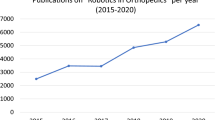
Abstract
Orthopedic surgery is one of the first surgical specialties to apply surgical robotics in clinical practice, which has become an interesting field over the years with promising results. Surgical robotics can facilitate total joint arthroplasty by providing robotic support to accurately prepare the bone, improving the ability to reproduce alignment, and restoring normal kinematics. Various robotic systems are available on the market, each tailored to specific types of surgeries and characterized by a series of features with different requirements and/or modus operandi. Here, a narrative review of the current state of surgical robotic systems for total joint knee arthroplasty is presented, covering the different categories of robots, which are classified based on the operation, requirements, and level of interaction with the surgeon. The different robotic systems include closed/open platform, image-based/imageless, and passive/active/semi-active systems. The main goal of a robotic system is to increase the accuracy and precision of the operation regardless of the type of system. Despite the short history of surgical robots, they have shown clinical effectiveness compared to conventional techniques in orthopedic surgery. When considering which robotic system to use, surgeons should carefully evaluate the different benefits and drawbacks to select the surgical robot that fits their needs the best.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Orthopedic surgery is one of the first surgical specialties to apply surgical robotics in clinical practice, which has become an interesting field over three decades with promising results [1,2,3,4]. Surgical robotics can facilitate total joint arthroplasty by providing robotic support to accurately prepare the bone, making the ligaments as competent as before osteoarthritic changes, improving the ability to reproduce alignment, and restoring normal kinematics [1].
Robotic surgery has gained popularity due to its extensive applications, which allow major orthopedics companies to introduce these devices to their portfolio by developing their own systems. Therefore, its adoption has grown along with approved surgical indications and the increased quality of supporting literature [3,4,5,6].
The orthopedic community has been striving for further innovations to improve patient satisfaction and decrease failure rates despite the promising results of total joint arthroplasty. The risk of failure suggests a room for improvement, with a survival rate at 10 years up to 98% and at 20 years up to 95% [7, 8]. Postoperative outcomes may be limited in some cases owing to technical errors, which can result in early implant failures [9]. Furthermore, a seemingly well-executed total joint arthroplasty from a surgical point of view may not translate to overall patient satisfaction and a natural wellbeing due to reasons that remain elusive [10,11,12,13,14,15,16,17,18,19,20]. Accordingly, several technological advances in computer navigation, patient-specific implants, and surgical robotics in the orthopedic field have been made recently due to the desire to decrease complications as well as increase patient satisfaction [9, 21,22,23]. However, as new technology continues to be incorporated into practice, it is vital to examine the reproducibility, precision, and accuracy of these advances. Proponents of robotic surgery have indicated that robotic systems help surgeons transition from preoperative planning to intraoperative steps, which can lead to greater accuracy and precision [2, 9, 24,25,26,27,28,29].
Surgical robots are mostly used in orthopedic surgery for knee and hip joint surgeries, as well as spine, shoulder, and ankle surgeries [13, 28]. This article focused on robotic applications in knee and hip arthroplasties due to their prevalence in the prosthetic implant market [27]. The aim of this article was to provide a narrative review of the current state of surgical robotic systems for total knee arthroplasty (TKA) and total hip arthroplasty (THA).
2 History of robotics
There are various definitions of “robot”. According to Webster’s Dictionary, a robot is defined as an automatic device that can accomplish a variety of tasks normally performed by humans or a machine. Modern robots are inspired by previous inventions including Egyptian water clocks and the wooden robot created by Giovanni Torriani; nevertheless, scientific advancements in the robotics field during the 20th century have exponentially progressed beyond these early developments.
Devol from Louisville (KY) invented the earliest modern robot in the early 1950s. He introduced a reprogrammable manipulator but failed to promote its use in commercial industries. In the late 1960s, Joseph Engelberger produced the “Unimate” robot and was successful in marketing it as an industrial robot, which led to advancements in the field of surgical robotics.
