Abstract
Drug resistance to practically all antimalarial drugs in use necessitate the development of new chemotherapeutics against malaria. In this aspect, traditionally used plants with folklore reputation are the pillar for drug discovery. Cuscuta reflexa being traditionally used in the treatment of malaria in Odisha, India we aimed to experimentally validate its antimalarial potential. Different solvent extracts of C. reflexa or column fractions from a promising solvent extract were evaluated for in vitro anti-plasmodial activity against Plasmodium falciparum strain Pf3D7. Potent fractions were further evaluated for inhibition of parasite growth against different drug resistant strains. Safety of these fractions was determined by in vitro cyto-toxicity, and therapeutic effectiveness was evaluated by suppression of parasitemia and improvement in survival of experimental mice. Besides, their immunomodulatory effect was investigated in Pf-antigen stimulated RAW cells. GCMS fingerprints of active fractions was determined. Column separation of methanol extract which showed the highest in vitro antiplasmodial activity (IC50 = 14.48 μg/ml) resulted in eleven fractions, three of which (F2, F3, and F4) had anti-plasmodial IC50 ranging from ≤ 10 to 2.2 μg/ml against various P. falciparum strains with no demonstration of in vitro cytotoxicity. F4 displayed the highest in vivo parasite suppression, and had a mean survival time similar to artesunate (19.3 vs. 20.6 days). These fractions significantly modulated expression of inflammatory cytokines in Pf-antigen stimulated RAW cells. The findings of the study confirm the antimalarial potential of C. reflexa. Exploration of phyto-molecules in GCMS fingerprints of active fractions is warranted for possible identification of lead anti-malarial phyto-drugs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human malaria, one of the deadliest infectious diseases of global health problems is caused by six species of Plasmodium (P. falciparum, P. vivax, P. malariae, P. ovale, P. Knowlesi and P. simium). Of these, P. falciparum malaria is often associated with severe manifestations of the disease, with a case fatality rate of 15–40% despite effective treatment with anti-malarial drugs [1]. Further, long term cognitive impairment is reported in as much as 25% of the survivors from cerebral malaria [2, 3]. This indicates that parasite clearance alone is insufficient to prevent mortality and cognitive deficits in malaria. During malaria infection, the host defence mechanism activates phagocytosis of parasite infected red blood cells (RBCs) by monocytes, macrophages, and dendritic cells including the production of pro-inflammatory cytokines [4, 5]. Although considered as part of the effective host defence at the initial stage of infection [6, 7], excessive and uncontrolled overproduction or persistent host inflammatory responses [5, 8] are deleterious to the host. It is complicated further by the emergence and spread of drug resistance P. falciparum to almost all the currently used antimalarial drugs including last effective artemisinin and its derivatives, particularly in South-East Asia [9]. Besides, delays in parasite clearance against isolates originating from West Africa and other parts of the globe are challenging [10]. Therefore, there is an urgent requirement to identify or develop effective antimalarial drugs having a regulatory effect on inflammatory cytokines production, which may resolve post survival malarial attributed deficits and be a powerful new therapeutic strategy.
In this aspect, plant derived compounds are the pillar for drug discovery, which constitutes the backbone of primary healthcare for about 70–95% of the world’s population and has the broadest range of therapeutic applications in curing various diseases [11]. According to an estimate, about 14–28% of 250,000 number of total plant species around the world are being used as drugs [12], of which 1854 plant species from 196 families and 1012 genera are recorded for treatment of malaria or fever globally [13]. Studies indicate that 25% of modern medicines could have been a reality because of their primary discovery in phytogenic natural sources based on their use in folklore and traditional medicine [11]. Interestingly, the most pharmacologically active and widely used antimalarials, such as quinine and artemisinin were obtained from plants having a folklore reputation for antimalarial properties, such as Cinchona calisaya and Artemisia annua, respectively [14]. Of the several medicinal plants used by indigenous people of Odisha, India for malaria treatment, use of Cuscuta reflexa Roxb. (Family: Convolvulaceae) is reported only in Mayurbhanj, Odisha [15], and it is not listed amongst 1854 plant species which are recorded for malaria and fever treatment globally. Based on its folklore reputation in the treatment of malaria in the locality and the lack of or limited investigation done on pharmacological and toxicological aspects, the present study was conducted to ascertain the antimalarial properties of Cuscuta reflexa, scientifically.
Cuscuta reflexa is an angiosperm parasite, popularly known as Amar bel or Devil’s Hair. Besides having mosquitocidal activity against malaria vectors [16], C. reflexa is reported to exhibit antibacterial, anti-helminthic, immunomodulatory, and hepatoprotective activity. Further, it is used in the treatments of various human ailments such as jaundice, diseases of the spleen, diabetes, epilepsy, ulcer and hair loss, etc. [17, 18]. The bioactivity of plant material is dependent on the nature of its constituent compounds and is influenced by the type of solvent used for extraction, their solubility in specific solvent systems and the methods used for extraction However, information on original extractor solvent (s) used, and method of traditional processing of Cuscuta reflexa for effective treatment of malaria (in which the bioactive phyto-constituents are more likely to be found) are lacking since its first report in 2010 [15]. To maximally extract the diverse group of plant metabolites of differential solvent solubility we used sequential-soxhlet extraction involving both polar and non-polar solvents. In order to attest the traditional use of C. reflexa and to validate its antimalarial activity scientifically, these solvent extracts were evaluated for their in vitro anti-plasmodial activity against chloroquine sensitive 3D7 strain of Plasmodium falciparum. Further, to characterise the more potent fractions having the ability to kill multiple drug resistant strains of Plasmodium falciparum, the most active solvent extract was column separated into different fractions, each of which were then evaluated for their in vitro anti-plasmodial activity against chloroquine sensitive 3D7, chloroquine resistant RKL-9 and artemisinin resistant R539T strains of Plasmodium falciparum. To determine the therapeutic effectiveness and safety of the potent fractions, their selectivity index (SI) was calculated after in vitro evaluation of these fractions for cyto-toxicity against normal cells. Since bioactive fractions/compounds exhibiting anti-plamsodial activity alone is insufficient, the potent fractions were further evaluated for their regulatory effect on inflammatory cytokines production in Pf-antigen stimulated RAW cells. Moreover, the antimalarial effect of potent fractions was evaluated in P. berghei infected Swiss-albino mice by examining the suppression of parasitemia and improvement in survival of infected mice in in vivo studies. Finally, the constituent compounds in potent fractions were identified in gas chromatography mass spectrometry (GC–MS) analysis. The findings of the study confirm antimalarial potential of C. reflexa in both in vitro and in vivo studies.
