Abstract
Background
As per estimates by WHO in 2021 almost half of the world’s population was at risk of malaria and > 0.6 million deaths were attributed to malaria. Therefore, the present study was aimed to explore the antimalarial activity of extracts derived from the leaves of the plant Anacardium occidentale L., which has been used traditionally for the treatment of malaria. Different extracts of A. occidentale leaves were prepared and tested for their inhibitory activity against recombinant P. falciparum transketolase (rPfTK) enzyme, in vitro. Further, growth inhibitory activity against cultivated blood stage P. falciparum parasites (3D7 strain), was studied using SYBR Green fluorescence-based in vitro assays. Acute toxicity of the hydro alcoholic extracts of leaves of A. occidentale (HELA) at different concentrations was evaluated on mice and Zebra fish embryos. HELA showed 75.45 ± 0.35% inhibitory activity against the recombinant PfTk and 99.31 ± 0.08% growth inhibition against intra-erythrocytic stages of P. falciparum at the maximum concentration (50 µg/ml) with IC50 of 4.17 ± 0.22 µg/ml. The toxicity test results showed that the heartbeat, somite formation, tail detachment and hatching of embryos were not affected when Zebra fish embryos were treated with 0.1 to 10 µg/ml of the extract. However, at higher concentrations of the extract, at 48 h (1000 µg/ml) and 96 h (100 µg/ml and 1000 µg/ml, respectively) there was no heartbeat in the fish embryos. In the acute oral toxicity tests performed on mice, the extract showed no toxicity up to 300 mg/kg body weight in mice.
Conclusion
The hydro-alcoholic extract of leaves of A. occidentale L. showed potent antimalarial activity against blood stage P. falciparum. Based on the observed inhibitory activity on the transketolase enzyme of P. falciparum it is likely that this enzyme is the target for the development of bioactive molecules present in the plant extracts. The promising anti-malarial activity of purified compounds from leaves of A. occidentale needs to be further explored for development of new anti-malarial therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malaria is a major public health problem caused by protozoan parasite belonging to Plasmodium spp. transmitted by the infective bite of female Anopheles mosquitoes. Plasmodium falciparum and Plasmodium vivax are mainly responsible for severe infection in humans. Almost half of the world's population is at risk of acquiring malaria and the total number of deaths due to malaria remains high at more than 0.6 million [1]. P. falciparum is the most prevalent malarial parasite in the African continent [2] and P. vivax is the dominant malaria parasite in most countries outside Sub-Saharan Africa, although it accounts for < 2% of global malaria cases. There has been a rising trend of P. falciparum malaria in India over the last five decades, and in 2021 an estimated 247million people in India were affected by malaria, resulting in 619 000 deaths [1].
Many antimalarial drugs like artemisinin, chloroquine, sulfadoxine and pyrimethamine combination, quinine, atovaquone-proguanil combination (Malarone) and primaquine are available for clinical use, but parasites have developed resistance against these drugs, thereby rendering them ineffective against malaria [3]. Currently, there are no alternate options as effective anti-malarial drugs, other than artemisinin and its derivatives [4]. However, in recent years, treatment failures with artemisinin combination therapies(ACTs) have been reported from South-East Asian countries and genetic basis for resistance to artemisinin has now been established [5]. Occurrence of artemisinin resistance is also suspected in South America but confirmatory studies are still going on [1]. This scenario, therefore, calls for the development of newer drugs against malarial parasites to augment the malaria control and elimination programs.
The drug discovery process can be hastened by adopting a targeted approach, especially with the dawn of molecular and genomic approaches to study parasite encoded proteins which has evolved as a promising approach [6]. Certain enzymes of parasites, such as transketolase have been identified as target for drug development [7]. Transketolase of P. falciparum is involved in the non-oxidative arm of the pentose phosphate pathway (PPP), catalysing the production of pentose sugar needed for nucleic acid synthesis. Hence, PfTK plays a very important role in the replication and survival of malaria parasites and therefore is a potential drug target. Further, it also has very low similarity with human transketolase thus making it a selective target for drug development.
Herbal preparations have been in traditional use for treating various infections, including malaria. Herbal treatments derived from Anacardium occidentale L. (the “cashew”) are popular forms of traditional medicine in some countries like Brazil, Colombia, Nigeria and Peru [8] and also in some tribal areas of India [9]. All parts of the cashew tree (especially leaf and stem bark) have been extensively used in traditional herbal medicine [8]. Thus, during the last few decades, medicinal properties of A. occidentale plant, have been extensively studied [10]. A. occidentale L. is well recognized as a source of alkyl-phenols, which are isolated from its fruit (the “cashew nuts”). These compounds have several biological activities such as antioxidant, mosquito larvicidal, anti-cancer, antibacterial, molluscicidal and schistosomicidal [11,12,13,14,15]. These compounds are also reported to have inhibitory activities against enzymes such as tyrosinase, acetylcholinesterase, glucosidase, aldose reductase, invertase, 15-lipoxygenase, and xanthine oxidase [16]. In the present study, we have explored the antimalarial activity of leaves extracts of A. occidentale L for their inhibitory activity against transketolase enzyme and blood stage of P. falciparum. Also, we assessed the acute toxicity of the extract in Zebra fish and mice models.
