Abstract
Banana is a tropical fruit that suffers from several postharvest diseases during transportation and storage. Anthracnose caused by the fungus Colletotrichum musae is the most destructive postharvest disease of banana. The aim of this study was to determine the antifungal activities of Aloe vera (AV) gel coating alone or in combination with garlic oil (GO) at two concentrations (AV + GO 0.05% and AV + GO 0.1%) in vitro and in vivo against anthracnose disease of banana fruit. The results showed that the AV gel coating incorporated with GO was more effective as a fungicide than AV gel alone. The highest antifungal activity was observed in AV + GO 0.1% treatment, which significantly inhibited the mycelial growth and spore germination by 87.7 and 91.2%, respectively, compared to the control. In vivo study indicated that AV gel combined with GO 0.1% effectively reduced anthracnose disease incidence (92.5%) and severity (81.0%) in artificially inoculated banana fruit after 15 days of storage. The same treatment delayed the changes in weight loss, firmness, soluble solids concentration, and titratable acidity. Moreover, AV gel coating and GO enhanced total phenolic contents and total antioxidant activities of banana fruit. These results suggested that AV gel combined with GO can be used as an effective biofungicide for controlling anthracnose disease of banana fruit.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Banana is the most popular tropical fruit and is economically important for local markets as well as for export worldwide. It is a highly perishable fruit and deteriorates rapidly after harvesting due to adverse physiological changes (Anthony et al. 2003). Banana is very susceptible to various pathogenic infections during storage and marketing. Anthracnose is one of the major postharvest diseases of banana caused by the pathogenic fungus Colletotrichum musae (Berk. and Curtis) Arx, resulting in significant economic losses (Ranasinghe et al. 2003). The fungus invades the immature banana in the field, where anthracnose disease symptoms appear at the advance stage of ripening, including lesions that look like brown sunken or black spots covered with salmon-coloured acervuli (Chillet et al. 2007).
Postharvest diseases of banana fruit are commonly controlled using chemical fungicides. However, the extensive application of chemical fungicides has led to environmental pollution, and fungicide residue on the fruit surface poses a great health risk to consumers. These ill effects of chemical fungicides have led to the development of alternative strategies for reducing postharvest diseases that pose minimal danger to the environment and human health. Recently, natural plant extracts have gained considerable attention due to their ability to prevent pathogenic infections and prolong the shelf life of fruits. They have a negligible effect on the environment and present little risk to human health compared to chemical fungicides. Natural plant extracts contain secondary metabolites, for example, phenols, flavonoids, alkaloids, or terpenoids, and have been used for centuries as a way to control postharvest fungi (Daniel et al. 2015).
Aloe vera gel is a biodegradable natural preservative use for reducing microbial growth and maintaining quality of fresh produce during storage. The mucilaginous gel of A. vera plants has been effective against gastrointestinal, ulcerous, kidney, and cardiovascular problems (Eshun and He 2005). The chemical composition of A. vera gel is mainly composed of polysaccharides, soluble sugars, phenolic compounds, enzymes, vitamins, amino acids, anthraquinones, and saponins (Boudreau and Beland 2006). When used as an edible coating, A. vera gel showed antifungal properties and inhibited decay incidence in grapes (Castillo et al. 2010), raspberry (Hassanpour 2015), and sour cherry (Ravanfar et al. 2014).
Essential oils obtained from plant materials have antimicrobial and fungicidal properties against several postharvest diseases of fruit and vegetables (Patrignani et al. 2015). Essential oils are gaining popularity due to their consumer acceptability and eco-friendly properties (Tzortzakis and Economakis 2007). Many studies showed that application of essential oil treatment inhibited the anthracnose disease in fruits, for example, avocados, mango, and papaya (Sellamuthu et al. 2013; Rabari et al. 2018; Sarkhosh et al. 2018). Garlic (Allium sativum L.) has been used in many cultures as a traditional medicine to improve the immune system, control cholesterol level, and as a treatment for cancer. Garlic comprises more than 200 compounds, some of which are enzymes, vitamins, proteins, minerals, saponins, and flavonoids (Goncagul and Ayaz 2010). Garlic essential oil has been used to inhibit plant pathogens. It was reported that garlic extract controlled anthracnose disease of pepper (Obagwu et al. 1997).
The incorporation of essential oils into polymer coatings is well known and confirmed to prevent microbial growth. The cinnamon oil incorporated into gum arabic and propolis edible coatings reduced the anthracnose disease of chili in vitro and in vivo (Ali et al. 2014). Similarly, the combined application of thyme oil and chitosan coating increased resistance against anthracnose disease in avocado fruit stored at low temperature (Bill et al. 2014). Plant extracts and essential oils are rich sources of biofungicides and efficiently control a wide range of fungi in fruit and vegetables. So, the aim of this study was to elucidate the effect of A. vera gel coating and garlic essential oil on anthracnose disease development and postharvest quality of banana fruit during storage.