Materials and Method
Preparation of Extracts and Fractions
After identification and authentication of C. reflexa by World Flora Online, 2022 (http://www.worldfloraonline.org/taxon/wfo-0001296658), 30 g of dried powder prepared by pulverization of shed dried whole plant of C. reflexa was used in sequential-Soxhlet extraction with different organic solvents based on their polarity [such as petroleum benzene (PB), chloroform (CH), ethyl acetate (EA) and methanol (M)] at 50 °C and for a minimum of 20 cycles with 300 ml of the solvent [19].The solvents from plant extracts were allowed to evaporate at RT and the percentage yield of extract was determined as [(weight of extract obtained − initial weight)/Initial weight]*100.
TLC Analysis of Bioactive Solvent Extracts
For selection of a suitable solvent system, 10 µl of crude extract in different solvent systems were run in analytical Thin Layer Chromatography (TLC) on a glass plate with silica gel (GF-254, SRL) [20] The solvent system CH: M: AQ in 4:4:2 ratio showed a distinct chromatogram (Supplementary table) and was used for bulk separation of crude extract through column chromatography.
Column Chromatography
Column chromatography was performed by loading 1 g/ml of crude extract on to the top of the column (Borosil-6101062, size 300 × 18 mm) prepared with slurry of silica (60–120 mesh size, SRL) in a selected solvent system. The compounds in the crude extract were separated through careful and continuous slow adding of solvents mixture on the top and collection of about 1 ml of eluents in separate test tubes by turning on the tap below of the column [21]. Each eluent was run in TLC, which was followed by the grouping of similar eluents into fractions. Solvents from the fractions were evaporated using a centrifuge concentrator (concentrator plus, Eppendorf, 5305000568) and percentage yields were calculated.
Drug Preparations
For in vitro assays, 100% DMSO was used to prepare a stock solution of 40 mg/ml for all solvent extracts and 30 mg/ml for different column fractions. Working solution for solvent extract (200 µg/ml) and column fractions (30 µg/ml) were prepared by appropriate dilution with culture media (containing 1% parasitemia and 2% hematocrit) to make the final concentration of DMSO in the working solution to be 0.1% (which is shown to be non-toxic and is commonly used in in vitro cell assays). This was followed by subsequent two-fold serial dilution with culture media to yield a range of final concentrations of drugs (ranging from 200 to 1.56 µg/ml) or fractions (ranging from 30 to 0.23 µg/ml). Intraperitoneal injections of 5% DMSO at a dose of 10 ml/kg for seven consecutive days are proven safe to mice in a recent study [22]. Therefore, for in vivo studies, a drug dose of 20 mg/kg was considered safe and given to test mice by injecting 200 µl of drug solution obtained after tenfold dilution (with PBS, pH −7.4) of a 30 mg/ml stock of fractions prepared in 100% DMSO. Similarly, stock for the standard drug artesunate (Sigma, A3731) was prepared with DMSO and amphotericin-B with incomplete culture media, whereas sterilized distilled water was used to dilute chloroquine (CQ).
Parasite Culture and In Vitro Anti-Plamsodial Assay
In vitro anti-plasmodial activities of various solvent extracts (PB, CH, EA and Methanol), and column fractions of crude methanol extract of C. reflexa (which showed highest in vitro anti-plasmodial activity) were assessed with different laboratory strains of P. falciparum using SYBR green-I based fluorescence assay following our established protocol [23]. Briefly, cultures of three different strains of P. falciparum such as 3D7, RKL-9 and R539T maintained in O positive human erythrocytes and complete RPMI-1640 (Sigma) media [supplemented with 2 g/l sodium bicarbonate (Sigma), 50 mg/l hypoxanthine (Sigma), 10 μg/ml gentamicin (Sigma), and 5 g/l Albumax I (Gibco)] at 37 °C under mixed gas environment (5% O2, 5% CO2 and 90% N2) were 5% sorbitol synchronized separately, prior to use in the growth inhibition assay. About 24 h post incubation, 100 µl of late ring stage or early trophozoite stage of P. falciparum diluted to 1% parasitemia and 2% hematocrit with complete culture media (and packed erythrocytes wherever needed) were incubated with serially diluted concentrations of test samples in triplicate to yield the final concentrations of the solvent extracts (200–1.56 µg/ml), column fractions (30–0.23 µg/ml), and standard antimalarial drugs (chloroquine at 100 nm and artesunate at 50 nm) in a 96-well plate. Untreated wells with parasitized erythrocytes were used as control. Growth inhibition due to plant extracts, fractions and standard drugs were assessed by SYBR-green based fluorescence assay [23] and fluorescence was recorded in a multimode microplate reader (Bio‐Rad) at an excitation and emission wavelength of 485 and 530 nm, respectively. Percent growth inhibition was calculated using the following formula: % Growth Inhibition = {(absorbance of control − absorbance of treated)/ (absorbance of control) * 100}.
In Vitro Cytotoxicity Assay Against Monocytes
A 40-mg/ml stock solutions of different column fractions was prepared in 100% DMSO. Column fractions possessing potent in vitro anti-plasmodial activity (such as F2, F3, F4, F5 and F8) were evaluated for cytotoxicity starting with a concentration of 100 µg/ml to 0.78 µg/ml in a two-fold dilution series in RPMI-1640 media supplemented with 10% foetal bovine serum following Ramu et al. [24] and incubated at 37 °C and 5% CO2. 72 h post incubation, 20 µl of MTT in PBS (5 mg/ml) was added to each well and it was incubated for 4 h at 37 °C in 5% CO2. Cell viability was checked by solubilisation of purple formazan product in viable cells after addition and gentle mixing of 100 µl of DMSO/well. Wells having amphotericin-B (29 µg/ml) were used as positive control, whereas wells without any column fractions/drugs were considered as control (100% growth). The absorbance was recorded at 570 nm on a microplate reader, and the percentage viability of cells was determined using the formula: % Cell viability = (Absorbance of control − Absorbance of treated/Absorbance of control)*100.
