Abstract
Brazilian green propolis extract (PP) and cinnamon oil (CM) as edible coatings can be used to maximize chilli shelf life due to their antifungal properties. Antifungal effects and quality control of 5 % PP, 0.1 % CM and the combination of PP and CM incorporated with 5 % gum arabic (GA) as base coating were investigated in vitro as well as in vivo. Coated chillies were stored in cold storage of 13 ± 2 °C (80–90 % relative humidity (RH)) for 28 days and 5 days at simulated marketing conditions (25 ± 2 °C, 60–70 % RH). Study revealed that PP and CM alone had fungicidal effect against Colletotrichum capsici, causal organisms of chilli anthracnose. However, combined treatment of PP and CM showed the most promising results against C. capsici with 100 % inhibition on mycelia growth and spore germination. In vivo studies revealed that combined treatment of PP and CM showed a significantly (p < 0.05) lower disease incidence (14 %) and severity score (1.5) after 33 days of storage, as well as a significant delay in the changes of weight loss, firmness, peel colour and soluble solids concentration compared with other treatments. It could be concluded that 5 % PP combined with 0.1 % CM incorporated with 5 % base coating of GA is an effective bio-fungicide for postharvest anthracnose control and retaining the quality of chilli.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chilli (Capsicum annuum L.) is a high demand non-climacteric fruit. Anthracnose is the devastating disease caused by Colletotrichum species and occurs as a pre-harvest or post-harvest fruit rot which causes major losses in chilli grown in tropical and subtropical climates (AVRDC 1998). Colletotrichum capsici being the most prevalent was first reported in India as a causal pathogen of chilli anthracnose (Sydow 1913). Damage symptoms are usually the circular rings of the acervuli within the brown lesions which then transform black from the development of setae and sclerotia (Roberts et al. 2001). Since chilli is a high demand crop and its varieties are commonly susceptible to anthracnose, growers often rely on fungicides application which leads to increases in health and environmental concerns. Therefore, a pressure has developed on the chilli industry to produce chillies without the use of synthetic chemical fungicide application.
Gum arabic (GA) is a dried, edible adhesive exudate from the stems and branches of Acacia senegal tree (Ali et al. 2009). In the novel, GA study on tomato by Ali et al. (2010), it was proven that GA is an edible coating that is able to delay fruit ripening but not confers any antifungal activity and therefore, could be considered only to act as a water and gas exchange barrier. Conversely, Brazilian green propolis extract (PP) is a sticky mixture from sap flows and tree buds which is gathered by honeybees (Bankova 2005) while cinnamon oil (CM) is the oil extract from cinnamon leaves (Maqbool et al. 2011). They are proven to have antifungal properties due to their major active components which are Artepillin-C and cinnamaldehyde, respectively (Messerli et al. 2009; Maqbool et al. 2011). The recent use of PP coating by Zahid et al. (2013) showed significantly lower anthracnose disease incidence (DI) on dragon fruits. Similarly, Maqbool et al. (2011) validated the antifungal properties of CM incorporated with GA in controlling post-harvest anthracnose of banana and papaya. In literature, no study has been reported on the use of PP combined with CM as a coating material against anthracnose and to increase the storage life of chilli. Therefore, the objectives of this study are to determine the antifungal properties of PP and CM coatings and to study the physicochemical changes of coated chilli during cold storage.
Materials and Methods
Plant Material
Mature red chillies (C. annum L.) MC 10 variety was purchased from the local store, Pasar Segar Ilham in Seri Kembangan, Selangor, Malaysia. GA powder (KB-120, food grade) was imported from Jumbo Trading Co., Ltd. Bangkok, Thailand. PP (aqueous) was obtained from Green Life Harvest Marketing (M) Sdn. Bhd., Kuala Lumpur, Malaysia, while CM was obtained from Tropical Bioscience Marketing Sdn. Bhd., Melaka, Malaysia.