Materials and Methods
Collection of Plant Material
The leaves of A. occidentale were collected from cashew grooves in Belagavi district (N 15.88668; E 74.52353; altitude ∼800 m from sea level), Karnataka, India. The plant was identified at the ICMR-National Institute of Traditional Medicine (ICMR-NITM), Belagavi, and an herbarium specimen (RMRC-1356) has been deposited at the institute.
Preparation of the Leaf Extract and Preliminary Phytochemical Test
The leaves of A. occidentale were shade dried and powdered using an electric grinder. Cold maceration of the extract was carried out for 72 h using different solvents viz., petroleum ether, dichloromethane, chloroform, ethyl acetate, methanol, and hydro alcoholic solution (70:30 water: ethanol). Leaves were extracted thrice with each solvent, pooled and then filtered through Whatman filter paper No. 1. The solvent was evaporated using a rotary evaporator. The extracts obtained were stored at 4 °C until further used. Various qualitative phytochemical tests for alkaloids (Dragendorff’s test), glycosides (Keller-kiliani test), amino acids (Ninhydrin test), saponins (Foamtest), flavonoids (Lead acetate test), tannins (Ferric chloride test), and terpenoid (Salkowski test) were carried out [17].
Expression and Purification of P. falciparum Transketolase (PfTk)
The recombinant PfTk was purified as described earlier [7]. Rosetta cells BL21(DE3) harboring the recombinant plasmid with transketolase gene insert were grown in Luria Bertani media (LB) at 37 °C, supplemented with Ampicillin (100 µg/ml) and Chloramphenicol (34 µg/ml), till OD 600 nm reached 0.6. The recombinant protein was induced with 1 mM isopropyl-β-thiogalactopyranoside (IPTG). Further, the culture was grown at 18 °C on a shaker incubator for 20 h and the cells were harvested by centrifugation at 11,000 g for 5 min. The cell pellet was suspended in lysis buffer (50 mM NaH2PO4, pH 8.0), 300mMNaCl, 10 mM imidazole, and 10% (v/v) glycerol) containing protease inhibitor cocktail (Sigma, USA) and the cells were lysed using ultra-sonication (Ultrasonic processor, Model-XL-2020, Germany). The lysate was centrifuged at 11,000 g at 4 °C for 30 min and the supernatant obtained was mixed with Ni-nitrilotriacetic acid (Ni–NTA) agarose (Qiagen, Germany) resin equilibrated with the lysis buffer. After incubation for 1 h at 4 °C, the resin was separated from the cell lysate by centrifugation (5600 g for 5 min at 4 °C) and washed with washing buffer with similar composition to the lysis buffer but containing 20 mM imidazole. The recombinant PfTK enzyme was then eluted with elution buffer containing 250 mM imidazole. The elute containing PfTK was further purified by ammonium sulfate precipitation (0–40% w/v). The protein pellet was resuspended in storage buffer (50 mM NaH2PO4 (pH 8.0), 300 mM NaCl, 10% (v/v) glycerol) and protein concentration was determined by Lowry’s method [18]. The purity of the protein was checked by SDS-PAGE [19] and western blotting using anti-His tag antibodies.
PfTKassay
The activity of the purified transketolase was determined by measuring the oxidation rate of carbanion intermediate in presence of ferricyanide [20]. The 1.0 ml reaction mix contained 50 mM glycyl-glycine buffer (pH 7.6), 2 mM magnesium chloride, 0.1 mM thiamine pyrophosphate, 0.5 mM potassium ferricyanide, 3 mM fructose-6-phosphates (F6P)/hydroxypyruvate (HP), 0.24 mg enzyme and different concentration of A. occidentale L. leaf extracts. The reaction was started by adding PfTk enzyme and extract, and then the ferricyanide reduction was monitored at 420 nm using a UV-1601 PC spectrophotometer (Shimadzu, Japan).
Cultivation of P. falciparum and Testing of Antimalarial Activity of Extracts
The culture of P. falciparum (3D7 strain) was propagated by the method of Trager and Jensen [21]. The parasites were cultured in optimal conditions of 5% CO2 at 37 °C, and maintained in RPMI 1640 supplemented with 0.5% Albumax II (Gibco™ AlbuMAX™), 100 µM hypoxanthine, 0.15% sodium bicarbonate, 25 mM HEPES buffer, 2 mM glutamine and 50 µg/ml gentamicin. The culture haematocrit was maintained at 2% using O+ve RBCs and in routine cultures the parasitaemia was monitored by Giemsa-stained thin blood smears and maintained at 2%–5%. Parasites were synchronized using 5% sorbitol to enrich for ring stage parasites before carrying out inhibition assays.