2 Materials and methods
2.1 Fruit materials
Banana (Musa sp. cv. Basarai) fruit at hard green mature stage were harvested from a commercial orchard located beside the Lasbela University of Agriculture, Water and Marine Science, Uthal, Balochistan, Pakistan and immediately transferred to the postharvest laboratory. Banana hands were selected that were free from visual defects and uniform in size, colour, and ripeness.
2.2 Isolation of the causal pathogen
Banana samples showing clear anthracnose symptoms were collected, and the causal pathogen C. musae was isolated by a single-spore isolation technique. The infected tissues were aseptically cut into small pieces (5 mm2) and disinfected with 0.01% sodium hypochlorite solution for 3 min. After that, the tissues were rinsed three times with distilled water and left to air-dry. The isolates were then transferred to sterile Petri dishes containing potato dextrose agar (PDA) medium. The Petri dishes were incubated at 25 °C and observed daily. To obtain pure fungus colonies, the fungus was subcultured on fresh PDA dishes. The pathogens were identified by microscope based on their cultural and morphological properties. The isolated pure fungi were maintained on PDA dishes at 4 °C for further studies.
2.3 A. vera gel coating and garlic essential oil
A. vera gel was obtained following the method of Navarro et al. (2011). Fresh A. vera leaves were harvested and transferred to the postharvest laboratory. The thick outer skin was removed to obtain the inner gel from each leaf. The gel was ground using a blender and filtered to discard fibrous tissue. The gel matrix was used for A. vera treatment. The garlic (Allium sativum) essential oils were purchased from Malkani oil products (Pvt) Karachi, Pakistan. Treatments for the experiment were as follows: (1) purified water as a control (2) AV (3) AV + GO 0.05%, and (4) AV + GO 0.1%.
2.4 In vitro antifungal assay
The antifungal effects of AV gel alone or combined with GO were tested based on the inhibition in radial mycelia growth on sterilized PDA media. Five-millimetre fungal discs from a pure culture of C. musae were cut with a sterile cork borer from the edge of actively growing culture and placed in the centre of a Petri dish comprising PDA mixed with AV, AV + GO 0.05%, and AV + GO 0.1%. PDA Petri dishes without plant extracts served as control. Petri dishes were incubated at 25 ± 2 °C for 7 days. The mycelia growth was observed every 24 h until fungal growth in the control filled the Petri dishes completely.
The spore germination inhibition test was conducted in vitro according to the method of Cronin et al. (1996). An aliquot (50 μL) of each of the AV, AV + GO 0.05%, and AV + GO 0.1% concentrations were transferred to a cavity slide. A spore suspension (50 μL) adjusted to 105 spore mL−1 using a hemocytometer was pipetted into the cavity slide. The slide was covered with a cover slip and incubated at 25 ± 2 °C for 24 h. Cavity slides containing sterile distilled water served as the control. Spore germination was observed under a microscope. At least 100 spores of each replicate were recorded. A spore was considered germinated if the length of the germ tube was equal to or longer than the spore diameter. The results were expressed on a percentage basis. Percentage of spore germination inhibition was calculated according to the following formula:
where gc is the spore germination in the control and gt is the spore germination in the treated Petri dish.
2.5 In vivo evaluation
The antifungal assay of AV gel coating and GO on anthracnose disease of banana fruit was determined in vivo. Banana fruit were surface sterilized with 0.01% sodium hypochlorite solution, washed with sterile distilled water, and air-dried at room temperature. Surface-sterilized banana fruit were uniformly wounded at two points with a sterilized cork borer (4-mm diameter) to a depth of 1–2 mm. Each wound site was then inoculated with 20 μL of spore suspension (105 spores mL−1) of C. musae and kept at ambient temperature for drying. Pathogen-inoculated banana fruit were then immersed in AV, AV + GO 0.05%, and AV + GO 0.1% solutions for 2–3 min. Control fruit were dipped in sterile distilled water without AV gel and GO treatments. The fruit were kept at ambient temperature to completely air-dry. After treatments, bananas were placed in plastic trays covered with a transparent polythene film and stored at 20 ± 2 °C and 80–90% relative humidity for 15 days. The effect of each treatment on disease incidence (DI) and disease severity (DS) was assessed after 3 days. Banana anthracnose disease incidence data were expressed as percentage of fruit indicating disease symptoms out of the total number of fruit in each treatment. Severity of the disease was assessed based on visual symptoms as described by Khaliq et al. (2016). The total disease symptoms of each fruit surface was scored on a 0–5 scale where 0 = no visible disease, 1 = 1–10% disease area, 2 = 11–25% disease area, 3 = 26–40% disease area, 4 = 40–50% disease area, and 5 = > 50% disease area.