Fungus Isolation and Culture Conditions
Chillies with disease symptoms were used for isolation of fungus. Symptomatic tissue (1 cm2) was surface-sterilized in 70 % ethanol for 2–3 min, followed by three rinses with distilled water. A second sterilization with 10 % sodium hypochlorite was done for 3–5 min and followed by three rinses with distilled water and then left to dry on sterile paper. The isolates were cultured aseptically on petri dishes containing potato dextrose agar (PDA) (Difco Brand, USA) and incubated at 25 ± 2 °C. Once the mycelial growth was observed, the colonies were re-isolated on fresh PDA dishes to obtain pure cultures. Identification of the isolates was based on their morphological (longitudinal curved shaped spores) and cultural distinctive features (Barnett and Hunter 1972). Re-isolation was performed on PDA slants in order to maintain inoculum.
Preparation of GA, PP and CM
Gum arabic with a concentration of 5 % (w/v) was used as a base coating and was prepared by dissolving 5 g of powder in 100 ml of purified water. The solution was stirred using a heated magnetic stirrer (Model: HTS-1003) set at 40 °C for 60 min, then filtered by muslin cloth to remove any undissolved impurities. After cooling, 0.2 ml of Tween 80 (0.2 %) was added as surfactant and the pH of the solutions was maintained to 5.6 with 1 N NaOH. PP (5 % v/v), CM (0.1 % v/v) and a combination of both were prepared by dispersing 5 ml PP, 0.1 ml CM and 5 ml + 0.1 ml PP + CM in the GA base coating, respectively. There were three treatments and controls, which included an untreated control, 5 % GA (second control), 5 % GA + 5 % PP, 5 % GA + 0.1 % CM and 5 % GA + 5 % PP + 0.1 % CM.
In vitro Antifungal Assay of PP and CM
The antifungal effects of PP, CM and the combination of both were determined based on the suppression in radial mycelia growth on PDA media. A fungal disc (5 mm diameter) from a pure culture of C. capsici was isolated from the edge of a 14 days old culture and positioned in the centre of a petri dish comprising of PDA with 5 % GA, 5 % GA + 5 % PP, 5 % GA + 0.1 % CM and 5 % GA + 5 % PP + 0.1 % CM. Only PDA plates were used as control. Petri dishes were incubated at 25 ± 2 °C. Radial mycelia growth was recorded every 2 days until the control dishes were completely covered with mycelia. The efficacy of treatments was assessed by measuring fungal colony growth (mm).
The in vitro conidial germination inhibition test was carried out by the cavity slide technique and the percentage inhibition in germination was computed by the method of Cronin et al. (1996). An aliquot (40 μl) of each of the GA, PP and CM concentrations was pipetted on a cavity slide. A freshly harvested spore suspension (10 μl) of C. capsici (adjusted to 105 conidia ml−1 using a hemocytometer) was pipetted into the cavity slide, and the slide was covered with a cover slip and kept in the dark for 7 h at 25 ± 2 °C. Cavity slides containing purified water served as the control. After incubation, the conidia were killed by adding 10 μl 2 % sodium azide to each cavity. Approximately 100 conidia were observed for germination in each treatment. A conidium was said to be germinated when its germ tube was half the length of the conidium.
In Vivo Antifungal Assay of PP and CM
Disease free chillies were washed with 0.05 % sodium hypochlorite, rinsed with distilled water and air-dried at 25 ± 2 °C. Each chilli was wounded in the centre of the fruit using a 5 mm cork borer before inoculation was carried out. A volume of 0.02 ml from conidial suspension of C. capsici (5 × 107 spores ml−1) was injected into the wounded chilli and then air-dried until complete drying. Chillies were then immersed for 1 min in their respective treatment and then kept at ambient temperature for drying. After treatments, chillies were placed in cardboard carton boxes and stored at 13 ± 2 °C and 80–90 % relative humidity (RH). The effect of treatments on DI and disease severity (DS) were evaluated weekly for 28 days during cold storage (13 ± 2 °C and 80–90 % RH) and then after 5 days at simulated marketing condition (SMC; 25 ± 2 °C, 60–70 % RH). DI data was presented as the percentage of chillies showing anthracnose out of the total number of chillies in each treatment, while DS was scored following the scale (1 = 0 % of fruit surface rotten; 2 = 1–25 %; 3 = 26–50 %; 4 = 51–75 % and 5 = 76–100 % rotten) (Sivakumar et al. 2002).