To test the anti-malarial activity of the extracts, inhibition assays were set up in 96 well plates with 200 µl of culture per well at ~ 2% parasitaemia. First the extracts were tested at 50 µg/ml concentration to identify those that inhibited malaria parasite growth along with the antimalarial drug chloroquine. Subsequently, for the extracts showing > 50% growth inhibition, a dose response assay was run with the starting concentration of the extract at 50 µg/ml and ending at ~ 50 ng/ml in a twofold dilution series. All assays were run in triplicates. After 48 h of incubation in optimal growth conditions, the plates were developed by adding 25 µl of SYBR Green I dye containing 10X lysis buffer. The plates were incubated for 1 h in dark and the fluorescence was measured using a multi-well plate reader with 485 nm and 530 nm as excitation and emission wavelengths.
Zebrafish Embryo Acute Toxicity
Zebrafish (Danio rerio), a member of the Cyprinidae family, originating from South Asia has been used as a model organism for biological research since the 1930s. Zebrafish are easy to breed and inexpensive to maintain and its embryos develop rapidly (5 days post-fertilization). Because the embryo is transparent, morphological structures and internal organs, including brain, eyes, heart, liver, and kidney can be easily visualized using light microscopy, without the need for surgery. Organ-specific and overall developmental toxicity can be assessed visually or quantified using dyes. Because of its small size, a single embryo can be maintained in fluid volumes of 100 µl in individual wells of microtiter plates and compound screening can be completed in a few days. Zebrafish are more closely related to humans and share many biological traits, genes, developmental processes, anatomy, physiology, and behavior. Its genome is ∼50% of the mouse genome and has extensive synteny with mammalian genomes. Further, its genes are ∼75% homologous to human genes on average and it’s orthologs for some genes that are known to play key roles in human diseases have been identified. These features make the zebrafish a unique vertebrate model for high-throughput chemical screening, which is useful for preclinical drug discovery and toxicological evaluation. A number of recent guidelines from the Organisation of Economic Co-operation and Development (OECD; Guidelines 203, 210, and 212; OECD, 1992a,c, 1998) recommended zebrafish for aquatic toxicity testing (For more information please refer [22]).
We used this model for acute toxicity testing of extracts of A. occidentale leaves. Adult zebra fish, obtained from the colony of ICMR-NITM were reared in 3 L housing tanks (10–15) with a light/dark cycle of 14/10 h at 28.5 °C. A newly fertilized embryo was placed in each well of a 24 well tissue culture plate (Corning costar TC) containing 2 ml sterile distilled water per well. Five different concentrations of the leaves extract of A. occidentale were made in the non-toxic volume of dimethyl sulfoxide (DMSO) made up to 100 ml of water. DMSO was used for dissolving the A. occidentale leaf extract. Three replicates of each treatment were run along with a positive control (3,4-dichloroaniline at 4 mg/L). Each plate contained four wells as internal control with water. Solvent control was parallelly maintained. Observations were recorded for lethality indicators. As per the recommendation of the Organization for Economic Co-operation and Development guidelines (OECD Test guidelines 236), four different parameters i.e., coagulation of fertilized eggs, lack of somite formation, lack of detachment of tail bud from the yolk sac, and lack of heartbeat were recorded up to 96 h.
Acute Oral Toxicity Study in Mice
The safety of the A. occidentale leaf extract was assessed through acute oral toxicity in BALB/c mice, after obtaining the approval by the Institutional Animal Ethics Committee (IAEC/ICMR-NTM BGM/2018/1) of the ICMR-NITM. The toxicity test was carried out following the OECD test guidelines No. 423. The animals were obtained from the ICMR-National Institute of Nutrition, Hyderabad, India, and maintained at the ICMR-NITM animal house facility. The mice were divided into 4 groups and each consisting of six mice (female). Three groups were overnight fasted (12 h) after which they were treated orally with different doses of the extracts and designated as test groups. The extracts and fractions were tested at the doses of 50, 300, 2000 mg/kg body weight (bw) and the 4th control group received vehicle (water) treatment. Thereafter, the animals were observed at hourly intervals for any change in general physical activities and behavior (changes in skin fur and eyes, and lethargy, sleep, activity/over activity), as also mortality if any, within 24 h and then daily for the next 14 days. At the end of the study, animals were sacrificed after anaesthetizing with Ketamine and major organs (heart, brain, lung, liver, and kidney) were isolated, their weight was recorded and the tissues were used for histopathology analysis. At the end of the study, animals were sacrificed humanly using ketamin injection and the brain, liver, kidney and lungs were preserved and held in 10% formalin for 2 days. Organs were dehydrated using different concentrations of the alcohol (70, 80, 90%, and absolute alcohol subsequently) for 12 h each. The tissues were cleaned using Xylene solution for 15–20 min and embedded in paraffin blocks. Five-micron sections of the tissue blocks were cut using microtome unit. The sections were placed on a microscopic slide and after drying they were stained with eosin and hematoxylin dyes. The stained sections were examined for infiltration of immune cells, inflammation and congestion if any, under a microscope.
