Abstract
Azadirachta indica A. Juss (neem tree) is used in Nigeria and India to control malaria. Due to the difficulty to obtain secondary metabolites from this plant, this study aimed to evaluate the in vitro antiplasmodial activity of extracts and fractions of A. indica cell culture on chloroquine-resistant Plasmodium falciparum (FCR3) strains. A. indica cell culture was carried on in stirred tank bioreactor. Biomass from this process was extracted with ethanol and subsequently fractionated with different solvents. Ethanolic extract and fractions obtained were tested in bioassays on chloroquine-resistant Plasmodium falciparum (FCR3) strains. Chloroquine was used as positive control. Finally, hexane fraction was analyzed by ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry, to tentatively identify some compounds that might be involved in antiplasmodial activity. The extract showed promising activity with a half-maximal (50%) inhibitory concentration (IC50) of 6.14 μg ml−1, which decreased with the time (IC50: 33.64 µg ml−1 for at > 18 months). Some extract fractions showed impressive activity against P. falciparum with IC50 values of 13.66 and 9.80 µg ml−1 for the hexane and dichloromethane fractions at > 18 months, which was higher than that of the crude extract during the same time frame. Also, 16 compounds were partially identified in hexane fraction. This is the first study reporting in vitro antiplasmodial activity of extracts of A. indica obtained by biotechnology way. The in vitro antiplasmodial activity of the A. indica cell culture ethanolic extract was demonstrated, and this extract could be a potential future alternative for treating and controlling malaria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malaria is a parasitic disease with negative health effects, especially in developing countries. In 2018, 228 million new cases and 405000 deaths due to this disease were estimated in these regions [1]. It is caused by parasites of the genus Plasmodium such as Plasmodium falciparum. It has a complex life cycle, which involves two hosts: an invertebrate (mosquitoes of the genus Anopheles) and vertebrate (humans). After infecting humans, the parasites move to the liver where they mature and subsequently move into the bloodstream to infect erythrocytes where they multiply, leading to lysis and infection of other erythrocytes [2].
The control and treatment of this disease are challenging because the parasite has developed multidrug resistance to currently used drugs, and few effective antimalarial medicines have been developed in the last few decades. Chloroquine is the most well-known drug for controlling malaria. Another potent substance is artemisinin, a product of Artemisia annua L. Presently, artemisinin derivatives are the most widely used to control malaria [2]; however, P. falciparum has developed resistance to these medications. Quinine is another natural-based medication that was originally isolated from Cinchona officinalis L. in 1944 and is very useful in severe malaria prevention and treatment.
Currently, research on new treatments to control this disease is focused on botanical-based drugs, which has led to the discovery and evaluation of numerous plants with antimalarial activity, which is still ongoing. Azadirachta indica A. Juss is a vernacular tree from India and Burma that only grows in tropical and subtropical regions and was introduced in Colombia several decades ago. Its growth is fast and can reach between 15 and 20 m high. A. indica has been widely used as an antimalarial remedy in Nigeria, India and other parts of Asia [3]. Also, it has been traditionally used in the African and Asian continents for thousands of years, and different parts of the plant such as leaves, seeds, flowers and stem have been used to treat acute and chronic diseases. A. indica has also been used as an antimicrobial agent, insecticide, larvicide, antiviral and spermicide [3]. Tablets and suspensions of the bark and leaf have been produced. Furthermore, the in vitro antimalarial activity of extracts of in vivo grown plants was demonstrated [4]. Additionally, it produces substances with high antimalarial activity against sexual and asexual stages of P. falciparum strains resistant to conventional drugs including chloroquine and pyrimethamine [5].
However, A. indica extracts are produced mainly from the oil of seeds. Seed production occurs annually, and only a little quantity is used for bioactive compound extraction. Moreover, the structural complexity of secondary metabolites produced by this tree makes their chemical synthesis difficult and increases their production costs [6]. Therefore, many researchers have attempted to produce A. indica compounds in vitro and numerous studies on the biotechnological production of them have been carried out. The effect of major nutrients and process type on growth and azadirachtin-related limonoid production in A. indica cell cultures has been studied [7, 8]. In addition, studies have established the role of combined elicitors in improving the secondary metabolite production of plant cell cultures [9]. Furthermore, other researchers determined the effect of three different hydrodynamic conditions corresponding to shake flasks (120 rpm) and stirred tank bioreactors (400 and 800 rpm) on A. indica cell cultures [10]. Thereupon, it could be possible to produce secondary metabolites of A. indica with antiplasmodial activity using biotechnology. Therefore, this study aimed to determine the in vitro antiplasmodial activity of ethanolic extracts and other solvent fractions of A. indica cell culture, obtained by biotechnology way, cultivated in bioreactors, not collected from plants in vivo. These findings would contribute to develop new effective drugs against multidrug-resistant strains of P. falciparum and novel strategies to control malaria.