2.6 Determination of postharvest quality
Physiological loss in weight was determined by weighing individual fruit initially and during each sampling day. The same fruit were measured each time. Weight loss was expressed as percent loss of initial weight. Fruit firmness was assessed following the method of Zheng et al. (2012) with slight modification. Fruit firmness of individual fruit was subjectively measured using a rating scale of 10 = hard, 8 = sprung, 6 = slight soft, 4 = soft, and 2 = oversoft. Firmness index was calculated using the following formula:
Soluble solids concentration was determined using a handheld refractometer (Alla 950032 B-ATC, France). Soluble solids concentration was expressed as a percentage of °Brix. titratable acidity was determined by the titration method. Ten grams of banana pulp was homogenized with 40 mL of distilled water and filtered through cheese cloth. The acidity was determined by titration with 0.1 mol L−1 NaOH to pH 8.1, using phenolphthalein (0.1%) as the indicator. The results were expressed as the percentage of citric acid per 100 g of fresh weight.
2.7 Measurement of total phenolic contents
The total phenolic contents in banana fruit were measured using the Folin–Ciocalteu method as described by Choi et al. (2006). Five grams of banana flesh sample was homogenized in 40 mL of 80% methanol and kept in a shaker for 1 h. The extracts were filtered using a double layer of cheese cloth. About 1 mL of sample extract was mixed with 1 mL of Folin–Ciocalteu reagent and allowed to stand for 10 min. Then, 10 mL of 7% sodium carbonate solution was added to the mixture. The solution was added to 13 mL of distilled water and mixed gently. After standing at room temperature for 90 min, the absorbance was read at 760 nm using a spectrophotometer (UV-1602, BMS, Canada) against the blank. A standard curve was prepared from various concentrations of gallic acid to estimate the total phenolic contents. The results were expressed as milligrams of gallic acid equivalent (GAE) per gram of fresh weight.
2.8 Determination of antioxidant activity
The 2,2-diphenyl-1-picryhydrazyl (DPPH) assay was used to measure radical scavenging activity as described by Brand-Williams et al. (1995). Briefly, 0.1 mL of banana flesh extract was mixed with 3.9 mL of 0.063 mM DPPH solution. The mixture was left in the dark at room temperature for 20 min, and the absorbance was read at 517 nm. The control was prepared as above without any sample extract. The capacity to scavenge DPPH radical was calculated by the following formula:
The ferric reducing antioxidant power (FRAP) assay was measured following the method of Benzie and Strain (1996). Briefly, the FRAP reagent was prepared by mixing 50 mL of acetate buffer (300 mM) at pH 3.6, 5 mL of 2,4,6-tripyridyl-s-triazine (TPTZ) solution (10 mM) prepared in hydrochloric acid (40 mM), and 5 mL of ferric chloride solution (20 mM). Three millilitres of FRAP reagent was added to 40 μL of sample extracts and incubated at 37 °C for 10 min. Absorbance was recorded at 593 nm against a control. The control sample contained 3 mL of FRAP reagent and 40 μL of 80% methanol. The standard curve was constructed using Trolox. The results were expressed as micromoles of trolox equivalent (TE) g−1 of fresh weight.
The 2,2-azinobis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) radical scavenging assay was conducted following the method of Re et al. (1999). ABTS reagent was prepared by mixing of 5 mL of 7 mM ABTS and 88 μL of 140 mM potassium persulfate solution. The mixture was allowed to stand in the dark at room temperature for 16 h. After incubation, the ABTS reagent was diluted with 50% methanol for an initial absorbance of about 0.70 ± 0.05 unites at 734 nm. After the addition of 30 μL sample extract or standard to 3 mL of ABTS reagent, the decrease of absorbance at 734 nm was measured at the end point of 6 min. A standard curve was obtained using an aqueous solution of Trolox. The results were expressed as micromoles of trolox equivalent (TE) g−1 of fresh weight.
2.9 Statistical analysis
All the experiments were conducted in a completely randomized design (CRD) with four replications. The data were subjected to analysis of variance using SAS version 9.1 software. The means were compared with Duncan’s multiple range test (DMRT) where significant differences occurred. For in vitro evaluation, five plates in each replicate were used; for in vivo and physicochemical studies, each replicate contained 30 fruit.
3 Results and discussion
3.1 In vitro antifungal assay of AV and GO
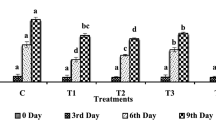
The results showed that A. vera gel coating and garlic essential oil had considerable antifungal activities against C. musae. A. vera gel coating alone or combined with garlic essential oil significantly inhibited the mycelial growth of C. musae compared to the untreated fruit (Fig. 1a). The maximum mycelial growth inhibition (87.7%) occurred in fruit treated with AV + GO 0.1% followed by AV + GO 0.05% (69.4%) and AV gel coating (32.7%). There was no significant effect between AV + GO 0.1% and AV + GO 0.05% treatments during the 7-day incubation period. However, the fungicidal activity of garlic essential oil against C. musae at a higher concentration (0.1%) was more evident. The results of in vitro bioassay indicated that AV gel coating and GO treatments were effective against the spore germination of C. musae. The spore germination was significantly (p ≤ 0.05) suppressed by the AV gel coating alone and combined with GO treatment (Fig. 1b). The composite treatments of AV and GO indicated more significant effects against the spore germination of C. musae compared to AV gel coating alone or control. The combined treatment of AV + GO 0.1% inhibited the spore germination up to (91.2%) followed by AV + GO 0.05% (80.1%) and AV gel coating alone (55.5%).