Results
Yield of Extracts of Leaves of A . occidentale and Preliminary Phytochemical Analysis
Leaves of A. occidentale L. were processed and extracted with various solvents. The yield of extracts (% w/w) with various solvents viz., petroleum ether, dichloromethane, chloroform, ethyl acetate, methanol and hydroalcoholic (70: 30 water: ethanol) solvents was 5.2%; 9.20%; 13.00%; 12.50%; 22.25% and17.32%, respectively. Preliminary phytochemical analysis was carried out for the hydro alcoholic extract, since it showed highest inhibition activity against the recombinant transketolase of P. falciparum (as given below). The analysis revealed the presence of alkaloids, glycosides, flavonoids, tannins, and terpenoids at varying amounts (Table 1).
Inhibition of Recombinant PfTk Enzyme by A. occidentale Leaf Extracts
The cloning and expression of recombinant PfTk enzyme was reported in a previous study [7]. The recombinant PfTK, expressed as a His-tagged protein in E. coli (Rosetta cells BL21(DE3)), was purified using Ni–NTA column. The purified protein was analyzed by SDS PAGE to confirm its size as ~ 70 kDa (Fig. 1) and used in inhibition assays for testing the inhibitory activity of different extracts. The hydroalcoholic extract (75.45 ± 0.35%) showed highest inhibitory activity against the enzyme, while chloroform, methanol and petroleum ether extracts showed moderate levels of inhibitory activity (53.00 ± 0.80%, 52.85 ± 1.85% and 35.00 ± 0.09%, respectively). The inhibitory activity of dichloro-methane (22.00 ± 1.50%) and ethyl acetate (18.88 ± 0.20%) extracts was very poor. Based on the inhibitory activity obtained, the hydroalcoholic extract was fractionated and the fractions were tested for activity against P. falciparum blood stage parasites in vitro.
The SDS–polyacrylamide gel of the Ni–NTA purified recombinant P. falciparum transketolase (PfTK). Protein fractions were electrophoresed on a 10% (w/v) SDS polyacrylamide gel under reducing conditions and stained with Coomassie Brilliant Blue R250. Lane 1–Molecular weight marker (Thermo Scientific, range 10–250 kDa), Lane 2–6—purified PfTK
Inhibition of P. falciparum Growth by Hydroalcoholic Extract of A. occidentale Leaf
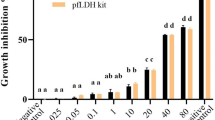
To evaluate the antimalarial activity, the hydroalcoholic extract was tested at different concentrations (from 50 µg/ml to 50 ng/ml). At the highest concentration tested the inhibition was 99.31 ± 0.08% from the fit of the dose response inhibition data, the IC50 was estimated as 4.17 ± 0.22 µg/ml (Fig. 2). Among the fractions of the hydroalcoholic extract tested, six (AO1 to AO6) showed significant inhibition activity (Table 2). Two of the fractions, AO2 and AO6, showed the highest activity, with close to 100% inhibition of parasite growth at 30 µg/ml concentration. A dose response assay was carried out for all the extracts to find out the IC50values. Interestingly, the fractions AO2 and AO6 showed potent antimalarial activity with the estimated IC50 values in the ng/ml range (Fig. 3).
Anti-malarial activity of hydroalcoholic extract of leaves of A. occidentale. The extracts were tested in a dose assay starting with a concentration of 50 µg/ml and going lower by twofold serial dilution. Data from representative assay with two replicates (both plotted separately) are shown. The growth inhibition obtained at 50 µg/ml concentration is 99.31 ± 0.08% with respect to control untreated P. falciparum. The IC50 obtained is 4.17 ± 0.22 µg/ml
Anti-malarial activity for the fractions AO2 (left) & AO6 (right) of hydroalcoholic extracts of A. occidentale leaves against blood stage of P. falciparum strain 3D7. This experiment was carried out in similar manner to that shown in Fig. 2 with the exception that the highest concentration was 30 µg/ml. The growth inhibition obtained at 30 µg/ml concentration for AO2 and AO6 was 99.97 ± 0.004% and 99.96 ± 0.005%,respectively, with respect to control untreated P. falciparum. The IC50 values obtained were 0.56 ± 0.03 µg/ml and 0.63 ± 0.03 µg/ml, respectively
Acute Toxicity Study of A. occidentale Leaves Hydroalcoholic Extract Against Zebra Fish Embryo
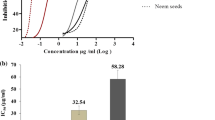
The Zebra fish embryo acute toxicity test was performed using different concentrations of the hydroalcoholic extract of leaves of A. occidentale along with positive (3,4-dichloroaniline) and negative (water) controls. In positive control group there was no somite formation and tail detachment after 24 h of treatment, whereas in A. occidentale leaf extract treatment and water control groups normal heartbeat, somite formation, tail detachment and hatching of embryos was observed (Table 3). However, in fish embryos treated with 100 µg/ml of extract, there was no heartbeat at 96 h. In fish embryos treated with 1000 µg/ml of extract, heartbeat was absent after 48 h only. All the embryos in control group showed no mortality even after 96 h. The LC50 of the extract was determined as 9.60 µg/ml.