Determination of Post-Harvest Quality Characteristics
Weight loss was measured by weighing the chilli fruit on Sartorius AG precision balance (Model: CP3202S, Goettingen, Germany) at day 0 and at the end of each storage interval. The total weight loss during that storage interval was measured by the difference between initial and final chilli weight, and it was computed as percentages on a fresh weight basis by the standard AOAC (1984) method.
Chilli firmness on point in the equatorial region of the whole piece of fruit was recorded on each sampling day using an Instron Universal Testing Machine with 6.00 mm plunger tip, single column model (Norwood, MA, USA) connected to a computer. It was evaluated by measuring the amount of force (N) to pierce a hole in the chilli. The machine was set for maximum compression with a speed of 20 mm min−1 as in the study of Zapata et al. (2008).
Peel colour on three points of the whole piece of fruit was analysed according to McGuire (1992) using the Hunter Lab System, Miniscan XE Plus colorimeter (Model: 45/0-5, Reston Virginia, USA). Values were expressed in chromaticity values recorded as L*, C* and h°.
Soluble solids concentration (SSC) (°Brix) was determined using a Palette Digital Refractometer (Model: PR-32α Atago Co., Ltd. Japan). Chilli samples (10 g) were ground and homogenized using a kitchen blender with 40 ml of distilled water and sieved with muslin cloth. A droplet of the filtrate was then placed on to refractometer, and it was calibrated with distilled water prior to taking readings. The readings were multiplied by the dilution factor to determine the original SSC (%) of the chilli pulp.
Statistical Analysis
In in vitro studies, four replicates of PDA prepared for each treatment with five plates in each replicate. In in vivo studies, each treatment had four replicates with 20 fruits in each replicate. Treated chillies were stored at 13 ± 2 °C (80–90 % RH) and each parameter was measured at 7 days interval for 28 days and then for 5 days at SMC (25 ± 2 °C, 60–70 % RH).
The treatments were organized in a Completely Randomized Design (CRD) throughout the experiment. Data were subjected to analysis of variance (ANOVA) and Post hoc multiple comparisons (Least Significant Difference (LSD) and Tukey tests) at p < 0.05 by using SPSS software (Version 16.0).
Results and Discussion
In vitro Antifungal Assay of PP and CM
The mycelial growth was observed to be inhibited gradually in 14 days. PP and CM tested alone showed significantly (p < 0.05) lower mycelia growth of C. capsici as compared to control (Fig. 1a). However, the highest inhibition (100 %) was observed with the combined treatment. Although CM alone had slightly higher mean suppression on mycelial growth of C. capsici (48 %) as compared to PP (42 %), there was no significant difference observed between these two treatments.
Similarly, the percentage of suppression in spore germination was more noticeable (p < 0.05) in the case of combined treatment (Fig. 1b). It was the best treatment for inhibition of spore germination followed by CM alone (58 and 61 %), PP alone (52 and 55 %) and control (17 and 26 %) in 24 and 72 h, respectively. PP alone was significantly (p < 0.05) less effective than CM in both 24 and 48 h. Both control and 5 % GA (second control) also showed no significant difference in mycelial growth and spore germination inhibition.
The results suggest that the combination of PP, GA and CM had the greatest in vitro antifungal effects against C. capsici, which could be attributed to the presence of active components in PP and CM. The active component of CM, cinnamaldehyde could interrupt biological processes as it inhibits respiratory electron transfer (production of ATP) and binds to nitrogen-containing compounds (e.g. proteins and nucleic acids) (Gupta et al. 2008). CM has been identified as an effective source of antifungal compounds when tested on papaya and banana (Maqbool et al. 2011), papaya (Berrera-Necha et al. 2008) and banana (Win et al. 2007). Artepillin-C is the active constituent for PP and research done by Zahid et al. (2013) on dragon fruits had proven that propolis possesses extremely potent antifungal properties. These active constituents might have acted synergistically to provide an effective barrier in limiting mycelia growth and spore germination of C. capsici due to its incapability to take up nutrients from PDA in the presence of PP and CM. This is similar to findings of Giovanelli (2008) who reported that propolis was able to inhibit spores germination of Colletotrichum gloeosporioides of avocado, and Maqbool et al. (2011) who found that CM was capable of suppressing spore germination of C. gloeosporioides of papaya and Cladosporium musae of banana, respectively.