In Vitro Inhibition of Inflammatory Cytokines Production
The inhibitory effects of potent fractions (F2, F3, F4 and F5) on the production of three different inflammatory cytokines (such as TNF-α, IL-10 and IL-12) by P. falciparum (Pf) antigen stimulated murine RAW 264.7 macrophage cells were assessed by qRT-PCR. Briefly, a running culture of RAW cells maintained in DMEM media following the previously described method [25] was trypsinized after a confluence of about 70–80% (in about 24 h culture). The cells were then seeded at 10,000 cells/200 µl/well in 4X12 well plates after appropriate dilution with complete DMEM media and cell counting. Allowing about 1 h of cell adherence, these cells were treated with Pf antigen (at a ratio of 1 RAW:10 trophozoites) alone, and in combination with different fractions (F2, F3, F4 and F5) at a final concentration corresponding to their respective anti-plasmodial IC50 values. While RAW cells un-stimulated with Pf antigen but treated with different fractions were considered as normal control, LPS (10 ng/ml) stimulated cells treated with these fractions were considered as their respective positive controls. About 24 h post-treatment at 37 °C, RNA was isolated from the cell pellets (TRIZOL reagent, Invitrogen) and quantified by Nanodrop. Using random hexamer primers, cDNA prepared from 1 µg of RNase-free DNase treated total RNA were evaluated for the expression of TNF-α, IL-12, and IL-10 in Power Up SYBR Green PCR master mix (Thermo Fisher, USA) by Real-Time PCR system (Applied Biosystem, CA, USA) following [26]. Melting curves were generated along with the mean CT values and confirmed the generation of a specific PCR product. Amplification of RNU6AP was used as internal control for normalization. Each experiment was done in triplicates.
In Vivo Studies
Experimental Animal and Parasite Inoculation
The in vivo experiment was performed in accordance with the internationally accepted principles for laboratory animal use and care (NIH publication no 85-23, revised 1985), and standard operation procedures of the Institutional Animal Ethics Committee (IAEC) of Jawaharlal Nehru University (JNU), Delhi (JNU/IBSC/2020/18).The male Swiss-albino mice, aged about 2 weeks and having an average body weight of ∼30 g housed at Central Laboratory Animal Resources, Jawaharlal Nehru University, Delhi were maintained under standard conditions of food, temperature (25 °C ± 3), relative humidity (55 ± 10%) and illumination (12 h light/dark cycles) throughout the experiment. Donor albino mouse previously infected with chloroquine sensitive lethal strain of Plasmodium berghei ANKA and having above 30% parasitemia (3 days post infection) was anaesthetized. About 1 ml of blood was then drawn from this infected mouse through the eye vein using a glass capillary. It was then transferred to a sterile vial containing 200 µl of citrate–phosphate-dextrose (Sigma, C7165) as anticoagulant. After centrifugation at 2000 rpm for 6 min, parasitemia in packed RBCs was determined. Following appropriate dilution, each mouse was intraperitoneally injected with 200 µl of suspension in PBS containing 107 infected RBCs.
In Vivo Suppressive Test and Survival Analysis
Infected mice were randomly divided into seven groups of three each, and the schizonticidal activity of fractions or standard drugs was evaluated following the method described by [27] with some modifications. Briefly, about 24 h post infection, five different column fractions (such as F2, F3, F4, F5 and F8) were injected intraperitoneally into five groups of mice separately at a dose of 20 mg/kg and continued for four consecutive days (D1–D4). The positive control group was similarly treated with the standard drug artesunate (ART) at a dose of 5 mg/kg, whereas the group of mice injected with 200 µl of 2% DMSO was used as the infected control group. The ART dose of 5 mg/kg was selected due to the non-reduction of parasitemia level in infected mice after intraperitoneal injection of a fixed daily dose of ART at 1.7 mg/kg (data not shown) based on previous study [28]. Since a single dose of 30 mg/kg of tested fractions did not result in mortality or adverse clinical symptoms within the first 24 h and up to a follow-up of 7 days (data not shown), a test dose of 20 mg/kg (four times the standard drug concentration) was also anticipated to be safe and selected for this study.
Giemsa-stained thin smear was prepared from the tail blood of all animals on a daily basis till day 6 (by which the first mouse in the control group died) to determine the parasitemia level. Percentage parasitemia was determined by counting infected erythrocytes/100 uninfected erythrocytes in at least 10 fields of 10% giemsa-stained thin smears. The % suppression of parasitemia was determined as [(average parasitemia of infected control − average parasitemia of infected test group)/ average parasitemia of infected control]*100. The mean survival time of mice in each group was determined over a period of 30 days after the day of infection (D0).
GC–MS Analysis
Prior to GC–MS analysis, a two-stage chemical derivatization was performed to make them suitable for analysis [29]. Briefly, 1 mg of dried fractions were dissolved in 100 µl of methoxy amine hydrochloride (20 mg/ml in pyridine) followed by vortex mixing for 2–5 min and subsequent incubation at RT for 1 h to convert aldehyde and ketones into their oximes or alkyloximes. Then, 100 µl of N-methyl-N-trimethylsilyltrifluoro(0) acetamide was added to this mixture in 1:1 ratio and incubated at 70 °C for 30 min to make the metabolites in the mixture less polar and more volatile, before samples were analysed in GC–MS (Shimadzu GC MS-QP-2010 plus system) at Advanced Instrumentation Research Facility, JNU, New Delhi. GC–MS analysis was conducted using RTx-5 SilMS columns (30 m × 0.25 mm id × 0.25 μm film thickness) with operating conditions as described previously [30]. The major and minor compounds present in each of these fractions were determined by comparing the mass spectra with data from National Institute of Standards and Technology, WILEY, Pesticide Library 3rd edition, Drug Library, GC-MS Metabolite Mass Spectral Database, and the Flavour and Fragrance Natural and Synthetic Compounds libraries based on their molecular weight and retention time.