In 1985, Puma 560 was the initial surgical robotic system used in neurosurgical biopsies with computed tomography, which could increase precision [30]. The ProBot system was influenced by Puma 560 and was developed for the accurate and precise dissection of soft tissue in the prostate, which demonstrated the practicability and predictability of soft tissue surgery by robots [31]. Therefore, surgical robotics has emerged as a prominent field in medicine.
3 Technology platform types
3.1 Closed platforms vs. open platforms
Robotic systems may have closed or open platforms, which may limit the surgeon’s freedom of choosing the type of prosthesis implant or manufacturer’s implant based on compatibility. Closed platforms require the use of implants from certain manufacturers during the surgical procedure, such as Mako SmartRobotics (Stryker, Kalamazoo, MI), Navio (Smith + Nephew, London, UK), ROSA Knee System (Zimmer-Biomet, Warsaw, IN), and OMNIBotics (Corin, Cirencester, UK). On the other hand, some systems have open platforms, allowing different implant companies and designs to be used depending on the surgeon’s choice or patient’s preference. The TSolution One Surgical System (formerly known as ROBODOC) (THINK Surgical, Freemont, CA) does not limit the pool of prosthesis models that can be implemented during the procedure.
The availability of closed or open platforms is important for orthopedic surgeons because this may influence their choice despite the patient’s preference; thus, the rationale of implant design could be superseded by the availability of models that are compatible with the robotic systems used. In comparison to closed platform systems, open platform systems may show reduced functionality, accuracy, and specificity because it is necessary for them to provide a higher level of generalization to be compatible with a wide range of prosthesis implants [9, 32]. However, the wide availability of models is associated with lower design specificity, and the lack of biomechanical-rationale data may adversely affect the accurate prediction of the kinematics derived from the positioning of the prosthesis. Indeed, some open systems depend on specific features rather than actual images of the patients without considering individual anatomical variations, which implies that some specificity and predictive value may be lost [4].
Orthopedic surgeons should carefully consider whether the advantages of open platform systems are enough to balance the loss of specificity and certain functionalities or whether the advantages of closed platform systems are worthwhile to give up the freedom of choosing the prosthesis implant models [33].
3.2 Image-based vs. imageless systems
Current robotic systems require a platform and preoperative plan on which to establish the operative procedure. Preoperative planning is a part of surgical robotics, differentiating it from other specialties that involve the use of robots. Robotic systems require the acquisition of the anatomy of patients to generate anatomical landmarks of the bone; however, this information can be provided using either image-based or imageless systems.
In image-based systems, computed tomography (CT) or magnetic resonance imaging (MRI) is performed to obtain preoperative data during the registration process. The patients’ actual geometries are used as a reference to determine the optimal component location and size, depth of the resected bone, target alignments, deformity correction, restoration of the posterior offset, and boundary of the osteophyte. All of the surgeon’s actions are guided during the operation by computer navigation. This approach considers the anatomy and deformities of the patients, which allows the surgeon to properly plan in advance and predict eventual outcomes. However, there are inevitable disadvantages including a higher cost of operation along with related complications and radiation exposure during CT [4, 34]. Some examples of image-based systems are the TSolution One, Mako, and ROSA systems.
On the other hand, imageless systems rely on the registration of the surfaces and landmarks of the bones of patients during the surgical procedure. The geometry of the patients and the surgical plan are generated on the spot, which is dependent on the surgeon’s accuracy. As a morphing procedure is used, approximation should be considered for patients with deformities that were not detected during the registration process. Imageless systems have some advantages including reduced cost, no preoperative exposure to CT radiation, and increased patient convenience. However, the lack of preoperative planning and outcome evaluation and the inability to verify the anatomic registration points during the procedure are the inevitable disadvantages of imageless systems. Some examples of imageless systems are the Navio, OMNIBotics, and ROSA systems. Interestingly, the ROSA system is equipped with a software that allows surgeons to choose between the image-based or imageless approach.