Material and Methods
Azadirachta indica A. Juss Cell Cultures
Friable calli were produced from A. indica A. Juss seeds collected from Cotové Agricultural Center (Universidad Nacional de Colombia) located in Santa Fe de Antioquia town (6°33′23″ N, 75°49′39″ W).
Friable calli were macerated and put into shake flasks (500 ml) with 100 ml of liquid culture medium (1 g of callus per flask), 25°C, dark conditions and agitated in an orbital shaker at 120 rpm. These cultures were the inoculum for the bioreactor. A 3l stirred tank bioreactor (Applikon® EZ-Control) was employed with 2l of working volume, an impeller of six pitched blades, a turbine rotating at 400 rpm and a porous stainless steel air diffuser. Culture was maintained at 25°C under dark conditions, and the pH was controlled at 5.80 with NaOH 0.25 M addition [8]. Biomass collected in day 14 was used for ethanolic extraction.
Ethanolic Extract Preparation
Ethanolic extracts of A. indica A. Juss cell cultures were prepared following the methodology described in previous research [11]. Seeds and leaves of the A. indica tree were naturally dried, macerated with ethanol (96%, v/v), and the extracts were finally combined and concentrated to dryness using a rotary evaporator (IKA® RV 10 Control) at a temperature lower than 40°C. The extracts were kept in the dark conditions until bioassays and fractionation [11]. Seeds and leaves of the A. indica tree were also evaluated in order to compare the results obtained from A. indica A. Juss cell cultures.
Fractionation of Ethanolic Extract
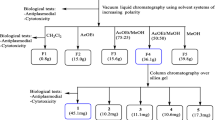
The ethanolic extract of A. indica A. Juss cell culture cultivated in the stirred tank bioreactor was further subjected to a liquid–liquid extraction process to separate the components according to their polarity. This was achieved using two immiscible solvent phases of different densities. The extract was sequentially separated with dichloromethane, ethyl acetate, hexane, an ethanol/methanol mix and water using a separatory funnel. The different fractions and crude extract were concentrated using a rotary evaporator (IKA® RV 10 Control), and then the antiplasmodial activity was subsequently evaluated.
In Vitro Antiplasmodial Activity
Chloroquine-resistant P. falciparum (FCR3) strains were cultured in vitro according to the method described by a precedent research [12] and in vitro antiplasmodial activity was assessed using the following methodology [13]. Seven concentrations of the extract were evaluated at a range between 1.56 and 100 μg ml−1 in duplicate, and each experiment was performed twice. Negative controls (untreated parasites) and positive controls (chloroquine diphosphate salt solid and aqueous-ethanolic bark extract of C. officinalis) were included. After 48 h of incubation (parasites and treatments), 10 µl SYBR Green I at 0.25X dilution was added to the samples, which were incubated in the dark at 25 °C for 20 min.
Parasite growth was determined using flow cytometry (BD Accuri® C6 Flow Cytometer USA) with a blue laser (488 nm), and a total of 10,000 cells were read. The half-maximal (50%) inhibitory concentrations (IC50) against parasite growth were obtained using the GraphPad Prism™ 5.01 software using a variable slope model (log [inhibitor] vs. response variable slope). The coefficient of variation (% CV) was also calculated, and a third test was carried out when %CV was > 20% [14]. Extracts were classified based on their activity (measured as the average IC50) [15].
