acquired at 45-s delay during the PPP and 70-s delay during the PVP. Iodinated contrast medium, 120 cc of optiray 350™, (Tyco Healthcare, Mallinckrodt, Hazelwood, MO, USA) is injected at 4 mL/s using a power injector with a saline chaser. The scan range for the CNC and PPP images is selected to cover the pancreas and duodenum, and the PVP scan range is from the dome of the diaphragm through the pubic symphysis. CARE Dose 4D (Siemens Medical Solutions, Forchheim, Germany) automatic tube current modulation is used during all acquisitions. 40 by 0.6 mm collimation with flying focal spot, 0.33 s rotation, and pitch of 0.9 is utilized. Axial and Coronal Images are reconstructed at 3 mm with a 50 % overlap and are reviewed on AGFA PACS 3 megapixel workstations (Agfa Healthcare, Mortsel, Belgium).
In our study, subjects underwent a single dual energy pancreas protocol. This involved modifying our standard MDCT protocol with the portal venous phase being replaced with the dual-energy CT acquisition performed at the same post-contrast delay. Each subject therefore received conventional non-contrast phase (120 kVp), pancreatic parenchymal phase (120 kVp), and portal venous phase (scanned in dual energy mode). Scan parameters for the dual energy acquisition were as follows: tube A (FOV 50 cm) 100 kVp and reference mA 210 and tube B (FOV 33 cm) 140 Vp and reference mA 162. 40 by 0.6 mm collimation was used with a 0.33 s rotation and pitch of 0.9. The low and high energy data sets were analyzed with post processing software (Syngo Dual Energy, Siemens Healthcare Liver VNC) on a CT multimodality workspace workstation (SW-Version VA20, Siemens Healthcare) to create virtual non contrast (VNC) series and a blended data set weighted at 120 kVp [0.5 (100 kVp):0.5 (140 kVp)]. Dedicated 100 and 140 kVp series were also directly obtained from each respective detector.
The data was reviewed independently by two radiologists with 12 and 14 years of experience. Review was performed for each subject using two separate approaches. The MDCT approach involved the CNC, 120 kVp PPP, and blended 120 kVp PVP series. The DECT approach involved the VNC, 100 kVp PVP, and blended 120 kVp PVP series. This approach capitalizes on the increased attenuation of contrast at 100 kVp to simulate the PPP and the VNC to replace the CNC. Readers were blinded to any previous reports or their prior interpretation using the alternative approach.
Subjective analysis
For all subjects and using both approaches, readers independently reported image quality for each series and overall confidence in detecting a pancreatic lesion. Quality was ranked on a four-point scale: 1—non-diagnostic, 2—significant diagnostic limitations, 3—sufficient quality, 4—excellent quality (sufficient quality was used to rank any study in which visible artifact or noise was present which did not affect diagnostic interpretation). Confidence was ranked on a five-point scale: 1—definitely no pancreatic lesion, 2—probably no pancreatic lesion, 3—indeterminate, 4—probable pancreatic lesion, 5—definite pancreatic lesion. An increase in confidence was recorded as a change in opinion away from point 3 in either direction.
Objective analysis
For all acquisitions and reconstructions, the following parameters were recorded: Image noise (measured as the standard deviation of a 1 cm2 ROI placed over an area of homogeneous retroperitoneal fat) and absolute attenuation difference between the normal enhancing pancreas and any observed pancreatic lesion. Contrast to noise (CNR) was then calculated as follows:
(HUpancreas: Mean attenuation of normal pancreas, HUlesion: Mean attenuation of pancreatic lesion). Dose length product (DLP) and Computed tomography dose index (CTDIvol) were also recorded for each subject from the manufacturer’s scanner dose report.
Statistical analysis
For the objective data, means were calculated for image noise, absolute attenuation difference, and CNR. These were tested for statistical significance using a student’s t test for paired samples following confirmation of a normal distribution.
The subjective data was analyze to construct confidence intervals for the proportions of, no change in confidence, defined as an identical level of diagnostic confidence between DECT and MDCT or, increase in confidence, defined as any increase in level of confidence between MDCT and DECT.
Results
Subjective analysis
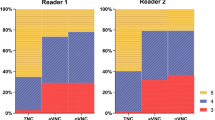
Reader 1 reported 90 % of the MDCT studies as excellent quality and 93 % of the DECT studies as excellent quality. Reader 2 reported 95 % of the MDCT studies as excellent quality and 95 % of the DECT studies as excellent quality. The virtual non-contrast images were ranked as sufficient quality or better by both readers with no diagnostic limitations (Fig. 2).
57-year-old female with increase in lipase. Axial MDCT 120 kVp non-contrast (A) image in the pancreatic parenchymal phase and axial DECT virtual non-contrast (B) image. There is similar conspicuity of the hypoattenuating lesion in the body of the pancreas (white arrow) with minimally increased noise in the VNC image.