3.3 Passive, active, and semiactive approach
There are three categories of robotic systems: passive, semiactive, and active [35]. Passive systems execute a procedure under the surgeon’s direct and continuous control. After preoperative planning, the registration is executed, and the robot provides the position of cutting guides under the direct supervision of the surgeon using the robotic system. Some examples of passive systems are the ROSA system and OMNIBotics, which is based on a combination of different devices that were developed by companies acquired by Corin (Omnilife Science, Praxim, iBlock) and assembled in a coherent ecosystem.
Active systems complete a portion of the procedure without the surgeon’s involvement. The robot performs bone resection by itself, requiring a low level of human interaction. An example of an active system is ROBODOC (initially developed by Curexo Technology, Fremont, CA), which is an image-based, active, and autonomous five-axis robotic system equipped with a mill that would prepare a cavity for the stem of the femur automatically, with the ORTHODOC workstation for preoperative planning. Other active systems include CASPAR “Computer Assisted Surgical Planning and Robotics” (Ortho-Maquet/URS, Schwerin, Germany) and Acrobot “Active Constraint Robot” (Acrobot Company Ltd., Hertford, UK).
Semiactive systems perform a task with the aid of the surgeon. With these systems, feedback is provided to facilitate surgeon control and contribute to operative safety. Semiactive systems create a haptic boundary, which assists the surgeon in executing bone resections according to plan. The haptic boundary could protect essential anatomical structures during bone preparation; thus, the surgeon cannot resect the bone outside the boundary of the preset volumetric parameters, limiting the treatment to only the planned level of resection in 3 dimensions. Auditory (beeping), tactile (vibratory), and visual (computer screen shows a change in color) signals are provided to the surgeon by “haptic” sensation. This technology allows the surgeon to achieve accurate bone resection within defined parameters with feedback and controls that improve precision and reduce errors. The process is based on the quantitative data instead of the intuition or rationale of the surgeon for clinical decision making. Some examples of semiactive systems are Mako SmartRobotics (Stryker, Kalamazoo, MI), Navio (Smith + Nephew, London, UK), and the new robotic system CORI, which is also developed by Smith + Nephew.
4 Discussion
Robotic surgery has already begun to alter the landscape of orthopedics. Robotic assistance was initially introduced to improve accuracy and precision, improve patient satisfaction, reduce revision rates, and obtain better outcomes. Surgical robotics can achieve these goals by improving the physician’s ability to produce reliable and reproducible results using an individualized operative approach. The restoration of normal joint kinematics, reproduction of alignment, and optimization of soft tissue balancing have been demonstrated as the benefits of surgical robotics in the orthopedic field [6, 36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51]. The existing evidence shows that robotic-assisted surgery can help physicians perform more reproducible and accurate procedures with patient-specific surgical planning regardless of the target of the component position or desired overall limb alignment [36, 37, 52,53,54,55]. Robotic-assisted orthopedic surgery is expected to become better and safer, and it would be eventually necessary for orthopedic surgeons to embark on the path of robotics in healthcare.
Although surgical robotics may still be in the early phases, certain limitations need to be addressed to define the future perspectives and applications of surgical robotics. Operating room staff including the surgeons should be educated of the advantages and safety features of surgical robotics in addition to the cost savings. The purchase of a surgical robot itself does not improve outcomes because the return on investment is not guaranteed. The surgical time for surgical robotics is considerably longer than that for conventional techniques, especially in the early phase of the learning curve, and closed platforms would not allow the use of an implant preferred by a surgeon [56, 57]. The current surgical robotic systems are designed to function based on a particular plan; thus, they cannot support a change in the surgical plan for unexpected situations such as an adjacent ligament injury due to accompanied fracture during surgery. Soft tissue balancing is not possible with the current systems even though they provide feedback for precise balancing during the operation. Moreover, the current systems perform planned cuts regardless of what they may be cutting. Therefore, orthopedic surgeons must use their instruments to retract and protect soft tissues that may be injured by the robot. As the existing robotic systems cannot distinguish the type of tissues, future designs will need to incorporate a failsafe mechanism to prevent inadvertent tissue injuries. Finally, despite the availability of registration data when using image-based or imageless systems, there is a possibility of incorrect registration, which may lead to catastrophic consequences.