Effect of A. vera gel coating and garlic essential oil on growth of the fungi C. musae for 7 days of incubation at 25 °C (a) and spore germination inhibition of C. musae for 24 h (b). Vertical bars represent standard deviation of means for four replicates. Means with different letters for each day and among treatments are significantly different at p < 0.05 using Duncan’s multiple range test. AVAloe vera, GO garlic oil
A. vera gel has many favourable properties, such as nontoxicity, high stability, hydrophilicity, and gel-forming abilities. It has been stated that A. vera gel coating suppressed the mycelial growth of Colletotrichum coccodes, Fusarium oxysporum, and Rhizoctonia solani (Jasso de Rodríguez et al. 2005). Similarly, A. vera gel significantly reduced the growth of Penicillium digitatum, Rhizopus stolonifera, and Botrytis cinerea of two nectarine cultivars (Navarro et al. 2011). The composite treatment of thyme oil and AV gel coating significantly suppressed the mycelia growth of Colletotrichum gloeosporioides and the addition of thyme oil into the AV gel enhanced the coating properties, and reducing the incidence of anthracnose disease in avocado fruit during storage (Bill et al. 2014). It is well known that A. vera gel inhibits bacteria, fungi, and viruses. One of the main biologically active ingredients of A. vera gel is aloin or barbaloin, and the antifungal activity of A. vera gel is associated with this compound (Alves et al. 2004).
Essential oil has antifungal activity against numerous fungi, such as R. stolonifera, Phytophthora citrophthora, Penicillium expansum, and B. cinerea (Camele et al. 2012). It has been reported that thyme essential oil controlled mycelial growth of C. musae in vitro and reduced disease severity in vivo of banana fruit during storage (Vilaplana et al. 2018). Benkeblia (2004) observed that garlic essential oil has antimicrobial properties and inhibited the activities of three fungi, Fusarium oxysporum, Aspergillus niger, and Penicillium cyclopium. Similarly, garlic essential oil showed good results against Colletotrichum sp., Phytophthora infestans, and Fosarium oxysporum (Seo et al. 2006). Essential oils consist of a complex mixture of ingredients, and it is hard to attribute the antifungal mode of action to one specific mechanism. It seems that they may cause breakdown of cell membrane, leakage of ions, depletion of proteins, deactivation of enzymes, and damage of genetic material (Burt 2004). The antifungal and antibacterial properties of garlic have been ascribed to the presence of active ingredients such as allicin, alliin, ajoene, dithiin, and diallylsulfide (Goncagul and Ayaz 2010). In this study, the antifungal activities of AV gel coating and GO could be attributed to the existence of bioactive compounds that inhibited the mycelial growth and spore germination of C. musae.
3.2 In vivo antifungal assay of AV and GO
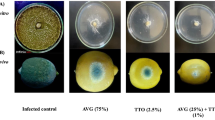
The results revealed that anthracnose disease symptoms of banana fruit gradually increased in all fruit as the storage period progressed (Fig. 2a). However, treated fruit significantly inhibited the development of anthracnose disease symptoms compared to the control throughout the storage period. Anthracnose disease incidence was 25.1%, 78.7%, and 92.5% lower in fruit subjected to AV, AV + GO 0.05%, or AV + GO 0.1%, respectively, than the control at the end of storage period. AV gel coating alone or added with GO exhibited higher fungicidal activities than the control (Fig. 2b). AV, AV + GO 0.05%, and AV + GO 0.1% significantly lowered disease severity in banana fruit to 30.0%, 60.1%, and 81.0%, respectively, compared to the untreated fruit at the end of the storage period. The highest antifungal effect was noted in the composite treatment of AV gel coating and GO during the whole storage period.
Effect of A. vera gel coating and garlic essential oil on disease incidence (a) and disease severity (b) of inoculated banana fruit during storage at 20 °C for 15 days. Vertical bars represent standard deviation of means for four replicates. Means with different letters for each day are significantly different at p < 0.05 using Duncan’s multiple range test
The in vitro assessment is essential to find out the antifungal properties of plant extracts against microorganisms. However, it is necessary to verify the positive effects of in vitro tests by in vivo investigations. Edible coatings retard the development of undesirable pathogens and can be used to control postharvest disease of fruits. Gum arabic coatings in combination with lemongrass and cinnamon oils indicated fungicidal effects against C. gloeosporioides and C. musae, causal pathogens of papaya and banana anthracnose, respectively (Maqbool et al. 2011). The specific mode of action of A. vera gel is not fully understood; however, the antifungal activity might be attributed to the presence of aloe-emodin and aloenin compounds along with other active ingredients (Ali et al. 1999).