In Vivo Antifungal Assay of PP and CM
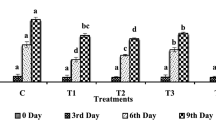
The PP, CM and the combination of both showed significantly (p < 0.05) higher fungicidal effects as compared with the control (Fig. 2a and b). Although coated chillies with PP and CM alone at 33 days were infected with anthracnose, disease symptoms were significantly less severe (score of 3.5 and 2.5) than the control. In addition, no significant difference was observed between the control and 5 % GA (second control). The DI of both 5 % PP and 0.1 % CM also showed no significant difference. However, the DS observed for CM was significantly less severe than PP. In contrast, the combined treatment of 5 % PP and 0.1 % CM not only delayed the early occurrence of anthracnose but also preserved the freshness of chillies during the first 3 weeks of storage, and later on showed only minor anthracnose symptoms after keeping at SMC for 5 days. Furthermore, there were no phytotoxic effects on chilli shown in all treatments. The highest fungicidal effect was observed in chillies coated with the combined treatment (DI of 14 % and DS score of 1.5), indicating infection on chillies surface close to zero at the end of the storage period.
Effect of different coating treatments on a disease incidence (DI in units of % fruit affected) and b disease severity (DS having a range of 1 = 0 % of fruit surface being decayed to 5 = 76–100 % of fruit surface being decayed) of inoculated chillies during storage (13 ± 2 °C, 80–90 % RH) for 28 days and then 5 days at simulated marketing conditions (25 ± 2 °C, 60–70 % RH). The vertical bars denote the standard error of means for four replicates
Although coating chillies with 5 % PP and 0.1 % CM alone showed fungicidal effect, the combined treatment was observed to have a synergistic effect on controlling fungal growth. This is supported by a study involving the application of a composite edible coating on papaya done by Maqbool et al. (2011) with 10 % GA, 0.4 % of CM and 0.5 % lemon grass oil expressing fungicidal effects against C. gloeosporioides. This indicates that the use of a combined treatment could offer further synergistic fungicidal effects compared to the use of one type of edible coating alone.
Determination of Post-Harvest Quality Characteristics
Weight loss was observed to gradually increase during the storage period. The lowest weight loss was detected in the combined treatment of PP and CM (27 %), followed by PP (47 %), CM (49 %), GA (87 %) and control (92 %) at the end of the storage period (Fig. 3a).
Firmness of chillies significantly (p < 0.05) declined with storage time for all treatments (Fig. 3b). At the end of the storage day 33, control chillies clearly displayed the lowest firmness (1.2 N) followed by chillies coated with 5 % GA (1.9 N), 0.1 % CM (6.5 N), 5 % PP (8.3 N) and the combined treatment (11.7 N).
Vapour pressure differences between produce and the surrounding storage atmosphere is the fundamental mechanism of weight loss from post-harvest fruits and vegetables (Yaman and Bayoindirli 2002), even though weight loss can also increase due to respiratory heat (Pan and Bhowmilk 1992). The effects of the coating as a semipermeable barrier against O2, CO2, moisture and solute mobilization possibly contributed to the decrease in weight loss and reduction of firmness, hence, decreasing water loss, respiration and oxidation rates (Baldwin et al. 1995; Park 1999). The high weight loss in control and 5 % GA chillies could be attributed to the coating not being thing enough to provide an adequate barrier against moisture loss due to pathogenic infection injury and chilli transpiration. In general, water loss is the main reason for loss of firmness in peppers. Moreover, decay organisms which produce pectolytic enzymes and infection itself may be the cause of accelerated softening. Conversely, combined coating of PP and CM was thicker than control where it entirely covered the chilli surface, inhibiting moisture loss and retaining firmness. The results correspond to the finding of Ben-Yehoshua (1969) who found that wax coatings on oranges were able to prolong the fruits shelf life through reduction in water loss and alteration of the internal atmosphere.
It was also observed that the lightness (L*) (data not shown) and hue angle (h°) progressively increased during storage in all treatments (Fig. 3c). The highest increase was observed in the control followed by 5 % GA, 0.1 % CM, 5 % PP and the combined treatment of PP and CM that constantly maintained their lightness and hue angle at the end of storage. On the other hand, chroma (C*) generally decreased during storage in all treatments (data not shown). The maximum decrease was observed in the control followed by 5 % GA, 0.1 % CM, 5 % PP and the combined treatment of PP and CM which retained their original C* values until the end of storage.