Future robotic innovations should focus on the improvement and simplification of surgical planning, setup, and workflow during surgery. Efforts should also be made to reduce the learning curve and achieve consistent surgical outcomes regardless of the surgeon’s experience or surgical volume [58, 59]. Advancements in technology may allow bone resection without requiring full joint visualization, leading to a shorter surgical duration and reduced postoperative complications [60]. The current surgical robotic systems use CT scans or radiographs for registering anatomical landmarks in a robotic-registered space to facilitate surgical planning and establish the boundaries of bone resection. A further step is to supersede the use of an imaging modality by applying the kinematics of the joint prior to a change in the joint caused by pathologic arthritis. Appropriate surgical planning will allow surgeons to develop the desired kinematic and anatomical framework. Considering the inherent limitations of previous implant designs that can be prepared with conventional instruments and requirements, future implant development may be different. In addition, the presence of additional robotic arms capable of performing retraction tasks and a reduction in the size of bulky robotic arms are necessary. Technological developments are expected to improve cost-effectiveness, reducing the economic burden on patients, hospitals, and society [61].
In conclusion, this article provides a narrative review of the different features of current surgical robotic systems for total joint arthroplasty. The main goal of a robotic system is to increase the accuracy and precision of the operation regardless of the type of system. Despite the short history of surgical robots, they have shown clinical effectiveness compared to conventional techniques in orthopedic surgery. When considering which robotic system to use, surgeons should carefully evaluate the different benefits and drawbacks to select the surgical robot that fits their needs the best.
References
Goldsmith MF. For better hip replacement results, surgeon’s best friend may be a robot. JAMA. 1992;267(5):613–4.
Lang JE, Mannava S, Floyd AJ, Goddard MS, Smith BP, Mofidi A, Seyler TM, Jinnah RH. Robotic systems in orthopaedic surgery. J Bone Joint Surg Br. 2011;93(10):1296–9.
Banerjee S, Cherian JJ, Elmallah RK, Pierce TP, Jauregui JJ, Mont MA. Robot-assisted total hip arthroplasty. Expert Rev Med Devices. 2016;13(1):47–56.
Banerjee S, Cherian JJ, Elmallah RK, Jauregui JJ, Pierce TP, Mont MA. Robotic-assisted knee arthroplasty. Expert Rev Med Devices. 2015;12(6):727–35.
Park SE, Lee CT. Comparison of robotic-assisted and conventional manual implantation of a primary total knee arthroplasty. J Arthroplasty. 2007;22(7):1054–9.
Bargar WL, Bauer A, Börner M. Primary and revision total hip replacement using the Robodoc system. Clin Orthop Relat Res 1998(354):82–91.
Callaghan JJ, Bracha P, Liu SS, Piyaworakhun S, Goetz DD, Johnston RC. Survivorship of a Charnley total hip arthroplasty. A concise follow-up, at a minimum of thirty-five years, of previous reports. J Bone Joint Surg Am. 2009;91(11):2617–21.
Jauregui JJ, Cherian JJ, Pierce TP, Beaver WB, Issa K, Mont MA. Long-term survivorship and clinical outcomes following total knee arthroplasty. J Arthroplasty. 2015;30(12):2164–6.
Jacofsky DJ, Allen M. Robotics in Arthroplasty: a Comprehensive Review. J Arthroplasty. 2016;31(10):2353–63.
Sakellariou VI, Poultsides LA, Ma Y, Bae J, Liu S, Sculco TP. Risk Assessment for Chronic Pain and patient satisfaction after total knee arthroplasty. Orthopedics. 2016;39(1):55–62.
Lavand’homme P, Thienpont E. Pain after total knee arthroplasty: a narrative review focusing on the stratification of patients at risk for persistent pain. Bone Joint J 2015, 97–b(10 Suppl A):45–48.