The causal pathogen of banana anthracnose disease was controlled by the application of clove and cinnamon oils (Ranasinghe et al. 2003). These results are in agreement with the findings of Begum et al. (2015), who reported that garlic essential oil inhibited the mycelial growth of Colletotrichum capsici in vitro and significantly reduced disease severity of chili. Similarly, garlic extracts and clove oil potentially reduced decay of artificially inoculated apple caused by B. cinerea and P. expansum (Daniel et al. 2015). The antifungal properties of essential oils are dependent on several factors, such as extraction methods, chemical ingredients, solubility, and the test microorganism. Different mechanisms have been proposed, but the most supported is associated with the essential oil’s volatile compounds resulting from plant secondary metabolism that control fungal pathogens. This study showed that AV gel coating combined with GO has the potential to control the anthracnose disease of banana fruit.
3.3 Postharvest quality attributes
Physicochemical properties of banana fruit are efficiently affected by AV gel coating in combination with GO during storage (Fig. 3). Weight loss of treated and untreated samples increased throughout the storage period. However, weight loss of banana fruit treated with AV + GO 0.05% (13.6%) and AV + GO 0.1% (11.2%) was significantly lower than the other treatments after 15 days of storage (Fig. 4a). No significant effects were noticed between the control and AV gel coating during the complete storage time, excluding on day 9. The effects A. vera gel coating were similar to other edible coatings, in that they slowed down the water loss and suppressed the respiration rate. It has been stated that the addition of thyme and rosehip essential oils to A. vera gel coating improved the postharvest quality of avocado and plum (Bill et al. 2014; Martínez-Romero et al. 2017). This study confirmed the previous report where acacia gum coating combined with lemongrass and cinnamon oils pointedly reduced weight loss of banana fruit during cold storage (Maqbool et al. 2011). A. vera gel is mainly composed of polysaccharides, which are highly effective as a moisture barrier. The addition of lipids into polysaccharides is known to enhance water barrier efficiency with increased lipid content and, in turn, reduce water loss. The mechanism for reduction of water loss is based on the hygroscopic water pressure between the fruit and environment. A. vera gel can form a thin layer of film on the banana surface, thereby sealing small wounds and reducing moisture loss.
Effect of A. vera gel coating and garlic essential oil on weight loss (a) and firmness (b) of banana fruit during storage at 20 °C for 15 days. Vertical bars represent standard deviation of means for four replicates. Means with different letters for each day are significantly different at p < 0.05 using Duncan’s multiple range test
Fruit texture is a major determinant of the quality and shelf life of banana. Textural changes are usually related to the degradation of pectin and hydrolysis of starch to sugar during fruit ripening. Our results showed that the treated and control banana fruit became soft due to ripening (Fig. 4b). The firmness of banana fruit treated with AV + GO 0.1% was 50.6% more followed by AV + GO 0.05% (41.2%) and AV (28.8%) than that of the control at the end of the storage period. A. vera gel coatings retarded the postharvest ripening process and reduced textural changes of table grape and sour cherry (Castillo et al. 2010; Ravanfar et al. 2014). Banana fruit textural changes and softening determine fruit shelf life, storability, and disease incidence. It has been known that changes in texture are related to moisture loss from the fruit surface, which decreases the cell turgor pressure. The softening process in banana fruit has been reported to be associated with the increased activities of polygalacturonase, pectin methylesterase, pectate lyase, and β-galactosidase enzymes (Amnuaysin et al. 2012). However, chitosan coating reduced weight loss and inhibited the activities of polygalacturonase and pectin methylesterase enzymes of mango fruit stored at ambient temperature (Khaliq et al. 2017). The explanation for the banana firmness maintenance might be related to the lower weight losses in the treated fruit. Moreover, the effect of A. vera gel coating and GO on inhibition of cell wall hydrolase enzymes responsible for banana softening could not be eliminated.
Soluble solids concentration is an important attribute that often determines fruit acceptability. Soluble solids concentration progressively increased in all samples throughout the storage period (Table 1), while the increase in soluble solids concentration was postponed in treated fruit. A. vera gel coating and garlic essential oil 0.1% significantly reduced soluble solids concentration compared to the control. However, no significant differences were observed between the control and other treatment during the entire storage period. In this study, the increase in soluble solids concentration was similar to those previously observed for sour cherry, banana, and mango (Ravanfar et al. 2014; Gutiérrez-Martínez et al. 2015; Khaliq et al. 2016). The increase in soluble solids concentration might be ascribed to conversion of carbohydrate into simple sugar and glucose. A. vera gel coating acted as a barrier to oxygen and carbon dioxide diffusion and inhibited the respiration rate in plum (Martínez-Romero et al. 2017). A possible explanation for the lower soluble solids concentration in treated banana may be the reduced respiration rate and metabolism, which reduced the conversion of starch into sugar.