Acute Oral Toxicity of A. occidentale Leaves Hydroalcoholic Extract in Mice
The acute oral toxicity of the hydroalcoholic extract of leaves of A. occidentale was tested at doses 50, 300, and 2000 mg/kg body weight in BALB/c mice. The results revealed that 3 different doses of extract tested did not incite any mortality and morbidity throughout the experimental period. Behavioral and physical observations of the experimental mice were found normal and did not exhibit any visible signs of abnormality (Table 4). Histopathological analysis of different organs of mice treated with different doses of the extract did not show any significant changes in the heart, liver, kidney, and brain. However, slight congestion of the lung was observed at 2000 mg/kg (Fig. 4). Sections of mice Brain, heart, kidney, liver, lung were stained with haematoxylin and eosin (scalebar = 100 μm). Mice receiving AO 50 mg, 300 mg, 2000 mg brain showed a normal histological structure of different neurons in the cerebral cortex, hippocampus and striatum. Normal atrium and ventricle are evident from mice treated with AO 50 mg, 300 mg, 2000 mg. Normal architecture of renal corpuscles with their glomeruli and renal tubules was seen after treatment with AO. Normal hepatocytes, central vein and sinusoidal space at magnification 40 × were observed. No haemorrhage or apparent tissue damage was found in the liver of mice in AO 50 mg and 300 mg treated groups, but in AO 2000 mg destruction of the vessel wall and focal haemorrhage (congestion) of lung were observed. Thus, the extract was found to be safe atleast up to 300 mg/kg.
Toxicity of hydroalcoholic extracts of A. occidentale leaves in mice. Histopathological analysis of different organs of mice treated with different doses (50, 300 and 2000 mg/kg body weight in BALB/c mice) of the extract did not show any significant changes in the heart, liver, kidney, and brain. Destruction of the vessel wall and focal haemorrhage (congestion) (indicated by ring) of the lung was observed at the highest dose (2000 mg/kg)
Discussion
Many antimalarial drugs such as chloroquine, mefloquine, amodiaquine, quinine, proguanil, sulfadoxine and pyrimethamine, primaquine, artemether, artesunate, arteether that target different stages of malaria parasites have been in use for treating clinical malaria cases. But malarial parasites have developed resistance against several of these drugs and currently there are no alternate options other than arteether. Artemisinin, initially extracted from the Chinese herb Artemisia annua, highlights the potential of plants as a source of chemotherapeutics. Artemisinin-based combination therapies (ACT) are currently being used in endemic countries worldwide. Over the past decade, unprecedented progress has been made in reducing malaria morbidity and mortality [23]. However, growing resistance to ACTs by malarial parasites, coupled with resistance to insecticides used in the control mosquito vectors, have negatively impacted the malaria control and long-term prospects for elimination [24]. P. falciparum has developed resistance to most antimalarial drugs in use, as also to the current ACT [25, 26]. Drug resistance development appears to be due probably to heavy drug use, parasite selection, cross resistance and genetic influences of drugs. Pharmaceutical companies do not evince interest in developing drugs for malaria and this is a matter of great concern, in the face of increasing drug resistance [27].There are very few drugs in various phases of trials in the thin pipeline of candidate antimalarials for treating malaria. Therefore, there is an urgent need to develop drugs against malaria parasites to augment the malaria control program. Herbal preparations have been traditionally used for treating certain infections, including malaria. Exploring herbal sources may yield new anti-malarial drugs and the discovery process can be hastened by adopting a targeted approach. Therefore, we attempted an approach which involved a drug target in malarial parasite and herbal source for discovering drug lead(s) for malaria.
We explored the possibility of A. occidentale leaves as source of anti-malarial drug(s), with the parasite transketolase enzyme as the target. We chose transketolase of P. falciparum as the target as it has been reported to play a vital role in the survival/replication of the malaria parasites [28]. It is a key enzyme of the non-oxidative arm of pentose phosphate pathway (PPP), which is an important metabolic pathway for yielding reducing agents in the form of NADPH and the production of pentose sugar needed for nucleic acid synthesis. Further, it exhibits very low similarity to the homologue in human host, making it a selective target for anti-malarial drug development. Key drug targets of P. falciparum such as dihydrofolate reductase (DHFR), Cytochrome b (CYTb), dihydroorotate dehydrogenase (DHOD) and Myosin A tail domain-interacting protein (MTIP) have been used for identifying drug leads. Notably, three tRNA synthetases were successfully used in identifying two potent antimalarial natural products viz., cladosporin and halofuginone [29].