Colour is a crucial component of quality and end users acceptability, especially in the context of chillies. Pigment degradation occurs in red chillies where the red lycopene pigment is degraded while anthocyanin is concomitantly synthesized during ripening (Aza-González et al. 2012).
There was a progressive increase in SSC during storage (Fig. 3d). The SSC was considerably highest (p < 0.05) in control (10.5 %) followed by GA (10.3 %), CM (6.8 %), PP (6.5 %) and the combined treatment of PP and CM (4.6 %). Generally, SSC will gradually increase during the storage period. It can be assumed that SSC is up regulated during ripening and down regulated in ripe fruits due to respiration. A probable explanation for the observed scenario in SSC rate is the considerable water loss experienced by fruits during storage (Hernández-Munoz et al. 2008). The higher deviations in SSC are certainly arising in those fruits which undergo greatest water loss. Also, the increase in SSC may be contributed by the solubilization of the cell wall hemicelluloses and polyuronides in mature fruits (Hernández-Munoz et al. 2008).
Conclusion
These studies showed that the combination of PP and CM were most effective against C. capsici in inhibiting mycelia growth and spore germination. In vivo studies also revealed that the combined treatment of PP with CM showed lower disease incidence and severity as well as significant delay in the changes of weight, firmness, peel colour and soluble solids concentration compared to other treatments. These results suggest that 5 % PP combined with 0.1 % CM incorporated into 5 % base coating of GA could be used as an effective eco-friendly fungicide to prolong shelf life of chilli by controlling chilli anthracnose and delaying the ripening process.
Abbreviations
- PP:
-
Brazilian green propolis extract
- CM:
-
Cinnamon oil
- GA:
-
Gum arabic
References
Ali, B. H., Ziada, A., & Blunden, G. (2009). Biological effects of gum arabic: a review of some recent research. Food and Chemical Toxicology, 47, 1–8.
Ali, A., Maqbool, M., Ramachandran, S., & Alderson, P. G. (2010). Gum arabic as a novel edible coating for enhancing shelf-life and improving postharvest quality of tomato (Solanum lycopersicum L.) fruit. Postharvest Biology and Technology, 58, 42–47.
AOAC. (1984). Official Methods of Analysis (14th ed.). Washington: Association of Official Analytical Chemists.
AVRDC. (1998). AVRDC Annual Report 1997, Tainan, Taiwan, pp. 54-57.
Aza-González, C., Núñez-Palenius, H. G., & Ochoa-Alejo, N. (2012). Molecular biology of chili pepper anthocyanin biosynthesis. Journal of the Mexican Chemical Society, 56, 93–98.
Baldwin, E. A., Nisperos-Carriedo, M., Shaw, P. E., & Burns, J. K. (1995). Effect of coatings and prolonged storage conditions on fresh orange flavour volatiles, degrees brix and ascorbic acid levels. Journal of Agricultural and Food Chemistry, 43, 1321–1331.
Bankova, V. (2005). Recent trends and important developments in propolis research. Journal of Evidence-Based Complementary and Alternative Medicine, 2, 29–32.
Barnett, H. L., & Hunter, B. B. (1972). Illustrated genera of imperfect fungi (p. 24). Broken Arrow: Burgess Publishing Co.
Ben-Yehoshua, S. (1969). Gas exchange, transportation and the commercial deterioration in storage of orange fruit. Journal of the American Society for Horticultural Science, 94, 524.
Berrera-Necha, L. L., Bautista-Baños, S., Flores-Moctezuma, H. E., & Rojas-Estudillo, A. (2008). Efficacy of essential oils on the conidial germination, growth of Colletotrichum gloeosporioides (Penz.) Penz. and Sacc and control of postharvest diseases in papaya (Carica papaya L.). Plant Pathology, 7, 1–5.
Cronin, M. J., Yohalem, D. S., Harris, R. F., & Andrews, J. H. (1996). Putative mechanism and dynamics of inhibition of the apple scab pathogen Venturia inaequalis by compost extracts. Soil Biology and Biochemistry, 28, 1241–1249.