Statistical Analysis
The quantitative values were expressed as mean ± SD. The comparison between two groups was performed by an unpaired t-test with Welch’s correction. The concentration of extracts/fractions/drugs at which there was 50% inhibition of parasite growth (IC50 value) or 50% reduction in cell viability (i.e., cell cytotoxicity, CC50 value) was determined by the dose–response curve. The associated SD for the IC50 value was calculated from the estimated standard error of mean (SEM) using the formula, SEM = SD/square root of n (where n is the number of repetitions of the study) [31]. The selectivity index (SI) of each fraction was calculated as the ratio of their respective CC50 value/ IC50 value. The resistance index (RI) was calculated as a ratio of the IC50 values of resistant strains (RKL-9, R539T) / IC50 value of sensitive strain (3D7). To improve homogeneity of variances, raw data were log transformed where necessary. In qRT-PCR, the Cycle-threshold (Ct) value corresponds to the PCR cycle at which the amplified product was first detected. Using the expression of RNU6AP as reference, gene-expression levels of TNF-α, IL-10 and IL-12 were expressed as fold change by 2−ΔΔCT comparative threshold method. Improvement in animal survival is presented in Kaplan–Meier plot and tested by Log-rank, Mantel-Cox test and Gehan-Breslow-Wilcoxon test. Suppression of parasitaemia in infected mice treated with different fractions were compared by post hoc analysis over a period of 6 days with untreated infected control by Dunnett’s multiple comparison test. In all cases, a two-tailed P value < 0.05 was considered significant. All statistical analyses were performed in GraphPad Prism (version 8.0.1, San Diego, USA).
Results
In Vitro Anti-Plasmodial Activity of Different Solvent Extracts
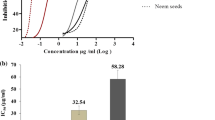
Methanol extract having a percentage yield (PY) of 10.6% showed the highest anti-Plasmodial activity against 3D7 (Plasmodium falciparum strain sensitive to chloroquine and artemisinin) with an IC50 value of 14.48 ± 1.96 μg/ml followed by extracts of chloroform (CH) (IC50 = 31.97; PY = 11%), Petroleum benzene (PB) (IC50 = 61.83; PY = 8%) and ethyl acetate (EA) (IC50 = 115.3; PY = 9.8%).
Fractionations of Methanol Extract, In Vitro Anti-Plasmodial Activity and Cytotoxic Activity
Column chromatography of methanol extract and subsequent grouping of similar fractions by TLC resulted in a total of 11 fractions, four of which (such as F2, F3, F4, F5) showed anti-plasmodial IC50 < 14.48 μg/ml. Rest of the fractions exhibited anti-plasmodial IC50 > 30 μg/ml (Table 1). These four potent fractions, along with one randomly chosen fraction (F8) among those with IC50 > 30 μg/ml were selected to investigate their anti-plasmodial activity against two more strains of P. falciparum such as R539T (artemisinin resistance strain) and RKL9 (chloroquine resistance strain). Besides, the cytotoxicity of these fractions was evaluated against THP-1 cell lines. The results of the percentage yield, anti-plasmodial activity and cytotoxicity of these fractions are shown in Table 1. Consistent with the results obtained with 3D7, all these potential fractions exhibited promising anti-Plasmodial IC50 ranging from < 10.0 to 2.2 μg/ml against R539T and RKL strains (except F5, which had an IC50 > 30.0 μg/ml against R539T). The F8 had an anti-plasmodial IC50 > 30 μg/ml in accordance with the results obtained with the 3D7 strain. CQ and ART had anti-plasmodial IC50 < 1 μg/ml against all the strains of P. falciparum. All the column fractions were non-cytotoxic for THP-1 (CC50 > 100 μg/ml). The SI value was determined to be above 10 μg/ml for column fractions (F2, F3 and F4) against different P. falciparum strains. Of note, the highest SI value was documented for F2 against all tested strains of P. falciparum.
In Vitro Inhibition of Inflammatory Cytokines Production
De-regulated inflammatory responses are the hallmark of severe malaria. To test whether these bioactive fractions affect inflammatory cytokine production, normal RAW cells and those stimulated with Pf antigen were treated with F2, F3, F4 and F5 at a concentration corresponding to their respective anti-plasmodial IC50 values. As shown in Fig. 1, Pf stimulated RAW cells produced several fold higher pro-inflammatory cytokines (TNF-α, IL-12) similar to LPS (10 ng/ml) treated positive control compared to unstimulated control (Fig. 1a and b), whereas the level of IL-10 between Pf stimulated RAW cells and unstimulated control was comparable (Fig. 1c). Interestingly, treatment with studied fractions significantly reduced the expression of TNF-α and IL-12 in both unstimulated and Pf stimulated RAW cells. However, in Pf stimulated RAW cells treated with F4, still the TNF-α level was two-fold high (1A), and level of IL-12 was the least (1B) compared to unstimulated control. On the contrary, while F3 treated RAW cells alone had comparable IL-10 level with that of untreated or Pf stimulated RAW cells; in Pf stimulated RAW cells treated with F3, the level of IL-10 reduced significantly (1C).
Effect of different fractions of methanol extracts on TNF-α (a), IL-12 (b) and IL-10 (c) production in RAW cells. RAW cells stimulated with Pf-antigen produced several fold higher pro-inflammatory cytokines (TNF-α, IL-12) similar to LPS treated positive control. Although treatment with studied fractions significantly reduced their expression, F4 treated RAW cells still had significant level of TNF-α (a). M: Untreated RAW cells, LPS: Lipo-polysaccharides treated at 10 ng/ml; F2, F3, F4 and F5 are different fractions of methanol extract treated at their respective IC50 values. Pf: P. falciparum antigen. Gene-expression levels are expressed as fold change by 2−ΔΔCT comparative threshold method using the expression of RNU6AP as reference. The results are representative of two independent experiments (n = 2) performed in triplicates
In Vivo Anti-Plasmodial Activity of Different Bioactive Fractions
Results of the in vivo suppression of parasitemia by different fractions at a dose of 20 mg/kg, and their effect on the mean survival time (MST) of P. berghei ANKA infected mice are summarized in Table 2 and Fig. 2. The results showed a significant reduction in parasitemia levels by all the tested fractions (above 60%) compared to the control, with the highest suppression demonstrated for F4 (85.93), followed by F3 (80.32) in a 4-day suppressive test. Interestingly, Dunnett’s multiple comparison tests revealed these suppressions were maintained throughout the study period of 6 days (Fig. 2a). Notably, mice treated with F4 had significantly higher MST of 19.3 ± 9.6 days (Log-rank P value < 0.0246 and Gehan-Breslow-Wilcoxon test P value < 0.0339), followed by F3 (MST of 17.0 ± 7.0 days), compared to the control group (MST of 6.67 ± 1.1). The F2 showed an MST of 11.0 ± 3.4. However, these observations were still less as compared to ART-treated mice (91.56% reduction in parasitemia, MST of 20.6 ± 9.5 days) (Fig. 2b).