Titratable acidity reflects the compositional changes and the possible storage life of fruit (Soradech et al. 2017). In this work, titratable acidity first increased in treated and untreated samples up to day 9 and then declined until the end of the storage period (Table 1). However, the highest decline in titratable acidity was detected in the control fruit. Banana fruit treated with AV + GO 0.1% significantly maintained higher titratable acidity than the untreated fruit throughout the storage period. This is consistent with a previous one, where titratable acidity increased in Robusta banana fruit (Kulkarni et al. 2011). The major organic acids, such as malic, citric, and ascorbic acids, are used for the determination of titratable acidity (Tovar et al. 2001). Organic acids are consumed as substrates during respiration (Soradech et al. 2017). The faster reduction of titratable acidity in control fruit may be due to the increased respiration rate of the fruit, which resulted in a greater utilization of organic acids. The higher titratable acidity values of treated banana fruit were probably due to the slowdown of the respiration process and reduced metabolism.
3.4 Total phenolic contents
Total phenolic contents in all samples first increased and then gradually declined as indicated in Table 2. The highest peak of total phenolic contents in control and treated banana fruit was observed on days 6 and 9, respectively. Banana fruit treated with AV combined with GO maintained the higher total phenolic contents throughout the storage period compared to the control and AV-gel-treated banana. It has been reported that phenolic compounds exhibited antioxidant and antifungal properties (Martins et al. 2015). Phytochemicals are thought to be nontoxic, biodegradable, and environmentally safe, and they have no residual effect. Total phenolic contents play an essential role in protecting against pathogens by interfering with the activity of phytopathogenic enzymes, affecting pathogen physiology and stimulating host plant resistance (Mohamed et al. 2016). Many studies show that plant extracts are a rich source of valuable compounds, which can be used for the control of plant disease and food protection. It was reported that chitosan coatings reduced decay symptoms and enhanced total phenolic contents of mango fruit during storage (Khaliq et al. 2017). A. vera gel coating combined with thyme oil significantly improved phenolic contents of avocado fruit (Bill et al. 2014). In this study, AV gel coating containing GO might be increased resistance against anthracnose disease by motivating the total phenolic contents of banana fruit.
3.5 Antioxidant activity
There are several methods to determine the antioxidant potential of tissue, and each of them has some limitations. Therefore, multiple methods are needed to generate an antioxidant profile. The lowest DPPH radical scavenging activity was observed in the control fruit and the highest in fruit treated with AV and GO during the whole storage period (Table 2). From day 9 to day 15, AV gel alone or combined with GO retained higher DPPH radical scavenging activity than the control fruit. The highest peak of FRAP activity was observed in control and AV-gel-treated fruit on days 6 and 9, respectively, whereas AV gel combined with GO showed on day 12 (Fig. 5a). Initially, the ABTS activity increased in fruit treated with AV, AV + GO 0.05% and control up to day 6, while it increased in fruit treated with AV + GO 0.1% up to day 9, and after that decreased until the end of storage (Fig. 5b). Similar behaviours were observed for antioxidant activity in banana fruit during ripening (Youryon and Supapvanich 2017). Previous studies have indicated that banana fruit is a good source of natural antioxidants (Rebello et al. 2014). A. vera gel contains various bioactive compounds, but it is considered that aloe-emodin is one of the key ingredients that contributes to antioxidant activity (Boudreau and Beland 2006). Phenolic compounds, carotenoids, and anthocyanins are the main natural plant antioxidants, which increase resistance against disease. It has been observed that thyme oil activated defence-related enzymes and inhibited anthracnose disease of avocado fruit (Sellamuthu et al. 2013). Similarly, A. vera gel coating increased resistance to decay through enhancing antioxidant capacity and antioxidant enzymes in raspberry fruit (Hassanpour 2015). In this sense, the high antioxidant activity in treated fruit was probably responsible for inhibition of anthracnose disease in banana fruit.
Effect of A. vera gel coating and garlic essential oil on FRAP (a) and ABTS (b) of banana fruit during storage at 20 °C for 15 days. Vertical bars represent standard deviation of means for four replicates. Means with different letters for each day are significantly different at p < 0.05 using Duncan’s multiple range test
4 Conclusions
Botanical fungicides effectively control postharvest diseases in banana fruit. The addition of GO to AV gel coating inhibited banana fruit metabolism, leading to increased resistance against anthracnose disease. A. vera gel coating enriched with GO enhanced total phenolic contents and antioxidant activities. Similarly, AV gel coating in combination with GO improved the postharvest quality attributes of banana by reducing changes in weight loss, soluble solids concentration, firmness, and titratable acidity. Therefore, the combined treatment of AV gel and GO can be an alternative technique to chemical fungicides for controlling anthracnose disease and maintaining postharvest quality of banana fruit.