Giovanelli, L. C. (2008). Evaluation of an Ethanolic Extract of Propolis as a Potential Pre- and Post-Harvest Fungicide for ‘Fuerte’ Avocado (Persea americana Mill.) Fruits and Orchards. Johannesburg: Faculty of Science, University of the Witwatersrand.
Gupta, C., Garg, A. P., Uniyal, R. C., & Kumari, A. (2008). Comparative analysis of the antimicrobial activity of cinnamon oil and cinnamon extract onsomefood-borne. African Journal of Microbiology Research, 9, 247–251.
Hernández-Munoz, P., Almenar, E., Valle, V. D., Velez, D., & Gavara, R. (2008). Effect of chitosan coating combined with postharvest calcium treatment on strawberry (Fragaria x ananassa) quality during refrigerated storage. Food Chemistry, 110, 428–435.
Maqbool, M., Ali, A., Alderson, P. G., Mohamed, M. T. M., Siddiqui, Y., & Zahid, N. (2011). Postharvest application of gum arabic and essential oils for controlling anthracnose and quality of banana and papaya during cold storage. Postharvest Biology and Technology, 62, 71–76.
McGuire, R. G. (1992). Reporting of objective color measurements. HortScience, 27, 1254–1260.
Messerli, S. M., Ahn, M. R., Kunimasa, K., Yanagihara, M., Tatefuji, T., Hashimoto, K., et al. (2009). Artepillin C (ARC) in Brazilian green propolis selectively blocks oncogenic PAK1 signaling and suppresses the growth of NF tumors in mice. Phytotherapy Research, 23, 423–427.
Pan, J. C., & Bhowmilk, S. R. (1992). Shelf-life of mature green tomatoes stored in controlled atmosphere and high humidity. Journal of Food Science, 57, 948–953.
Park, H. J. (1999). Development of advanced edible coatings for fruits. Trends in Food Science and Technology, 10, 254–260.
Roberts, P.D., K.L. Pernezny & T.A. Kucharek. (2001). Anthracnose caused by Colletotrichum spp. on pepper. Available at: http://edis.ifas.ufl.edu. Assessed on 12th May 2013.
Sivakumar, D., Hewarathgamagae, N. K., Wijeratnam, R. S. W., & Wijesundera, R. L. C. (2002). Effect of ammonium carbonate and sodium bicarbonate on anthracnose of papaya. Phytoparasitica, 30, 1–7.
Sydow, H. (1913). Beitrage zur kenntnis der PilzHora des Siidlichen Ostindiens-I. Anv. Myc, XI, p. 329.
Win, N. K. K., Jitareerat, P., Kanlayanarat, S., & Sangchote, S. (2007). Effects of cinnamon extract, chitosan coating, hot water treatment and their combinations on crown rot disease and quality of banana fruit. Postharvest Biology and Technology, 45, 333–340.
Yaman, O., & Bayoindirli, L. (2002). Effects of an edible coating and cold storage on shelf-life and quality of cherries. Lebensmittel-Wissenschaft and Technologie, 35, 146–150.
Zahid, N., Ali, A., Siddiqui, Y., & Maqbool, M. (2013). Efficacy of ethanolic extract of propolis in maintaining postharvest quality of dragon fruit during storage. Postharvest Biology and Technology, 79, 69–72.
Zapata, P. J., Guillén, F., Martínez-Romero, D., Castillo, S., Valero, D., & Serrano, M. (2008). Use of alginate or zein as edible coatings to delay postharvest ripening process and to maintain tomato (Solanum lycopersicon Mill) quality. Journal of the Science of Food and Agriculture, 88, 1287–1293.
Acknowledgments
We are grateful to the Ministry of Agriculture, Malaysia (MOA) for providing financial support under project grant No. 05-02-12-SF1003.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ali, A., Chow, W.L., Zahid, N. et al. Efficacy of Propolis and Cinnamon Oil Coating in Controlling Post-Harvest Anthracnose and Quality of Chilli (Capsicum annuum L.) during Cold Storage. Food Bioprocess Technol 7, 2742–2748 (2014). https://doi.org/10.1007/s11947-013-1237-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-013-1237-y