Effect of different column fractions of methanol extract on in vivo anti-plasmodial activity (a) and survival of Swiss-albino mice (b) infected with P. berghei. F4 treated mice (n = 3) exhibited highest suppression of parasitemia throughout the study period of 6 days (a) compared to untreated infected control as revealed by Dunnett’s multiple comparison test. In addition, treatment with F4 improved the survival of infected mice in 30 days follow-up study (b) similar to ART-treated mice as shown in Kaplan–Meier survival curve. Different fractions (F2-F5 and F8) were treated at 20 mg/kg. ART: artesunate was treated at 5 mg/kg. Dunnett’s multiple comparison test P value < 0.05 is represented by *, P value < 0.01 by **, P value < 0.001 by *** and P value < 0.0001 by ****
GC–MS Analysis of Potent Anti-Plasmodial Fractions of C. reflexa
GC–MS analysis revealed 34 constituent compounds in F2, 80 in F3 and 121 in F4 (S1). Of these, compound 1-(4-isopropylphenyl)-2-methylpropylacetate was found to be common in both F2 and F4, whereas about 37 compounds were observed in both F3 and F4 (S1). There were four major constituent compounds in F2 (such as myristicin, elimicin, Bis(2-ethylhexyl)phthalate and 2H-Pyran-2-dodecanoicacid,6-(17,19-dimethylheneicosyl)), three in F3 (such as D-( +)-Talose- pentakis(trimethylsilyl)ether-methyloxime, 9,12-octadecadienoicacid(z,z)-trimethylsilyl ester and oelsaeure-trimethylsilylester) and four in F4 (such as benzoicacid,3,4,5-tris(trimethylsiloxy)-trimethylsilyl ester, bis-o-trimethylsilyl-palmitinicacid-glycerin-(2)-monoester including the first two compounds of F3) which had their percentage share ≥ 5% in respective fractions. Similarly, there were five compounds in F2, 22 in F3 and 20 in F4 having percentage share between 1 ≤ 5% in respective fractions. Other components were found in less than 1% quantity (S1 and SF1-SF3).
Discussion
With an aim to scientifically confirm the potential of C.reflexa for malaria treatment, when different solvent extracts of C.reflexa were evaluated for their in vitro anti-plasmodial activity against Pf3D7, methanol extract showed promising anti-plasmodial activity, having an IC50 of 14.48 μg/ml. According to a recent classification [32], plant extract possessing IC50 < 5 µg/ml is described to have strong activity, whereas those with 5 ≤ IC50 < 15 µg/ml is described to have good or promising activity. Interestingly, three out of eleven column fractions (such as F2, F3 and F4) of methanol extract exhibited excellent anti-plasmodial activity (IC50 < 10) against three different tested strains of P. falciparum such as 3D7, RKL-9 and R539T with no demonstration of in vitro cytotoxicity up to a concentration of 100 µg/ml. This indicates a selective index higher than tenfold, which is considered specific against infected erythrocytes but non-toxic to the host [32]. These findings attest to the traditional use of C.reflexa for malaria control and highlight the importance of potent column fractions for their containment of possible lead antimalarial phyto-drug. Although in vitro study revealed F2 (high SI value) to be a more effective anti-plasmodial fraction, the same could not be replicated by in vivo studies. Instead, the F4 displayed the highest in vivo anti-plasmodial activity with an 85.93% reduction in parsitemia in a 4-day suppressive test. Further, P. berghei-infected mice treated with F4 had comparable MST with that of artesunate-treated mice (19.3 days vs. 20.6 days). This was followed by F3 and F2-treated mice. A similar trend was established through Dunnett’s multiple comparison tests over the study period of 6 days. These observed discrepancies of studied fractions (F2, F3 and F4) in in vitro and in vivo studies though could not be explained by the available data at present; all had significant anti-malarial effects and could be the source of active phyto-molecules for anti-malaria therapeutics.
It is an established fact that hyper-parasitemia is associated with severe malaria in both human studies and experimental mice [33]. However, some mild malaria patients are documented to be tolerant to high-level parasitemia. On the contrary, malaria patients with low-level parasitemia are succumbed to severity in certain cases [33, 34]. These findings suggest parasitemia alone is insufficient in disease progression to severity in malaria. This is evidenced by the observation of a considerably high case fatality rate despite effective antimalarial treatment for severe malaria patients [1]. Therefore, a combination of parasite density and the host immune response to infection is crucial. While pro-inflammatory cytokines (TNF-α, IL-12, IFN-γ etc.) are proven beneficial during the acute phase of malaria infection, an increased level of anti-inflammatory cytokines (TGF-β and IL-10) is shown to limit the progression of mild malaria to a life-threatening severe complication [6, 7, 35], whereas deregulated expression leading to an imbalance between pro-inflammatory and anti-inflammatory cytokines or an extreme level of either, is associated with heightened severity and mortality [5, 8]. Unfortunately, we could not collect blood samples from control and treated mice for gene expression analysis of relevant cytokines and thus the effect of studied fractions on on in vivo production of inflammatory cytokines could not be analysed. Alternatively, to explore the effect on inflammatory cytokines production in vitro, when un-stimulated or Pf antigen stimulated RAW cells treated with these active column fractions were examined for pro-inflammatory (TNF-α, IL-12) and anti-inflammatory (IL-10) cytokines production (Fig. 1), a significant reduction in the expression level of these cytokines were observed. This suggests that, besides having direct anti-plasmodial activity, the studied fractions might also affect inflammatory cytokines production in infected mice thereby modulating their survival time. However, in Pf antigen stimulated RAW cells treated with F4, the expression of TNF-α was still significantly high compared to untreated control (Fig. 1a). Although we could not investigate the change in expression of cytokines profile in in vivo studies, our observation of improved survival in F4 treated P. berghei infected mice, and previous findings of malaria protective association of early pro-inflammatory cytokine burst [6, 7, 35,36,37] advocate possible protective maintenance of TNF-α in F4 treated infected mice, adequate enough to suppress malaria infection. On the contrary, despite possessing high in vitro anti-plasmodial activity (Table 1), the impact of F2 in drastically reducing the expression of these early phase malaria protective cytokines might have compromised its overall effectiveness on malaria associated immunopathology and subsequent outcomes. However, more promising antiplasmodial activity with high SI index results being demonstrated for F2 in in vitro study compared to F3 and F4, possible containment of important anti-plasmodial phyto-molecules in F2 cannot be ruled out.