Analysis of Fractions by Ultra-Performance Liquid Chromatography/Quadrupole Time-of-Flight Mass Spectrometry (UPLC-Qtof-MS)
Hexane fraction was analyzed by UPLC-Qtof-MS. UPLC was performed with a Waters ACQUITY UPLC system (Waters Corporation, Milford, MA, EE. UU.), equipped with a quaternary solvent delivery system, a sample manager coupled to a Xevo-G2-XS-Q-Tof and mass spectrometer equipped with an electrospray interface (Waters Corporation). The separation was achieved on a Zorbax Eclipse Plus chromatography column (150 × 4.6 mm, 5.0 μm) as stationary phase. The temperature of the column was maintained at 30 °C. The flow of the mobile phase was 0.5 ml/min, and its composition was controlled by a gradient of Acetonitrile (A)/Water: 0–10 min, 15% A; 10–20 min, 30% A; 20–30 min, 60% A; 30–35 min, 65% A; 35–40 min, 75% A; 40–60 min, 100% A. The injection volume was 50 μl. The mass spectrometer was operated in positive ionization mode. The desolvation temperature was 486 °C, the flow rate was 1000 l/h, and the temperature of the source was 110 °C. The capillary and cone voltages were 500 and 30 V, respectively. A mass range was scanned from 50 to 1200 Da with an acquisition rate of 0.5 s, and the inter-scan delay was 0.01 s, for a time of analysis of 70 min.
Results and Discussion
Ethanolic Extracts and Fractions
The extract of the A. indica A. Juss cell culture had a viscous consistency and strong garlic smell. It had a dark brown color as reported by other researchers [11]. The extract was obtained at a ratio of 0.11 g extract/g dried cells and was separated into a 0.56 g dichloromethane fraction (25.9% of the crude extract), 0.54 g hexane fraction (28.5%), 0.16 g ethyl acetate fraction (8.4%), 0.14 g ethanol/methanol fraction (7.3%) and 0.49 g aqueous fraction (25.9%). All fractions preserved organoleptic features of the ethanolic extract. At present, there are no reports on metabolites with antiplasmodial activity produced by A. indica cell culture, and compounds are obtained from the leaves and seed oil of A. indica.
In Vitro Antiplasmodial Activity of A. indica A. Juss Cell Culture Ethanolic Extract
The in vitro antiplasmodial activity of A. indica A. Juss cell culture ethanolic extract in the bioreactor scale and that of other agents against P. falciparum are shown in Fig. 1. The IC50 of the ethanolic extract of the A. indica cell culture was 6.14 μg ml−1, while that of the seed and leaf extracts were 58.28 and 32.54 μg ml−1, respectively. The IC50 values of C. officinalis and chloroquine were 0.20 and 0.04 μg ml−1, respectively, which were the controls.
a Percentage inhibition of chloroquine-resistant Plasmodium falciparum (FCR3) growth vs ethanolic extract concentrations of Azadirachta indica A. Juss cell culture and other agents. b In vitro antiplasmodial activity of Azadirachta indica A. Juss cell culture ethanolic extract and other agents against chloroquine-resistant P. falciparum (FCR3)
This study is the first to report the antiplasmodial activity of A. indica cell culture extract. The extract showed activity against P. falciparum at a concentration of 6.14 μg ml−1, which is considered promising because its IC50 was ≤ 15 μg ml−1 [13, 16]. This result is also comparable to data described in other research [17]. They confirmed that a A. indica leaf acetone–water extract had in vitro antimalarial activity at 5 μg ml−1, which was also within the “promising” range [13, 16]. Extracts inhibited the malaria parasite adhesion in infected erythrocytes, while other studies of A. indica seed oil proved its antiplasmodial activity [5, 17].
Studies have found that the oil obtained from A. indica seed (0.025% v/v) inhibited the parasitic growth at levels approximately comparable to those obtained with chloroquine at 10−5 M [5]. They also demonstrated that trophozoites and schizonts were highly susceptible to A. indica seed oil (antiplasmodial effect). In addition, the activity of the A. indica cell culture extract was higher than that described for A. indica seed and leaf extracts and considerably lower than those reported for C. officinalis and chloroquine (Fig. 1).