When comparing the DECT review method to the MDCT method, there was an increase in confidence in 14% (± 9 % [95 % CI]) of cases for reader 1 and 9 % (± 7 % [95 % CI]) of cases for reader 2 (Fig. 3). For reader 1, there were 5 instances where a lesion was felt to be probably or definitely present on DECT but not on MDCT, and one instance of the opposite. For reader 2, there were no recorded instances. None of these instances had a pathology correlate to confirm whether or not a true lesion was present. There was however imaging follow-up available for all cases ranging from 6 months to 2 years. No interval change or new lesion development was observed.
Diagnostic performance
Pathologic correlation was available for 23 of the 60 cases. The range of observed lesions is listed in Table 1. The sensitivity for detection of the pathologically proven cases was 100 % for both DECT and MDCT.
Objective analysis
Mean noise, absolute attenuation difference (pancreas—observed lesion), and CNR is reported in Table 2. The mean noise for the 100 kVp DECT images was slightly greater than the 120 kVp MDCT images, 15.89 vs. 11.31 (p < 0.001). There was no statistically significant difference between the mean absolute attenuation of pancreas and lesion, 73.53 (DECT) vs. 71.32 (MDCT) (p = 0.76). Despite this, the higher noise of the DECT studies resulted in lower CNR for the 100 kVp DECT images compared to the 120 kVp MDCT images, 4.73 vs. 6.50 (p < 0.05).
Patient dose
The average dose for the MDCT pancreatic protocol was 44.96 (CTDIvol) and 895 (DLP). The average dose for the DECT pancreatic protocol was 13.43 (CTDIvol) and 381 (DLP).This equates to an absolute dose savings of 31.52 (CTDIvol) (p < 0.001), 514 (DLP) (p < 0.001) or an abdominal effective dose savings of 7.7 mSv when using the DECT pancreatic protocol instead of the MDCT approach.
Discussion
Our study has demonstrated an overall increase in diagnostic confidence by two readers when evaluating subjects undergoing CT for known or suspected pancreatic neoplasm using a single phase DECT approach as oppose to a conventional MDCT multiphase protocol. No pathology proven lesions were missed by either imaging approach. There were five instances by reader 1 which would result in follow-up imaging when using DECT vs. no follow-up for the MDCT approach. There was additionally one event with the reverse scenario where MDCT would have resulted in follow-up but not DECT. Given the lack of pathologic correlation of these specific cases, it is difficult to determine the ultimate clinical outcome of these examples. These subjects did however demonstrate stability on follow up imaging.
There was a small observed absolute difference between enhancing and hypoattenuating tissues when using 100 kVp DECT images performed in the portal venous phase compared to 120 kVp MDCT images performed in the pancreatic parenchymal phase. The greater absolute conspicuitiy of hypoattenuating lesions observed at 100 kVp may have been the primary reason for the increase in reader confidence. There was therefore felt to be concordance between the objective and subjective results of this study. It is also assumed that as the readers became more familiar with lesion appearances on the DECT images their overall confidence increased. This effect was however not directly observed.
The average measured CNR was greater for the MDCT images compared to DECT images. This was due to the lower overall noise in the standard 120 kVp images, which is inherently increased in the lower kVp images. This did not affect diagnostic interpretation or subjective image quality.
Our study was performed in the portal venous phase for the DECT acquisitions as opposed to the optimal pancreatic phase. The reason for this was to allow for a paired review so that subjects would receive the benefit of the current gold standard CT protocol in pancreatic investigation prior to the validation of the DECT approach. Performing the DECT study in the pancreatic phase could further increase the absolute differences between normal pancreas and hypoattenuating lesions such that the CNR would surpass the MDCT approach. This could potentially lead to an increase in pancreatic neoplasm detection and diagnosis.
The DECT imaging protocol used in our study was performed as a single acquisition. In combination with the dose savings for incorporating the VNC, this could result in an overall decrease of two imaging phases and significant decreased dose. In the patient with terminal pancreatic carcinoma, this is perhaps negligible; however, in the average patient undergoing initial investigation or continued follow-up of an indeterminate pancreatic lesion, this saving in cumulative radiation burden becomes very important. The limitations of this study include the relatively small number of subjects and limited number of pathology proven cases. The number of subjects in this study reflects all patients referred for investigation of known or suspected pancreatic lesion from the periods of Oct 2011–Dec 2012. In order to increase the number of subjects, a multicenter approach could be considered as well as a longer period of subject recruitment. The limited number of pathology proven cases is likely due to a high number of negative studies and subjects with findings that warrant a conservative approach with follow-up imaging instead of surgery or biopsy. Additional consideration could include the incorporation of a split bolus technique, which allows for acquisition of a single scan performed with contrast delays equivalent to both PPP and PVP combined. This allows for a dose saving equivalent to the omitted separate portal venous phase. In combination with the use of spectral CT performed on a single source fast kVp switching system, Brook et al. have produced early results yielding equivalent or slightly improved rates of conspicuity compared to the standard MDCT approach. The results of this study support the use of DECT in the investigation of pancreatic neoplasms. This approach allows for identical rates of detection with improved confidence in image interpretation with a potential of significant dose reduction to the patient.