References
Ali MIA, Shalaby NMM, Elgamai MHA, Mousa ASM (1999) Antifungal effects of different plant extracts and their major components of selected Aloe species. Phytother Res 13:401–407
Ali A, Chow WL, Zahid N, Ong MK (2014) Efficacy of propolis and cinnamon oil coating in controlling post-harvest anthracnose and quality of chilli (Capsicum annuum L.) during cold storage. Food Bioprocess Technol 7:2742–2748
Alves DS, Pérez-Fons L, Estepa A, Micol V (2004) Membrane-related effects underlying the biological activity of the anthraquinones emodin and barbaloin. Biochem Pharmacol 66:549–561
Amnuaysin N, Jones ML, Seraypheap K (2012) Changes in activities and gene expression of enzymes associated with cell wall modification in peels of hot water treated bananas. Sci Hortic 142:98–104
Anthony S, Abeywickrama K, Wijeratnam SW (2003) The effect of spraying essential oils of Cymopogon nardus, Cymbopogon flexuosus and Ocimum basilicum on postharvest diseases and storage life of Embul banana. J Hortic Sci Biotechnol 78:780–785
Begum S, Yumlembam RA, Marak TR, Nath SP (2015) Integrated management of anthracnose of chilli caused by colletotrichum capsici in west Bengal condition. Bioscan 10:1901–1904
Benkeblia N (2004) Antimicrobial activity of essential oil extracts of various onions (Allium cepa) and garlic (Allium sativum). LWT Food Sci Technol 37:263–268
Benzie IFF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 239:70–76
Bill M, Sivakumar D, Korsten L, Thompson AK (2014) The efficacy of combined application of edible coatings and thyme oil in inducing resistance components in avocado (Persea americana Mill.) against anthracnose during post-harvest storage. Crop Prot 64:159–167
Boudreau MD, Beland FA (2006) An evaluation of the biological and toxicological properties of Aloe barbadensis (Miller), Aloe vera. J Environ Sci Health C 24:103–154
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. LWT Food Sci Technol 28:25–30
Burt S (2004) Essential oils: their antibacterial properties and potential applications in foods. Int J Food Microbiol 94:223–253
Camele I, Altieri L, De Martino L, De Feo V, Mancini E, Rana GL (2012) In vitro control of post-harvest fruit rot fungi by some plant essential oil components. Int J Mol Sci 13:2290–2300
Castillo S, Navarro D, Zapata PJ, Guillén F, Valero D, Serrano M, Martínez-Romero D (2010) Antifungal efficacy of Aloe vera in vitro and its use as a preharvest treatment to maintain postharvest table grape quality. Postharvest Biol Technol 57:183–188
Chillet M, Hubert O, de Lapeyre de Bellaire L (2007) Relationship between physiological age, ripening and susceptibility of banana to wound anthracnose. Crop Prot 26:1078–1082
Choi Y, Lee SM, Chun J, Lee HB, Lee J (2006) Influence of heat treatment on the antioxidant activities and polyphenolic compounds of Shiitake (Lentinus edodes) mushroom. Food Chem 99:381–387
Cronin MJ, Yohalem DS, Harris RF, Andrews JH (1996) Putative mechanism and dynamics of inhibition of apple scab pathogen Venturia inaequalis by compost extracts. Soil Biol Biochem 28:1241–1249
Daniel CK, Lennox CL, Vries FA (2015) In vivo application of garlic extract in combination with clove oil to prevent postharvest decay caused by Botrytis cinerea, Penicillium expansum and Neofabraea alba on apples. Postharvest Biol Technol 99:88–92
Eshun K, He Q (2005) Aloe vera: a valuable ingredient for the food, pharmaceutical and cosmetic industries: a review. Crit Rev Food Sci Nutr 44:91–96
Goncagul G, Ayaz E (2010) Antimicrobial effect of garlic (Allium sativum) and traditional medicine. J Anim Vet Adv 9:1–4
Gutiérrez-Martínez P, Avila-Peña RC, Sivakumar D, Bautista-Baños S (2015) Postharvest evaluation of goldfinger banana (FHIA-01) at different storage temperatures followed by an acclimation time. Fruits 70:73–179
Hassanpour H (2015) Effect of Aloe vera gel coating on antioxidant capacity, antioxidant enzyme activities and decay in raspberry fruit. LWT Food Sci Technol 60:495–501
Jasso de Rodríguez D, Hernández-Castillo D, Rodríguez-García R, Angulo-Sánchez JL (2005) Antifungal activity in vitro of Aloe vera pulp and liquid fraction against plant pathogenic fungi. Ind Crops Prod 21:81–87
Khaliq G, Mahmud TMM, Ding P, Ghazali HM, Ali A (2016) Storage behaviour and quality responses of mango (Mangifera indica L.) fruit treated with chitosan and gum arabic coatings during cold storage conditions. Int Food Res J 23:141–148
Khaliq G, Nisa MU, Ramzan M, Koondhar N (2017) Textural properties and enzyme activity of mango (Mangifera indica L.) fruit coated with chitosan during storage. J Agric Stud 5:32–50
Kulkarni SG, Kudachikar VB, Keshava Prakash MN (2011) Studies on physico-chemical changes during artificial ripening of banana (Musa sp) variety ‘Robusta’. J Food Sci Technol 48:730–734