A. indica extract from the tree is considered as nontoxic; therefore, in 2012, the in vivo antiplasmodial activity of aqueous and ethanolic A. indica leaf extracts was studied using Plasmodium berghei infected BALB/c mice. In this study, authors found that the IC50 of the ethanolic extract was > 1 g/kg body weight of the native mice. This indicates the lack of cytotoxicity and proves the extract is clinically safe [18]. Some researchers reported behavioral signs of toxicity in mice treated with 4 g/kg aqueous crude leaf extract of the Siamese neem tree. However, there was no mortality at all doses used, and the authors highlighted that aqueous crude extract of the Siamese neem tree had antihemolytic activity against P. berghei infected mice [19]. Other researchers studied neem toxicity and reported that only a very high dose caused mortality and that in general, the toxicity of leaf and bark extracts and isolated limonoids was very low [20]. The neem tree is regarded as a valuable tree and can be rationally used in the development of traditional drugs and pesticides [20].
In vitro Antiplasmodial Activity of Ethanolic Extract Fractions of A. indica A. Juss Cell Culture Suspension
The fractionation was carried out 18 months after evaluating the in vitro antiplasmodial activity of the ethanolic extracts. Figure 2a shows the results of the evaluation of the in vitro antiplasmodial activity of ethanolic extract fractions of A. indica A. Juss cell culture and crude extract. Figure 2b presents the information used to calculate the IC50 for each treatment, and the values of the hexane and dichloromethane fractions were 13.66 and 9.80 μg ml−1, respectively. In addition, the IC50 of the ethanolic extract was 33.65 μg ml−1, whereas the ethyl acetate, ethanol/methanol and water fractions were not active (IC50 > 100 μg /ml−1; data are not shown).
a Percentage inhibition of chloroquine-resistant Plasmodium falciparum (FCR3) growth vs ethanolic extract and some fractions concentrations of Azadirachta indica A. Juss cell culture. b In vitro antiplasmodial activity of ethanolic extract and some fractions of Azadirachta indica A. Juss cell culture against chloroquine-resistant P. falciparum (FCR3)
The activity of the A. indica cell culture was similar to that reported for NeemAzal®, a product formulated with A. indica seeds oil [21]. Research studies using an ookinete assay showing increased activity of NeemAzal® against early sporogonic stages compared to that of azadirachtin A [21]. The IC50 value determined for NeemAzal® was 6.80 µg ml−1, which is approximately half of that of azadirachtin (IC50 12.40 µg ml−1). This result and that obtained in previous studies led us to propose that more than one compound may mediate the antiplasmodial activity, perhaps a group of compounds of the same chemical family. In addition, azadirachtin blocked the development of motile male malarial gamete in vitro at a concentration of 72.07 µg ml−1 and completely inhibited in vitro exflagellation of human malaria P. falciparum parasites [22]. Other researchers in 2012 also reported the antiplasmodial activity of neem. They found the bark extract and leaf extract of A. indica showed an IC50 = 29.77 and an IC50 = 47.20 µg ml−1, respectively [23]. In contrast, the antiplasmodial activity of A. indica cell culture extract, obtained by biotechnology way, showed in the present study (IC50 = 6.14 μg ml−1) was higher than the activity reported by bark extract and leaf extract.
Some studies have reported the degradation of A. indica compounds, especially azadirachtin, which leads to loss of activity [24]. This phenomenon was observed in the study evaluating the antiplasmodial activity of ethanolic extracts of A. indica cell culture 18 months later. The initial evaluation showed an IC50 of 6.14 µg ml−1, whereas the subsequent evaluation showed a value of 33.64 µg ml−1. The antiplasmodial activity likely decreased because of compound degradation. A. indica cell culture extract degradation was reported by other researchers who found that the half-life of the extract exposed to ultraviolet (UV) light (368 nm) was 302 min, proving its photosensitivity [11]. Comparing the results, the activity of the fraction activity at > 18 months was higher than that of the extract during the same time frame. This result was possibly obtained because the bioactive compound concentration in the fractions was higher than that in the crude extract. Although we have identified the bioactive compound in A. indica cell culture extracts, we expect that they are limonoids. Researchers reviewed the components of A. indica cell culture extracts which belong to the class of limonoids [3]. Epoxyazadiradione and deacetylnimbin, which are both limonoids, have been reported as compounds in A. indica tree with antiplasmodial activity [25, 26].
Analysis of Fractions by UPLC-Qtof-MS
The assignment of metabolites was carried out by analyzing the retention times and MS spectra, together with fragmentation mechanism. Then, they were compared with the reference data related with the species (A. indica A. Juss) that are reported in literature and databases such as ChemSpider and PubChem. In this way, 16 metabolites were partially identified as limonoids, diterpenoids and fatty acids derivatives. Table 1 summarizes this information.