To the best of our knowledge, this is the first study that confirms experimentally the potential of C. reflexa for malaria treatment. Interestingly, acute oral doses of methanol extract of C. reflexa are shown to be non-toxic against Swiss-albino mice [38, 39], though not documented in malaria infected mice yet. Phytochemical characterization of active column fractions through GC–MS analysis revealed the presence of several compounds in F2 alone (such as Terpinen-4-ol, myristicin, hexadecanoic acid-methylester- pentacosane and methyl isoeugenol), which are either reported to occur in the bioactive fractions of different plant extracts or shown to exhibit promising anti-malarial activity in previous studies [40,41,42,43,44,45,46]. Besides, the 1-(4-isopropylphenyl)-2-methylpropylacetate observed in both F2 and F4 also been found in active fractions of Syzygiumcumini methanolic extract [47] indicating its likeliness to be a drug-candidate for malaria. Further, out of 37 compounds found in both F3 and F4 (S2), eight compounds (such as 9,12-octadecadienoicacid(z,z)-trimethylsilyl ester, Trimethylsilyl ethers derivatives (TMS) of stearic acid, azelaic acid 2 TMS derivatives, TMS derivatives of Butane-dioic-acid, Phenyl-phosphonic-acid, Malic acid, meso-Erythritol and Xylitol) are reported to exhibit anti-malarial activity (see S2 for details). Of these, the compound 9,12-octadecadienoicacid(z,z)-,trimethylsilyl ester was observed as above 5% in the entire compound collection in F3 (7.0%) and F4 (5.35%). Interestingly, it is also documented as a major peak in bioactive methanol extract of Achyranthes aspera, a plant commonly used in the treatment of chronic malaria [48] However, their target sites or mechanism of action are mostly unknown. Since, both F3 and F4 harbour several anti-malarial compounds in common, the reported anti-malarial activity and immune-pathological effects leading to a significant reduction in parasitemia and improved survival of malaria infected mice by F4, followed by F3 is not surprising and could be due in part to their bioactive constituent components. However, whether the compound (s) exhibiting potent in vivo anti-plasmodial activity are the same or different from those having protective immune-pathological effects remains to be determined. Therefore, anti-plasmodial activity and toxicological analysis of all the major constituents in each of these fractions (S1) require determination. In silico analysis of all major phyto-compounds against important and validated drug targets of P. falciparum in virtual screening may help in identifying the potential drug-like candidates in these fractions. However, discovering a single-active and safe antimalarial drug from plant sources is often very difficult due to their synergistic association with diverse compounds in the extract. Interestingly, certain plant extracts are proven to be more effective than their individual constituent compounds against malaria [49]. This has led to the use of active extract in monotherapy or in combination therapy with standard antimalarial drug [49, 50]. However, identification of single-active phyto-compound would help in synthesizing the more active structural analogs as observed in the case of most active antimalarial drugs such as CQ and artemisinin. Although Soxhlet-extraction is not the traditional practice of preparing herbal medicine, our approach of sequential Soxhlet-extraction with different solvents of increasing polarity extends the prospect of isolating maximum and diverse phyto-constituents in different solvent fractions. Moreover, Soxhlet-extraction being conducted at 50 °C, future exploration of bioactive fractions might lead to isolation of temperature stable interesting anti-malarial compound. Although the explanations relating to the observed discrepancies in active fractions in in vivo and in vitro studies are only speculative, direct and concrete evidence is required to be generated through detailed analysis of major constituent compounds in in vitro and well-designed in vivo studies with additional toxicological analyses.
Conclusions
In conclusion, the results obtained in this study through multiple approaches establish the anti-malarial effect of C. reflexa and authenticate its traditional use in the treatment of malaria. The methanol extract is more promising, especially the column fractions F4 along with two other fractions such as F2 and F3. Besides having anti-plasmodial activity against multiple strains of P. falciparum, these fractions also exhibited protective immunomodulatory activity with no cytotoxicity. There were several compounds found to be common in F3 and F4. Although F2, F3 and F4 contain several promising anti-malarial compounds, their mechanisms of action are unknown. Further, a large number of compounds in these fractions are yet to be explored for containment of possible lead antimalarial phyto-drug. Therefore, all major phyto-compounds in these fractions should be analysed in silico against validated drug targets of P. falciparum in virtual screening to identify the potential drug-like candidates. Information on important hit compounds from this screening would be helpful in synthesising druggable molecules, which can be explored further for lead anti-malarial drug development after appropriate study design.
Data Availability
All data are available with corresponding authors.
Code Availability
Not applicable.