Liddle AD, Pandit H, Judge A, Murray DW. Patient-reported outcomes after total and unicompartmental knee arthroplasty: a study of 14,076 matched patients from the National Joint Registry for England and Wales. Bone Joint J 2015, 97–b(6):793–801.
Buckwalter JA, Saltzman C, Brown T. The impact of osteoarthritis: implications for research. Clin Orthop Relat Res 2004(427 Suppl):S6–15.
Dillon CF, Rasch EK, Gu Q, Hirsch R. Prevalence of knee osteoarthritis in the United States: arthritis data from the Third National Health and Nutrition Examination Survey 1991-94. J Rheumatol. 2006;33(11):2271–9.
Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, Gabriel S, Hirsch R, Hochberg MC, Hunder GG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26–35.
Prevalence of doctor-diagnosed. Arthritis and arthritis-attributable activity limitation–United States, 2010–2012. MMWR Morb Mortal Wkly Rep. 2013;62(44):869–73.
Murphy L, Helmick CG. The impact of osteoarthritis in the United States: a population-health perspective: a population-based review of the fourth most common cause of hospitalization in U.S. adults. Orthop Nurs. 2012;31(2):85–91.
King J, Stamper DL, Schaad DC, Leopold SS. Minimally invasive total knee arthroplasty compared with traditional total knee arthroplasty. Assessment of the learning curve and the postoperative recuperative period. J Bone Joint Surg Am. 2007;89(7):1497–503.
Felson DT, Lawrence RC, Dieppe PA, Hirsch R, Helmick CG, Jordan JM, Kington RS, Lane NE, Nevitt MC, Zhang Y, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133(8):635–46.
Leopold SS. Minimally invasive total knee arthroplasty for osteoarthritis. N Engl J Med. 2009;360(17):1749–58.
Dalton DM, Burke TP, Kelly EG, Curtin PD. Quantitative analysis of Technological Innovation in knee arthroplasty: using Patent and Publication Metrics to identify Developments and Trends. J Arthroplasty. 2016;31(6):1366–72.
Lonner JH, Moretti VM. The evolution of image-free robotic assistance in Unicompartmental knee arthroplasty. Am J Orthop (Belle Mead NJ). 2016;45(4):249–54.
Matassi F, Pettinari F, Frasconà F, Innocenti M, Civinini R. Coronal alignment in total knee arthroplasty: a review. J Orthop Traumatol. 2023;24(1):24.
Cobb J, Henckel J, Gomes P, Harris S, Jakopec M, Rodriguez F, Barrett A, Davies B. Hands-on robotic unicompartmental knee replacement: a prospective, randomised controlled study of the acrobot system. J Bone Joint Surg Br. 2006;88(2):188–97.
de Steiger RN, Liu YL, Graves SE. Computer navigation for total knee arthroplasty reduces revision rate for patients less than sixty-five years of age. J Bone Joint Surg Am. 2015;97(8):635–42.
Mancino F, Jones CW, Benazzo F, Singlitico A, Giuliani A, De Martino I. Where are we now and what are we hoping to achieve with robotic total knee arthroplasty? A critical analysis of the current knowledge and future perspectives. Orthop Res Rev. 2022;14:339–49.
Innocenti B, Bori E. Robotics in orthopaedic surgery: why, what and how? Arch Orthop Trauma Surg. 2021;141(12):2035–42.
Karthik K, Colegate-Stone T, Dasgupta P, Tavakkolizadeh A, Sinha J. Robotic surgery in trauma and orthopaedics: a systematic review. Bone Joint J 2015, 97–b(3):292–299.
Bagaria V, Sadigale OS, Pawar PP, Bashyal RK, Achalare A, Poduval M. Robotic-assisted knee arthroplasty (RAKA): the technique, the Technology and the transition. Indian J Orthop. 2020;54(6):745–56.