Peak 1 was tentatively identified as a tricyclic diterpenoid known as nimbionone (C18H22O4; 302.89 uma) [27]. Second peak was identified as a nimbosterol (C29H50O; 414. 25 uma), a β-sitosterol derivative [27] and next four peaks as a nimbidic acid (C26H34O7; 458.28 uma) [27], 28-deoxonimbolide (C27H32O6; 452.35 uma), 1-Acetyl-7-tigloylnimbidinin (C33H42O8; 566.43 uma) and 3-deacetylazadirachtin (C33H42O15; 678.49 uma) [28]. All of them were limonoids. Nimbidic acid and 28-deoxonimbolide are nimbolide derivatives and for this compound it has been reported high in vitro antiplasmodial activity (1.74 μg/ml) [29]. For peaks 10 and 11, two options of compounds were possible. The first one was a pair of diterpenoids partially identified as azadricin (C19H26O; 270.31 uma) (Peak 10) and nimbionol (C18H24O2; 304.30 uma) (Peak 11) [28]. The second option consisted in two fatty acids derivatives known as hexadecenoic acid (Peak 10) (C16H32O2; 270.31 uma) and araquidonic acid (C20H32O2; 304.30 uma) (Peak 11) [27, 30].
On the other hand, peak 12 was partially identified as a diterpenoid nimbionol derivative, and there are two compounds from A. indica that could coincide with this compound, demethylnimbionol and demethylnimbinol (C17H22O4; 290.20 uma) [28]. Peaks 15, 16, 19, 20 and 22 correspond to compounds partially identified as limonoids. They are nimocin (C26H34O4; 410.10 uma), meldenindiol (C26H34O4; 410.10 uma), isonimocinolide (C28H36O7; 484.12 uma), azadirachtin M (C32H42O13; 634.14 uma) and azadirachtin O (C35H46O15; 706.17 uma), respectively [27, 28]. The last two compounds are related with azadirachtin. Although this compound does not have a good activity on the intraerythrocytic stages of P. falciparum, it has a good biological activity on the sporogonic phases of the parasite.
Peak 17 corresponds with a meliacine derivative (C32H48O8; 560.12 uma) [28] and peak 18 with a fatty acid derivative, lignoceric acid (C24H48O2; 368.43 uma) [30]. Finally, it was not possible to identify peaks 7,8,9,13,14,23,24,25 and 26 by this analysis.
Conclusion
The in vitro antiplasmodial activity of A. indica A. Juss cell culture ethanolic extract was demonstrated in this study. The IC50 value of the extract was considered promising, as it was ≤ 15 µg ml−1. The extract activity decreased in a time-dependent manner (IC50, 33.64 µg ml−1 at > 18 months). Nevertheless, the hexane and dichloromethane fractions of the extract showed impressive activity against P. falciparum, which was higher than that of the crude extract. Therefore, A. indica cell culture extract may be used as an active ingredient and potential drug to control malaria. Currently, our research group is attempting to identify the optimal A. indica cell culture conditions that improve the production of secondary metabolites with antiplasmodial activity. 16 compounds were partially identified in hexane fraction. Moreover, it would be necessary to identify the bioactive components and elucidate their action mechanism. On the other hand, it is necessary to confirm the nontoxic effect of the plant cell culture extract and its fractions. To the best of our knowledge, this is the first report on the in vitro antiplasmodial activity of extracts of A. indica cell culture obtained by biotechnology way. This extract could be a potential future alternative to treat and control malaria.