References
Dondorp AM, Fanello CI, Hendriksen IC, Gomes E et al (2010) Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT): an open-label, randomised trial. Lancet 376(9753):1647–1657. https://doi.org/10.1016/s0140-6736(10)61924-1
Oluwayemi IO, Brown BJ, Oyedeji OA, Oluwayemi MA (2013) Neurological sequelae in survivors of cerebral malaria. Pan Afr Med J. https://doi.org/10.11604/pamj.2013.15.88.1897
Schiess N, Villabona-Rueda A, Cottier KE, Huether K et al (2020) Pathophysiology and neurologic sequelae of cerebral malaria. Malar J 19(1):1–12. https://doi.org/10.1186/s12936-020-03336-z
Chua CLL, Ng IMJ, Yap BJM, Teo A (2021) Factors influencing phagocytosis of malaria parasites: the story so far. Malar J 20(1):1–15. https://doi.org/10.1186/s12936-021-03849-1
Dobbs KR, Crabtree JN, Dent AE (2020) Innate immunity to malaria—the role of monocytes. Immunol Rev 293(1):8–24. https://doi.org/10.1111/imr.12830
Gbedande K, Carpio VH, Stephens R (2020) Using two phases of the CD 4 T cell response to blood-stage murine malaria to understand regulation of systemic immunity and placental pathology in Plasmodium falciparum infection. Immunol Rev 293(1):88–114. https://doi.org/10.1111/imr.12835
Leão L, Puty B, Dolabela MF, Povoa MM et al (2020) Association of cerebral malaria and TNF-α levels: a systematic review. BMC Infect Dis 20(1):1–17. https://doi.org/10.1186/s12879-020-05107-2
Popa GL, Popa MI (2021) Recent advances in understanding the inflammatory response in malaria: a review of the dual role of cytokines. J Immunol Res. https://doi.org/10.1155/2021/7785180
Woodrow CJ, White NJ (2017) The clinical impact of artemisinin resistance in Southeast Asia and the potential for future spread. FEMS Microbiol Rev 41(1):34–48. https://doi.org/10.1093/femsre/fuw037
Ikeda M, Kaneko M, Tachibana SI, Balikagala B et al (2018) Artemisinin-resistant Plasmodium falciparum with high survival rates, Uganda, 2014–2016. Emerg Infect Dis 24(4):718. https://doi.org/10.3201/eid2404.170141
El-Sheikha AF (2017) Medicinal plants: ethno-uses to biotechnology era. Biotechnology and Production of Anti-Cancer Compounds. https://doi.org/10.1007/978-3-319-53880-8_1
Al-Sokari S, El-Sheikha FA (2015) In vitro antimicrobial activity of crude extracts of some medicinal plants from Al-Baha region in Saudi Arabia. J Food Nutr Sci 3(1):74. https://doi.org/10.11648/j.jfns.s.2015030102.24
Woon-Chien T et al (2016) Research on medicinal plants for malaria. Medicinal Plants Malaria. https://doi.org/10.1201/b19026-3
Karunamoorthi K, Sabesan S, Jegajeevanram K, Vijayalakshmi J (2013) Role of traditional antimalarial plants in the battle against the global malaria burden. Vector-Borne Zoonotic Dis 13(8):521–544. https://doi.org/10.1089/vbz.2011.0946
Rout S, Panda S (2010) Ethno-medicinal plant resources of Mayurbhanj district, Orissa. https://doi.org/10.1080/09735070.2009.11886333
Bhan S, Mohan L, Srivastava CN (2015) Efficacy of Cuscuta reflexa extract and its synergistic activity with Temephos against mosquito larvae. Int J Mosq Res 2:34–41
Mishra S, Alhodieb FS, Barkat MA, Hassan MZ et al (2022) Antitumor and hepatoprotective effect of Cuscuta reflexa Roxb in a murine model of colon cancer. J Ethnopharmacol 282:114597. https://doi.org/10.1016/j.jep.2021.114597
Verma N, Yadav RK (2018) Cuscuta reflexa: a paracitic medicinal plant. Plant Arch 18:1938–1942
Ojha SB, Roy S, Das S, Dhangadamajhi G (2019) Phytochemicals screening, phenolic estimation and evaluation for anti-oxidant, anti-inflammatory and anti-microbial activities of sequentially Soxhlet extracted coconut testa. Food Nutr Sci 10(08):900. https://doi.org/10.4236/fns.2019.108065
Ginovyan M, Ayvazyan A, Nikoyan A, Tumanyan L et al (2020) Phytochemical screening and detection of antibacterial components from crude extracts of some armenian herbs using TLC-bioautographic technique. Curr Microbiol 77(7):1223–1232. https://doi.org/10.1007/s00284-020-01929-0
Komsta L, Waksmundzka-Hajnos M, Sherma J (eds) (2013). CRC Press, p 20
Zhu Y-P, Song Y-R, Quan W, Xu X-X et al (2021) Letter to the editor: administration of TGF-ß inhibitor mitigates radiation-induced fibrosis in a mouse model. Clin Orthop Relat Res 479(8):1862–1863. https://doi.org/10.1097/corr.0000000000001815
Sah RK, Pati S, Saini M, Singh S (2021) Erythrocyte sphingosine kinase regulates intraerythrocytic development of Plasmodium falciparum. Sci Rep 11(1):1–16. https://doi.org/10.1038/s41598-020-80658-7
Ramu D, Jain R, Kumar RR, Sharma V et al (2019) Design and synthesis of imidazolidinone derivatives as potent anti-leishmanial agents by bioisosterism. Arch Pharm (Weinheim) 352(4):1800290. https://doi.org/10.1002/ardp.201800290
Chaurasiya A, Garg S, Khanna A, Narayana C et al (2021) Pathogen induced subversion of NAD+ metabolism mediating host cell death: a target for development of chemotherapeutics. Cell Death Discov 7(1):1–21. https://doi.org/10.1038/s41420-020-00366-z
Madan E, Puri M, Muthuswami R, Zilberstein D et al (2021) Leishmania parasite arginine deprivation response pathway influences the host macrophage lysosomal arginine sensing machinery. BioRxiv. https://doi.org/10.1101/2021.09.01.458453
Fidock DA, Rosenthal PJ, Croft SL, Brun R et al (2004) Antimalarial drug discovery: efficacy models for compound screening. Nat Rev Drug Discov 3(6):509–520. https://doi.org/10.1038/nrd1416
Peters W, Fleck S, Robinson B, Stewart L et al (2002) The chemotherapy of rodent malaria LX: The importance of formulation in evaluating the blood schizontocidal activity of some endoperoxide antimalarials. Ann Trop Med Parasitol 96(6):559–573. https://doi.org/10.1179/000349802125001744
Moros G, Chatziioannou AC, Gika HG, Raikos N et al (2017) Investigation of the derivatization conditions for GC–MS metabolomics of biological samples. Bioanalysis 9(1):53–65. https://doi.org/10.4155/bio-2016-0224