References
Siegel R, Naishadham D, Jemal A (2012) Cancer Statistics 2012. Cancer J Clin 62:10–29
Klapman J, Malafa MP (2008) Early detection of pancreatic cancer: why, who, and how to screen. Cancer Control 15(4):280–287
Witkowski ER, Smith JK, Tseng JF (2013) Outcomes following resection of pancreatic cancer. J Surg Oncol 107(1):97–103
Shrikhande SV, Barreto SG, Goel M, Arya S (2012) Multimodality imaging of pancreatic ductal adenocarcinoma: a review of the literature. HPB 14(10):658–668
Raman SP, Horton KM, Fishman EK (2012) Multimodality imaging of pancreatic cancer-computed tomography, magnetic resonance imaging, and positron emission tomography. Cancer J 18(6):511–522
Al-Hawary MM, Francis IR, Chari ST, et al. (2014) Pancreatic ductal adenocarcinoma radiology reporting template: consensus statement of the society of abdominal radiology and the american pancreatic association. Radiology 270(1):248–260
Kim JH, Park SH, Kim MH, et al. (2010) Visually isoattenuating pancreatic adenocarcinoma at dynamic-enhanced CT: frequency, clinical and pathologic characteristics and diagnosis at imaging examinations. Radiology 257:87–96
Bashir MR, Gupta RT (2012) MDCT evaluation of the pancreas: nuts and bolts. Radiol Clin N Am 50(3):365–377
Lu DS, Vedantham S, Krasny RM, et al. (1996) Two-Phase helical CT of pancreatic tumors: pancreatic versus hepatic phase enhancement of tumor, pancreas, and vascular structures. Radiology 199:697–701
Erkan M, Reiser-Erkan C, Michalski CW, et al. (2009) Cancer—Stellate cell interactions perpetuate the Hypoxia-Fibrosis cycle in pancreatic ductal adenocarcinoma. Neoplasia 11(5):497–508
Graser A, Johnson TR, Chandarana H, Macari M (2009) Dual energy CT: preliminary observations and potential clinical applications in the abdomen. Eur Radiol 19(1):13–23
Coursey CA, Nelson RC, Boll DT, et al. (2010) Dual-Energy multidector CT : how does it work, What can it tell us, and When can we use It in abdominopelvic imaging. Radiographics 30:1037–1055
Neville AM, Gupta RT, Miller CM, et al. (2011) Detection of renal lesion enhancement with Dual-Energy methods. Radiology 259(1):173–183
Chu AJ, Lee JM, Lee YJ, et al. (2012) Dual-source, dual-energy multidetector CT for the evaluation of pancreatic tumours. BJR 85:e891–e898
Johnson TR, Krauss B, Sedlmair M, et al. (2007) Material differentiation by dual energy CT: initial experience. Eur Radiol 17(6):1510–1517
De Cecco CN, Buffa V, Fedeli S, et al. (2010) Dual energy CT (DECT) of the liver: conventional versus virtual unenhanced images. Eur Radiol 20(12):2870–2875
Silva A, Morse BG, Hara AK, et al. (2011) Dual-Energy (spectral) CT: applications in abdominal imaging. Radiographics 31:1031–1046
Fletcher JG, Takahashi N, Hartman R, et al. (2009) Dual-energy and dual-source CT: is there a role in the abdomen and pelvis? Radiol Clin N Am 47(1):41–57
Brooks RA (1977) A quantitative theory of the hounsfield unit and its application to dual energy scanning. J Comput Assist Tomogr 1(4):487–493
Macari M, Spieler B, Kim D, et al. (2010) Dual-source dual-energy MDCT of pancreatic adenocarcinoma: initial observations with data generated at 80 kVp and at simulated weighted-average 120 kVp. Am J Roentgenol 194(1):W27–W32
Acknowledgments
There were no received grants and no disclosures relating to this project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Quiney, B., Harris, A., McLaughlin, P. et al. Dual-energy CT increases reader confidence in the detection and diagnosis of hypoattenuating pancreatic lesions. Abdom Imaging 40, 859–864 (2015). https://doi.org/10.1007/s00261-014-0254-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-014-0254-2