Here, we have explored the leaves of A. occidentale (cashew) because of its reported medicinal properties and use in traditional medicine practice [10]. Unlike other studies in which the fruit, bark and other plant parts were tested, we have used the leaves for exploring the presence of anti-malarial compounds as it is readily available in large quantities, renewable and usually disposed off as a waste. According to the concept of green chemistry, it is desirable that an abundant and cheap source of raw material, especially natural waste product, should be utilized as it also ensures environmental friendliness. Leaves or nutshell extracts from cashew have long been used as a folk medicine to treat inflammation and other conditions including asthma, ulcers, and cancer.
In this study, we utilized the recombinant PfTk enzyme as a target for initial screening for the inhibitory activity of the extracts/fractions of the leaves of A. occidentale. Out of six extracts of leaves of A. occidentale prepared using different solvents, the hydroalcoholic extract was found to have highest enzyme inhibition activity. Therefore, this extract was tested against late-ring and early trophozoite stages of P. falciparum 3D7 strain in fluorescence based in vitro assay, where the extract showed a very high activity, both in terms of growth inhibition (99.31 ± 0.08% at 50 µg/ml) and IC50 (4.17 ± 0.22 µg/ml). The percentage inhibition activity is higher than that reported for 80% ethanol extracts of several plants of Tanzania and extract of Berasama abyssinica, which gave a maximum of 86.67 ± 11.32% activity when tested at 100 µg/ml against P. falciparum Dd2 strain [30]. An ethanol extract of B. abyssinica leaves was reported to have good in vitro anti-plasmodial activity with IC50 of 2.5 µg/ml [31], while methanolic extract of stem bark of the same plant had IC50 of 11.00 µg/ml [32]. A chloroform extract of Maesa lanceolate was found to have very good anti-plasmodial activity with IC50 of 2.5 µg/ml against clinical isolates of P. falciparum [33]. The anti-plasmodial activity of plant extracts may vary depending upon the plant and its parts, geographic location of the plant etc. Bark, leaves, fruit infusions and decoction have been used in traditional medicine as anti-malarial treatments [8]. Several medicinal plants, including A. occidentale L. are being used by various tribes of Bankura, West Bengal, India for the treatment of malaria [9]. But, extracts prepared from A. occidentale L. were reported to be inactive in vitro against P. falciparum by Lima et al. [8]. However, in the present study the hydroalcoholic extract of leaves of A. occidentale showed anti-malarial activity in vitro against P. falciparum. Based on the IC50 it’s activity can be considered as moderate as per the classification of Lemma et al. [34], who categorized the herbs as very good, good, moderate and inactive if the IC50 values were < 0.1 μg/ml, 0.1-1 μg/ml, > 1-5 μg/ml and > 5 μg/ml, respectively.
The hydroalcoholic extract of leaves of A. occidentale did not affect the heartbeat, somite formation, tail detachment and hatching of Zebra fish embryos in acute toxicity test. This indicates the safety of extract in this animal model. Similarly, the extract when tested at doses 50–2000 mg/kg body weight in BALB/c mice, it was found to be safe to without causing any mortality and morbidity or change in behavioral and physical activities. Histopathological analysis of major organs of extract treated mice did not show any changes, except lung showing congestion at a very high dose (2000 mg/kg) (Fig. 4). The ethanolic extract of stem bark of A. occidentale was reported to play a modulatory role in the inflammatory response mediated by the down regulation of IL-1 and TNF-α [35], In the current study, the extract caused lung congestion in mice which may be due to excessive dose administered (2000 gm/kg body weight), which might have resulted in severe immunomodulation. However, it can be concluded that the hydroalcoholic extract of leaves of A. occidentale was found to be safe up to a dose of 300 mg/kg.
Bioactivity guided fractionation of this extract was done and upon testing, two of the fractions (AO2 & AO6) showed very promising antimalarial activity against blood stage parasites. As stated above A. occidentale L. is well recognized as a rich source of alkyl-phenols, which have several biological activities [15]. Further studies are required to identify the compound responsible for the observed antimalarial activity in the leaves of A. occidentale and delineate the mechanism by which the activity of PfTK enzyme is inhibited. Identifying the compounds from the extracts with anti-malarial activity will help to develop suitable formulations as a phytopharmaceutical drug which can then be further taken up for preclinical and clinical studies, and eventually for clinical practice either through traditional route (such as Ayurveda or Siddha) and allopathic route.
Conclusion
The in vitro assays showed that the hydroalcoholic extract of leaves of A. occidentale has promising activity against P. falciparum and its target enzyme Transketolase. The extract and its two fractions of the extract showed significant activity against P. falciparumin in vitro tests. The extract was found to be non-toxic in preliminary toxicity studies in Zebra fish embryos and mice. Therefore, because of the anti-malarial potential and that the leaves of A. occidentale can be obtained in large quantities perennially, it is worthwhile to explore it as a natural anti-malarial drug source.