Davies B. A review of robotics in surgery. Proc Inst Mech Eng H. 2000;214(1):129–40.
Murphy D, Challacombe B, Khan MS, Dasgupta P. Robotic technology in urology. Postgrad Med J. 2006;82(973):743–7.
Bautista M, Manrique J, Hozack WJ. Robotics in total knee arthroplasty. J Knee Surg. 2019;32(7):600–6.
Sousa PL, Sculco PK, Mayman DJ, Jerabek SA, Ast MP, Chalmers BP. Robots in the operating room during hip and knee arthroplasty. Curr Rev Musculoskelet Med. 2020;13(3):309–17.
Smith-Bindman R, Lipson J, Marcus R, Kim KP, Mahesh M, Gould R, Berrington de González A, Miglioretti DL. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med. 2009;169(22):2078–86.
Netravali NA, Shen F, Park Y, Bargar WL. A perspective on robotic assistance for knee arthroplasty. Adv Orthop. 2013;2013:970703.
Conditt MA, Roche MW. Minimally invasive robotic-arm-guided unicompartmental knee arthroplasty. J Bone Joint Surg Am. 2009;91(Suppl 1):63–8.
Plate JF, Mofidi A, Mannava S, Smith BP, Lang JE, Poehling GG, Conditt MA, Jinnah RH. Achieving accurate ligament balancing using robotic-assisted unicompartmental knee arthroplasty. Adv Orthop. 2013;2013:837167.
Song EK, Seon JK, Park SJ, Jung WB, Park HW, Lee GW. Simultaneous bilateral total knee arthroplasty with robotic and conventional techniques: a prospective, randomized study. Knee Surg Sports Traumatol Arthrosc. 2011;19(7):1069–76.
Song EK, Seon JK, Yim JH, Netravali NA, Bargar WL. Robotic-assisted TKA reduces postoperative alignment outliers and improves gap balance compared to conventional TKA. Clin Orthop Relat Res. 2013;471(1):118–26.
Koulalis D, O’Loughlin PF, Plaskos C, Kendoff D, Cross MB, Pearle AD. Sequential versus automated cutting guides in computer-assisted total knee arthroplasty. Knee. 2011;18(6):436–42.
Suero EM, Plaskos C, Dixon PL, Pearle AD. Adjustable cutting blocks improve alignment and surgical time in computer-assisted total knee replacement. Knee Surg Sports Traumatol Arthrosc. 2012;20(9):1736–41.
Lonner JH, Smith JR, Picard F, Hamlin B, Rowe PJ, Riches PE. High degree of accuracy of a novel image-free handheld robot for unicondylar knee arthroplasty in a cadaveric study. Clin Orthop Relat Res. 2015;473(1):206–12.
Lonner JH. Indications for unicompartmental knee arthroplasty and rationale for robotic arm-assisted technology. Am J Orthop (Belle Mead NJ). 2009;38(2 Suppl):3–6.
Coon TM. Integrating robotic technology into the operating room. Am J Orthop (Belle Mead NJ). 2009;38(2 Suppl):7–9.
Lonner JH, John TK, Conditt MA. Robotic arm-assisted UKA improves tibial component alignment: a pilot study. Clin Orthop Relat Res. 2010;468(1):141–6.
Pearle AD, O’Loughlin PF, Kendoff DO. Robot-assisted unicompartmental knee arthroplasty. J Arthroplasty. 2010;25(2):230–7.
Citak M, Suero EM, Citak M, Dunbar NJ, Branch SH, Conditt MA, Banks SA, Pearle AD. Unicompartmental knee arthroplasty: is robotic technology more accurate than conventional technique? Knee. 2013;20(4):268–71.
Tamam C, Plate JF, Augart M, Poehling GG, Jinnah RH. Retrospective Clinical and Radiological Outcomes after Robotic Assisted Bicompartmental Knee Arthroplasty. Adv Orthop 2015, 2015:747309.