Gokhale M, Gautam D, Khanna A (2017) A comparative GC-MS analysis of bioactive compounds in the different fractions of root extract of oroxylum indicum (L) vent. Anal Chem Lett 7(3):410–20. https://doi.org/10.1080/22297928.2017.1351889
Lee DK, In J, Lee S (2015) Standard deviation and standard error of the mean. Korean J Anesthesiol 68(3):220. https://doi.org/10.4097/kjae.2015.68.3.220
Habibi P, Shi Y, Fatima Grossi-de-Sa M, Khan I (2022) Plants as sources of natural and recombinant antimalaria agents. Mol Biotechnol 64(11):1177–1197. https://doi.org/10.1016/j.biotechadv.2018.02.002
World Health Organisation (2015) Guidelines for the treatment of malaria. World Health Organization. https://apps.who.int/iris/handle/10665/162441
Sowunmi A, Walker O, Salako L (1992) Hyperparasitaemia: not a reliable indicator of severity or poor prognosis in falciparum malaria in children in endemic African countries. Ann Trop Paediatr 12(2):155–158. https://doi.org/10.1080/02724936.1992.11747561
Malaguarnera L, Pignatelli S, Musumeci M, Simporè J et al (2002) Plasma levels of interleukin-18 and interleukin-12 in Plasmodium falciparum malaria. Parasite Immunol 24(9–10):489–492. https://doi.org/10.1046/j.1365-3024.2002.00485.x
Dodoo D, Omer F, Todd J, Akanmori B et al (2002) Absolute levels and ratios of proinflammatory and anti-inflammatory cytokine production in vitro predict clinical immunity to Plasmodium falciparum malaria. J Infect Dis 185(7):971–979. https://doi.org/10.1086/339408
Mitchell AJ, Hansen AM, Hee L, Ball HJ et al (2005) Early cytokine production is associated with protection from murine cerebral malaria. Infect Immun 73(9):5645–5653. https://doi.org/10.1128/iai.73.9.5645-5653.2005
Chatterjee D, Sahu RK, Jha AK, Dwivedi J (2011) Evaluation of antitumor activity of Cuscuta reflexa Roxb (Cuscutaceae) against Ehrlich ascites carcinoma in Swiss albino mice. Trop J Pharm Res 10(4):447–454. https://doi.org/10.4314/tjpr.v10i4.10
Thomas S, Shrikumar S, Velmurugan C, Kumar BA (2015) Evaluation of anxiolytic effect of whole plant of Cuscuta reflexa. World J Pharm Sci 4:1245–1253
Amlabu WE, Nock IH (2018) Antimalarial efficacy of Vitellaria paradoxa Gaertn (Family: Sapotaceae) leaves and stem bark. FUW Trends Sci Technol J 3:605–609
Ezim O, Alagbe O, Idih F (2021) Antimalarial activity of ethanol extract of Mucuna pruriens leaves on Nk65 Chloroquine sensitive strain of plasmodium berghei. J Complement Altern Med Res 13(4):1–7. https://doi.org/10.9734/jocamr/2021/v13i430229
Fujisaki R, Kamei K, Yamamura M, Nishiya H et al (2012) In vitro and in vivo anti-plasmodial activity of essential oils, including hinokitiol. Southeast Asian J Trop Med Public Health 43(2):270–279
Khan H, Saeed M, Muhammad N, Tariq SA et al (2013) Antimalarial and free radical scavenging activities of aerial parts of Polygonatum verticillatum (L.) All. and identification of chemical constituents by GC-MS. Pak J Bot 45:497–500. https://doi.org/10.1007/s00044-011-9637-x
Khan M (2016) Antimalarial, non alkaloidal molecules from preliminary elucidation of nauclea diderechi extract. J Pharm Pharma 3:1–7. https://doi.org/10.15436/2377-1313.16.012
Lam NS, Long X, Su X, zhuan, Lu F, (2020) Melaleuca alternifolia (tea tree) oil and its monoterpene constituents in treating protozoan and helminthic infections. Biomed Pharmacother 130:110624. https://doi.org/10.1016/j.biopha.2020.110624
Wangchuk P, Keller PA, Pyne SG, Taweechotipatr M et al (2013) GC/GC-MS analysis, isolation and identification of bioactive essential oil components from the Bhutanese medicinal plant Pleurospermum amabile. Nat Prod Commun 8(9):1934578X1300800930. https://doi.org/10.1177/1934578x1300800930
Sachdeva C, Mohanakrishnan D, Kumar S, Kaushik NK (2020) Assessment of in vitro and in vivo antimalarial efficacy and GC-fingerprints of selected medicinal plant extracts. Exp Parasitol 219:108011. https://doi.org/10.1016/j.exppara.2020.108011
Prakash V (2022) To perform gas chromatography and Mass spectroscopy (GC-MS) analysis of Achyranthes aspera L leaf extract. J Drug Deliv Therap. 12(1-S):1–3. https://doi.org/10.22270/jddt.v12i1-s.5299
Rasoanaivo P, Wright CW, Willcox ML, Gilbert B (2011) Whole plant extracts versus single compounds for the treatment of malaria: synergy and positive interactions. Malaria J. https://doi.org/10.1186/1475-2875-10-s1-s4
Willcox M (2011) Improved traditional phytomedicines in current use for the clinical treatment of malaria. Planta Med 77(06):662–671. https://doi.org/10.1055/s-0030-1250548
Acknowledgements
We thankfully acknowledge the financial support from Science and Technology Department, Govt. of Odisha to GD and from Drug and Pharmaceuticals Research Programe (DPRP) to SS. SBO is supported by DST-INSPIRE fellowship. We sincerely thank Advanced Instrumentation and Research Facility (AIRF), JNU, New Delhi for providing the facility of GCMS/MS. We thank Central Instrumentation Facility (CIF) of Special Centre for Molecular Medicine (SCMM), JNU New Delhi.
Funding
This study is supported by the Science and Technology Department, Govt. of Odisha to GD (27552800232014/20) and from Drug and Pharmaceuticals Research Program to SS (P/569/2016–1/TDT, SS).
Author information
Authors and Affiliations
Contributions
Conceptualization, GD and SS; Methodology, SS, GD, SBO and RS; Investigation, SBO, RS, EM, RB and SR; Writing- original draft, SBO, GD and SS; Writing-review and editing, SS and GD. Funding acquisition, SS and GD; Supervision, SS and GD. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Conflict of interest
All authors have no conflict of interest.
Ethical Approval
Approved by IAEC of Jawaharlal Nehru University (JNU), Delhi (JNU/IBSC/2020/18).
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ojha, S.B., Sah, R.K., Madan, E. et al. Cuscuta reflexa Possess Potent Inhibitory Activity Against Human Malaria Parasite: An In Vitro and In Vivo Study. Curr Microbiol 80, 189 (2023). https://doi.org/10.1007/s00284-023-03289-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-023-03289-x