Data availability
The raw data is available with the senior author (M.K).
References
World Health Organization. World Malaria report – 2021. Page 18. https://www.who.int/news-room/fact-sheets/detail/malaria. Accessed Feb 2022
Cui L, Mharakurwa S, Ndiaye D, Rathod PK, Rosenthal PJ (2015) Antimalarial drug resistance: literature review and activities and findings of the ICEMR network. Am J Trop Med Hyg 93(3 Suppl):57. https://doi.org/10.4269/ajtmh.15-0007
Talapko J, Škrlec I, Alebić T, Jukić M, Včev A (2019) Malaria: the past and the present. Microorganisms 7(6):179. https://doi.org/10.3390/microorganisms7060179
Yasri S, Wiwanitkit V (2021) Artemisinin resistance: an important emerging clinical problem in tropical medicine. Int J Physiol Pathophysiol Pharmacol 13(6):152–157
Ariey F, Witkowski B, Amaratunga C, Beghain J, Langlois AC, Khim N et al (2014) A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature 505:50–55. https://doi.org/10.1038/nature12876
Selzer PM, Brutsche S, Wiesner P, Schmid P, Müllner H (2000) Target-based drug discovery for the development of novel anti-infectives. Int J Med Microbiol 290:191–201. https://doi.org/10.1016/S1438-4221(00)80090-9
Joshi S, Singh AR, Kumar A, Misra PC, Siddiqi MI, Saxena JK (2008) Molecular cloning and characterization of Plasmodium falciparum transketolase. Mol Biochem Parasitol 160(1):32–41. https://doi.org/10.1016/j.molbiopara.2008.03.005
Lima RB, Silva LF, Melo MR, Costa JS, Picanço NS, Lima ES, Vasconcellos MC, Boleti AP, Santos JM, Amorim RC, Chaves FC (2015) In vitro and in vivo anti-malarial activity of plants from the Brazilian Amazon. Malaria J 14(1):1–4. https://doi.org/10.1186/s12936-015-0999-2
Sinhababu A, Banerjee A (2015) Medicinal plants for the treatment of malaria used by various tribes of Bankura, West Bengal, India. Res Rev: J Bot 4(1):33–36
Salehi B, Gültekin-Özgüven M, Kirkin C, Özçelik B, Morais-Braga MFB, Carneiro JNP, Bezerra CF, Silva TG, Coutinho HDM, Amina B, Armstrong L, Selamoglu Z, Sevindik M, Yousaf Z, Sharifi-Rad J, Muddathir AM, Devkota HP, Martorell M, Jugran AK, Cho WC, Martins N (2020) Antioxidant, antimicrobial, and anticancer effects of Anacardium plants: an ethnopharmacological perspective. Front Endocrino 11:295. https://doi.org/10.3389/fendo.2020.00295
Kubo I, Komatsu S, Ochi M (1986) Molluscicides from the cashew Anacardium occidentale and their large-scale isolation. J Agric Food Chem 34:970–97300295. https://doi.org/10.1021/jf00072a010
Himejima M, Kubo I (1991) Antibacterial agents from the cashew Anacardium occidentale (Anacardiaceae) nut shell oil. J Agric Food Chem 39(2):418–421. https://doi.org/10.1021/jf00002a039
Kubo I, Nitoda T, Tocoli FE, Green IR (2011) Multifunctional cytotoxic agents from Anacardium occidentale. Phytother Res 25:38–45. https://doi.org/10.1002/ptr.3109
Oliveira MS, Morais SM, Magalhães DV, Batista WP, Vieira IG, Craveiro AA, de Manezes JE, Carvalho AF, de Lima GP (2011) Antioxidant, larvicidal and anti acetylcholinesterase activities of cashew nut shell liquid constituents. Acta Trop 117:165–170. https://doi.org/10.1016/j.actatropica.2010.08.003
Alvarenga TA, de Oliveira PF, de Souza JM, Tavares DC, Andrade E, Silva ML, Cunha WR, Groppo M, Januário AH, Magalhães LG, Pauletti PM (2016) Schistosomicidal activity of alkyl-phenols from the Cashew Anacardiumoccidentale against Schistosoma mansoni adult worms. J Agric Food Chem 64:8821–8827. https://doi.org/10.1016/j.bjp.2018.11.003
Gimenez VM, Alvarenga TA, Groppo M, Silva ML, Cunha WR, Januário AH, Smilkstein MJ, Riscoe MK, Pauletti PM (2019) Antiplasmodial evaluation of Anacardium occidentale and alkyl-phenols. RevistaBrasileira de Farmacognosia 29:36–39. https://doi.org/10.1016/j.bjp.2018.11.003
Shaikh JR, Patil MK (2020) Qualitative tests for preliminary phytochemical screening: an overview. Int J Chem Stud 8(2):603–608. https://doi.org/10.22271/chemi.2020.v8.i2i.8834
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275. https://doi.org/10.1016/S0021-9258(19)52451-6
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(5259):680–685. https://doi.org/10.1038/227680a0