Schulz AP, Seide K, Queitsch C, von Haugwitz A, Meiners J, Kienast B, Tarabolsi M, Kammal M, Jürgens C. Results of total hip replacement using the Robodoc surgical assistant system: clinical outcome and evaluation of complications for 97 procedures. Int J Med Robot. 2007;3(4):301–6.
Nawabi DH, Conditt MA, Ranawat AS, Dunbar NJ, Jones J, Banks S, Padgett DE. Haptically guided robotic technology in total hip arthroplasty: a cadaveric investigation. Proc Inst Mech Eng H. 2013;227(3):302–9.
Domb BG, El Bitar YF, Sadik AY, Stake CE, Botser IB. Comparison of robotic-assisted and conventional acetabular cup placement in THA: a matched-pair controlled study. Clin Orthop Relat Res. 2014;472(1):329–36.
Liow MH, Xia Z, Wong MK, Tay KJ, Yeo SJ, Chin PL. Robot-assisted total knee arthroplasty accurately restores the joint line and mechanical axis. A prospective randomised study. J Arthroplasty. 2014;29(12):2373–7.
Yildirim G, Fernandez-Madrid I, Schwarzkopf R, Walker PS, Karia R. Comparison of robot surgery modular and total knee arthroplasty kinematics. J Knee Surg. 2014;27(2):157–63.
Bukowski BR, Anderson P, Khlopas A, Chughtai M, Mont MA, Illgen RL. 2nd: Improved Functional Outcomes with robotic compared with manual total hip arthroplasty. Surg Technol Int. 2016;29:303–8.
Illgen RLN, Bukowski BR, Abiola R, Anderson P, Chughtai M, Khlopas A, Mont MA. Robotic-assisted total hip arthroplasty: outcomes at Minimum two-year Follow-Up. Surg Technol Int. 2017;30:365–72.
Kayani B, Konan S, Huq SS, Tahmassebi J, Haddad FS. Robotic-arm assisted total knee arthroplasty has a learning curve of seven cases for integration into the surgical workflow but no learning curve effect for accuracy of implant positioning. Knee Surg Sports Traumatol Arthrosc. 2019;27(4):1132–41.
Vermue H, Luyckx T, Winnock de Grave P, Ryckaert A, Cools AS, Himpe N, Victor J. Robot-assisted total knee arthroplasty is associated with a learning curve for surgical time but not for component alignment, limb alignment and gap balancing. Knee Surg Sports Traumatol Arthrosc. 2022;30(2):593–602.
Kayani B, Konan S, Pietrzak JRT, Huq SS, Tahmassebi J, Haddad FS. The learning curve associated with robotic-arm assisted unicompartmental knee arthroplasty: a prospective cohort study. Bone Joint J 2018, 100–b(8):1033–1042.
Tay ML, Carter M, Bolam SM, Zeng N, Young SW. Robotic-arm assisted unicompartmental knee arthroplasty system has a learning curve of 11 cases and increased operating time. Knee Surg Sports Traumatol Arthrosc. 2023;31(3):793–802.
Favroul C, Batailler C, Canetti R, Shatrov J, Zambianchi F, Catani F, Servien E, Lustig S. Image-based robotic unicompartmental knee arthroplasty allowed to match the rotation of the tibial implant with the native kinematic knee alignment. Int Orthop. 2023;47(2):519–26.
Clement ND, Deehan DJ, Patton JT. Robot-assisted unicompartmental knee arthroplasty for patients with isolated medial compartment osteoarthritis is cost-effective: a markov decision analysis. Bone Joint J 2019, 101–b(9):1063–1070.
Funding
The authors declare that no fund, grant, or other support was received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to this review. The idea was conceived by Jong Keun Seon. Literature search, data analysis, and drafting of the manuscript were performed by Hong Yeol Yang and Jong Keun Seon. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, H.Y., Seon, J.K. The landscape of surgical robotics in orthopedics surgery. Biomed. Eng. Lett. 13, 537–542 (2023). https://doi.org/10.1007/s13534-023-00321-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13534-023-00321-8