References
World Health Organization (2019) World malaria report 2019. Geneva. Licence: CC BY-NC-SA 3.0 IGO
Biamonte MA, Wanner J, Le KG (2013) Recent advances in malaria drug discovery. Bioorg Med Chem Lett 23:2829–2843. https://doi.org/10.1016/j.bmcl.2013.03.067
Gupta SC, Prasad S, Tyagi AK, Kunnumakkara AB, Aggarwal BB (2017) Neem (Azadirachta indica): an Indian traditional panacea with modern molecular basis. Phytomedicine 34:14–20. https://doi.org/10.1016/j.phymed.2017.07.001
Adebayo JO, Krettli AU (2011) Potential antimalarials from Nigerian plants: A review. J Ethnopharmacol 133:289–302. https://doi.org/10.1016/j.jep.2010.11.024
Dhar R, Zhang K, Talwar G, Garg S, Kumar N (1998) Inhibition of the growth and development of asexual and sexual stages of drug-sensitive and resistant strains of the human malaria parasite Plasmodium falciparum by Neem (Azadirachta indica) Fractions. J Ethnopharmacol 61:31–39. https://doi.org/10.1016/s0378-8741(98)00012-9
Sidhu OP, Behl HM (1996) Seasonal variation in azadirachtins in seeds of Azadirachta indica. Curr Sci 70:1084–1086
Raval K, Hellwig S, Prakash G, Ramos-Plasencia A, Srivastava A, Büchs J (2003) Necessity of a two-stage process for the production of azadirachtin-related limonoids in suspension cultures of Azadirachta indica. J Biosci Bioeng 96:16–22. https://doi.org/10.1016/S1389-1723(03)90091-0
Vásquez-Rivera A, Chicaiza-Finley D, Hoyos R, Orozco-Sánchez F (2015) Production of limonoids with insect antifeedant activity in a two-stage bioreactor process with cell suspension culture of Azadirachta indica. App Biochem Biotechnol 177:334–345. https://doi.org/10.1007/s12010-015-1745-5
Prakash G, Srivastava AK (2008) Statistical elicitor optimization studies for the enhancement of azadirachtin production in bioreactor Azadirachta indica cell cultivation. Biochem Eng J 40:218–226. https://doi.org/10.1016/j.bej.2007.12.017
Villegas-Velásquez S, Martínez-Mira AD, Hoyos R, Rojano B, Orozco-Sánchez F (2017) Hydrodynamic stress and limonoid production in Azadirachta indica cell culture. Biochem Eng J 122:75–84. https://doi.org/10.1016/j.bej.2017.03.004
Zuleta-Castro C, Rios D, Hoyos R, Orozco-Sánchez F (2017) First formulation of a botanical active substance extracted from neem cell culture for controlling the armyworm. Agron Sustain Dev 37:40. https://doi.org/10.1007/s13593-017-0448-4
Trager W, Jensen JB (2005) Human malaria parasites in continuous culture. J Parasitol 91:484–486. https://doi.org/10.1645/0022-3395(2005)091[0484:HMPICC]2.0.CO;2
Desjardins RE, Canfield CJ, Haynes JD, Chulay JD (1979) Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob Agents Chemother 16:710–718. https://doi.org/10.1128/aac.16.6.710
Reed GF, Lynn F, Meade BD (2002) Use of coefficient of variation in assessing variability of quantitative assays. Clin Diagn Lab Immunol 9:1235–1239. https://doi.org/10.1128/cdli.9.6.1235-1239.2002
Jonville M, Kodja H, Humeau L, Fournel J, De Mol P, Cao M (2008) Screening of medicinal plants from Reunion Island for antimalarial and cytotoxic activity. J Ethnopharmacol 120:382–386. https://doi.org/10.1016/j.jep.2008.09.005
Jansen O, Angenot L, Tits M, Nicolas JP, De Mol P, Nikiéma JB, Frédérich M (2010) Evaluation of 13 selected medicinal plants from Burkina Faso for their antiplasmodial properties. J Ethnopharmacol 130:143–150. https://doi.org/10.1016/j.jep.2010.04.032
Udeinya IJ, Mbah AU, Chijioke CP, Shu EN (2004) An antimalarial extract from Neem leaves Is antiretroviral. Trans R Soc Trop Med Hyg 98:435–437. https://doi.org/10.1016/j.trstmh.2003.10.016
Oseni L, Akwetey G (2012) An in-vivo evaluation of antiplasmodial activity of aqueous and ethanolic leaf extracts of Azadirachta indica in Plasmodium berghei infected BALB/c mice. Int J Pharm Sci Res 3:1406–1410. https://doi.org/10.13040/IJPSR.0975-8232.3(5).1406-10
Somsak V, Chachiyo S, Jaihan U (2015) Anti-hemolysis of aqueous crude extract of siamese neem tree (Azadirachta indica) during Plasmodium berghei infection in mice. Malar Cont Elimination 4:1–4. https://doi.org/10.4172/2470-6965.1000129