Kochetov GA (1982) Determination of transketolase activity via ferricyanide reduction. Methods Enzymol 89:43–44. https://doi.org/10.1016/S0076-6879(82)89009-5
Trager W, Jensen JB (1976) Human malaria parasites in continuous culture. Science 193(4254):673–675. https://doi.org/10.1126/science.781840
Zhang C, Willett C, Fremgen T (2003) Zebrafish: an animal model for toxicological studies. Current Protocols in Toxicology UNIT 1.7 (2003) 1.7.1–1.7.18, 2003 John Wiley & Sons, Inc
Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, Dalrymple U et al (2015) The effect of malaria control on P. falciparum in Africa between 2000 and 2015. Nature 526:207–211. https://doi.org/10.1038/nature15535
The malERA Refresh Consultative Panel on Insecticide and DrugResistance (2017) The malERA refresh consultative panel on insecticide and drug resistance malERA: an updated research agenda for insecticide and drug resistance in malaria elimination and eradication. PLoS Med 14(11):e1002450. https://doi.org/10.1371/journal.pmed.1002456
Antony HA, Parija SC (2016) Antimalarial drug resistance: an overview. Trop Parasitol 6(1):30. https://doi.org/10.4103/2229-5070.175081
Menard D, Dondorp A (2017) Antimalarial drug resistance: a threat to malaria elimination. Cold Spring Harbor Perspect Med 7(7):a025619. https://doi.org/10.1101/cshperspect.a025619
KagerP A,Wetsteyn JC Malaria and drug resistance. Ed. TijdschrGeneeskd, 1996, 20;140:151–5]. PMID: 8618636.
Hasan M, Mazumder M, Hasan H, Chowdhury AS, Datta A, Khan M (2015) Molecular-docking study of malaria drug target enzyme transketolase in Plasmodium falciparum 3D7 portends the novel approach to its treatment. Source Code Biol Med 10(1):1–4. https://doi.org/10.1186/s13029-015-0037-3
Flannery EL, Chatterjee AK, Winzeler EA (2013) Antimalarial drug discovery—approaches and progress towards new medicines. Nat Rev Microbiol 11(12):849–862. https://doi.org/10.1038/nrmicro3138
Nondo RS, Zofou D, Moshi MJ, Erasto P, Wanji S, Ngemenya MN, Titanji VP, Kidukuli AW, Masimba PJ (2015) Ethnobotanical survey and in vitro antiplasmodial activity of medicinal plants used to treat malaria in Kagera and Lindi regions. Tanzania J Med Plants Res 9(6):179–192. https://doi.org/10.5897/JMPR2014.5685
Ngemenya M, Titanji V, Akam T, Yong N, Tane P, Fanso-free S, Berzins K (2005) Antiplasmodial activity and toxicity of extracts and products from selected medicinal plants used in Cameroon [MIM-MN-187588]. Acta Trop 95:S193–S194. https://doi.org/10.1155/2022/4661753
Kassa M, Mohana R, Hunde A (1996) Antimalarial activity of Bersama abyssinica against Plasmodium falciparum. Ethiop Phar J 14:16–21
Katuura E, Waako P, Tabuti JR, Bukenya-Ziraba R, Ogwal-Okeng J (2007) Antiplasmodial activity of extracts of selected medicinal plants used by local communities in western Uganda for treatment of malaria. Afr J Ecol 45:94–98. https://doi.org/10.1111/j.1365-2028.2007.00864.x
Lemma MT, Ahmed AM, Elhady MT, Ngo HT, Vu TL, Sang TK, Campos-Alberto E, Sayed A, Mizukami S, Na-Bangchang K, Huy NT (2017) Medicinal plants for in vitro antiplasmodial activities: a systematic review of literature. Parasitol Int 66(6):713–720. https://doi.org/10.1016/j.parint.2017.09.002
Vilar MS, de Souza GL, Vilar Dde A, Leite JA, Raffin FN, Barbosa-Filho JM, Nogueira FH, Rodrigues-Mascarenhas S, Moura TF (2016) Assessment of phenolic compounds and anti-inflammatory activity of ethyl acetate phase of Anacardium occidentale L. Bark. Molecules 21(8):1087. https://doi.org/10.3390/molecules21081087
Acknowledgements
We thank the Department of Science and Technology, New Delhi for providing Women Scientist A fellowship (SR/WOS-A/LS-456/2016) is gratefully acknowledged.
Funding
Department of Science and Technology, New Delhi, SR/WOS-A/LS-456/2016, Meenakshi Kaushik.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kaushik, M., Hoti, S.L., Saxena, J.K. et al. Antimalarial Activity of Anacardium occidentale Leaf Extracts Against Plasmodium falciparum Transketolase (PfTK). Acta Parasit. 68, 832–841 (2023). https://doi.org/10.1007/s11686-023-00718-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11686-023-00718-6