Maqbool M, Ali A, Alderson PG, Mohamed MTM, Siddiqui Y, Zahid N (2011) Postharvest application of gum arabic and essential oils for controlling anthracnose and quality of banana and papaya during cold storage. Postharvest Biol Technol 62:71–76
Martínez-Romero D, Zapata PJ, Guillén F, Paladines D, Castillo S, Valero D, Serrano S (2017) The addition of rosehip oil to Aloe gels improves their properties as postharvest coatings for maintaining quality in plum. Food Chem 217:585–592
Martins N, Barros L, Henriques M, Silva S, Ferreira ICFR (2015) Activity of phenolic compounds from plant origin against Candida species. Ind Crops Prod 74:648–670
Mohamed MSM, Saleh AM, Abdel-Farid IB, El-Naggar AA (2016) Growth, hydrolases and ultrastructure of Fusarium oxysporum as affected by phenolic rich extracts from several xerophytic plants. Pestic Biochem Physiol 141:57–64
Navarro D, Díaz-Mula HM, Guillén F, Zapata PJ, Castillo S, Serrano M, Valero D, Martínez-Romero D (2011) Reduction of nectarine decay caused by Rhizopus stolonifer, Botrytis cinerea and Penicillium digitatum with Aloe vera gel alone or with the addition of thymol. Int J Food Microbiol 151:241–246
Obagwu J, Emechebe AM, Adeoti AA (1997) Effects of extracts of garlic (Allium sativum) bulb and neem (Azadirachta indica) seed on the mycelial growth and sporulation of Collectotrichum capsici. J Agric Technol 5:51–55
Patrignani F, Siroli L, Serrazanetti DI, Gardini F, Lanciotti R (2015) Innovative strategies based on the use of essential oils and their components to improve safety, shelf-life and quality of minimally processed fruits and vegetables. Trends Food Sci Technol 46:311–319
Rabari VP, Chudashama KS, Thake VS (2018) In vitro screening of 75 essential oils against Colletotrichum gloeosporioides: a causal agent of anthracnose disease of mango. Int J Fruit Sci 18:1–13
Ranasinghe LS, Jayawardena B, Abeywickrama K (2003) Use of waste generated from cinnamon bark oil extraction as a postharvest treatment of Embul banana. J Food Agric Environ 1:340–344
Ravanfar R, Niakousari M, Maftoonazad N (2014) Postharvest sour cherry quality and safety maintenance by exposure to hot-water or treatment with fresh Aloe vera gel. J Food Sci Technol 51:2872–2876
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26:1231–1237
Rebello LPG, Ramos AM, Pertuzatti PB, Barcia MT, Castillo-Muñoz N, Hermosín-Gutiérrez I (2014) Flour of banana (Musa AAA) peel as a source of antioxidant phenolic compounds. Food Res Int 55:397–403
Sarkhosh A, Schaffer B, Vargas AI, Palmateer AJ, Lopez P, Soleymani A, Farzaneh M (2018) Antifungal activity of five plant-extracted essential oils against anthracnose in papaya fruit. Biol Agric Hortic 34:18–26
Sellamuthu PS, Sivakumar D, Soundy P, Korsten L (2013) Essential oil vapours suppress the development of anthracnose and enhance defence related and antioxidant enzyme activities in avocado fruit. Postharvest Biol Technol 81:66–72
Seo S, Lee J, Park J, Han K, Jang H (2006) Control of powdery mildew by garlic oil in cucumber and tomato. Res Plant Dis 12:51–54
Soradech S, Nunthanid J, Sontaya Limmatvapirat S, Luangtana-anan M (2017) Utilization of shellac and gelatin composite film for coating to extend the shelf life of banana. Food Control 73:1310–1317
Tovar B, García HS, Mata M (2001) Physiology of pre-cut mango. II. Evolution of organic acids. Food Res Int 34:705–714
Tzortzakis NG, Economakis CD (2007) Antifungal activity of lemongrass (Cymbopogon citrates L.) essential oil against key postharvest pathogens. Innov Food Sci Emerg Technol 8:253–258
Vilaplana R, Pazmiño L, Valencia-Chamorro S (2018) Control of anthracnose, caused by Colletotrichum musae, on postharvest organic banana by thyme oil. Postharvest Biol Technol 138:56–63
Youryon P, Supapvanich S (2017) Physicochemical quality and antioxidant changes in ‘Leb Mue Nang’ banana fruit during ripening. Agric Nat Resou 51:47–52
Zheng X, Ye L, Jiang T, Jing G, Li J (2012) Limiting the deterioration of mango fruit during storage at room temperature by oxalate treatment. Food Chem 130:279–285
Acknowledgements
This research was supported by Lasbela University of Agriculture, Water and Marine Sciences, Uthal, Balochistan, Pakistan.
Author information
Authors and Affiliations
Contributions
GK conceived the study idea and wrote the paper. HTA provided the technical inputs. IA and MW crossed checked the references and performed statistical analysis.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Communicated by Eun Jin Lee, Ph.D.
Rights and permissions
About this article
Cite this article
Khaliq, G., Abbas, H.T., Ali, I. et al. Aloe vera gel enriched with garlic essential oil effectively controls anthracnose disease and maintains postharvest quality of banana fruit during storage. Hortic. Environ. Biotechnol. 60, 659–669 (2019). https://doi.org/10.1007/s13580-019-00159-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13580-019-00159-z