Raj A (2014) Toxicological effect of Azadirachta indica. Asian J Multidiscip Stud 2:29–33
Dahiya N, Chianese G, Abay SM, Taglialatela-Scafati O, Esposito F, Lupidi G, Bramucci M, Quassinti L, Christophides G, Habluetzel A, Lucantoni L (2016) In vitro and ex vivo activity of an Azadirachta indica A. Juss seed kernel extract on early sporogonic development of Plasmodium in comparison with azadirachtin A, its most abundant constituent. Phytomed 23:1743–1752. https://doi.org/10.1016/j.phymed.2016.10.019
Jones IW, Denholm AA, Ley SV, Lovell H, Wood A, Sinden RE (1994) Sexual development of malaria parasites is inhibited in vitro by the neem extract azadirachtin, and its semi-synthetic analogues. FEMS Microbiol Lett 120:267–273. https://doi.org/10.1111/j.1574-6968.1994.tb07044.x
Ravikumar S, Inbaneson SJ, Suganthi P (2012) In vitro antiplasmodial activity of ethanolic extracts of South Indian medicinal plants against Plasmodium falciparum. Asian Pac J Trop Med 2(3):180–183. https://doi.org/10.1016/S2222-1808(12)60043-7
Johnson S, Dureja P, Dhingra S (2003) Photostabilizers for azadirachtin-A (a neem-based pesticide). J Environ Sci Health B 38:451–462. https://doi.org/10.1081/PFC-120021665
Tapanelli S, Chianese G, Lucantoni L, Yerbanga RS, Habluetzel A, Taglialatela-Scafati O (2016) Transmission blocking effects of neem (Azadirachta indica) seed kernel limonoids on Plasmodium berghei early sporogonic development. Fitoterapia 114:122–126. https://doi.org/10.1016/j.fitote.2016.09.008
Yadav PA, Pavan C, Siva B, Babu KS, Devi A, Singh P, Rao AV (2017) Synthesis and evaluation of anti-plasmodial and cytotoxic activities of epoxyazadiradione derivatives. Eur J Med Chem 134:242–257. https://doi.org/10.1016/j.ejmech.2017.04.016
Singh KK, Phogat S, Dillon RS, Tomar A (2008) Neem: a treatise. I.K. International Publishing House Pvt. Ltd., New Delhi, pp 232–316
Deepa V, Sreekumar S, Biju CK (2016) Validation of Russell’s viper venom detoxification Activity of Azadirachta indica through in silico Method. J Pharm Biol Sci 11(2):35–46. https://doi.org/10.9790/3008-1102033546
MacKinnon S, Durst T, Arnason JT, Angerhofer C, Pezzuto J, Sanchez-Vindas PE, Poveda LJ, Gbeassor M (1997) Antimalarial activity of tropical Meliaceae extracts and gedunin derivatives. J Nat Prod 60:336–341. https://doi.org/10.1021/np9605394
Skellon JH, Thorburn S, Spence J, Chatterjee SN (1962) The fatty acids of neem oil and their reduction products. J Sci Food Agric 13(12):639–643. https://doi.org/10.1002/jsfa.2740131204
Acknowledgments
The authors thank Universidad Nacional de Colombia at Medellín, Laboratories of Bioconversiones and Biotecnología Vegetal, Grupo Malaria of Universidad de Antioquia. Zuleta-Castro expresses her gratitude to Colciencias (Departamento Administrativo de Ciencia, Tecnología e Innovación) for sponsoring her doctoral studies and to Pablo E. Murillo for his valuable help. All work was funded by Universidad Nacional de Colombia and Universidad de Antioquia. Colciencias (Departamento Administrativo de Ciencia, Tecnología e Innovación) sponsored the doctoral studies of C. Zuleta-Castro and the other authors were paid for the work as part of their employment.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Significance Statement A. indica produces substances with antimalarial activity. This is the first report showing this effect by neem cell culture extracts. In addition, it contributes to the development of new effective drugs against multi-drug resistant strains of P. falciparum, through an alternative production of bioactive metabolites of A. indica.
Rights and permissions
About this article
Cite this article
Zuleta-Castro, C., Ríos, A., Durango, D. et al. In Vitro Antiplasmodial Activity of an Azadirachta indica Cell Culture Extract. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 91, 81–88 (2021). https://doi.org/10.1007/s40011-020-01204-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-020-01204-z