Abstract
Purpose
Computer navigation increases accuracy and precision of component alignment in total knee arthroplasty (TKA) compared to the manual technique, but is often associated with increases in surgical time. In a previous cadaver study, we demonstrated a significant improvement in guide positioning precision, final bone cut precision, and procedure length when using adjustable cutting blocks (ACB) compared to conventional cutting blocks (CCB) in computer-navigated TKA. The aim of this study was to evaluate the use of ACB in vivo.
Methods
We radiographically compared component alignment and mechanical leg alignment, as well as tourniquet time, in 94 patients who underwent TKA using either ACB (N = 30) or CCB (N = 64).
Results
Postoperative mechanical alignment variability was significantly less in the ACB group (SD = 1.7°) than in the CCB group (SD = 2.7°). Tourniquet time was significantly reduced by 14.8 min in the ACB group compared to the CCB. Differences in component alignment were not significant.
Conclusion
ACB for TKA significantly reduced postoperative mechanical alignment variability and tourniquet time compared to conventional navigated instrumentation, while providing equal or better component alignment.
Level of evidence
III.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Long-term implant survivorship in total knee arthroplasty (TKA) is dependent on numerous factors, including patient selection, implant design, soft tissue balancing, and surgical technique. Previous research has also identified alignment of the tibial and femoral components and mechanical leg alignment as important predictors of long-term survivorship [17, 18]. Computer navigation increases accuracy and precision of component and limb alignment in TKA compared to the manual technique [5, 13, 15, 19]. However, the use of computer navigation technology is often associated with an increase in surgical time ranging from 11 to 24 min on average [3, 5].
Approximately, eighty percent of TKAs performed in the United States are currently executed by surgeons who perform 50 or less TKAs per year [7]. Reports also suggest that short-term outcomes in TKA are related to surgeon procedure volume [4]. Therefore, the idea of using computer navigation as a tool to help decrease the number of alignment outliers and related revision procedures has merit. However, in order for computer-assisted surgery to be cost-effective in low-procedure volume scenarios, a significantly higher reduction in malalignment and associated revision rate is necessary [22].
In a previous cadaver study [12], we demonstrated a significant improvement in guide positioning precision, final bone cut precision, and procedure length when using adjustable cutting blocks (ACB) compared to conventional cutting blocks (CCB) in computer-navigated TKA. The aim of this study was to evaluate the use of ACB in vivo. We hypothesized that the use of ACB would (1) improve tibial and femoral component positioning; (2) improve postoperative mechanical leg alignment; and (3) decrease tourniquet time, when compared to CCB.
Materials and methods
This study was approved by the Institutional Review Board. This was a retrospective cohort study of consecutive patients who underwent navigated primary TKA by a single surgeon between 2006 and 2010. Prior to surgery, all patients in the TKA group had evidence of end-stage knee osteoarthritis in at least two compartments (Kellgren–Lawrence score, ≥3) [8]. Patients were excluded if a revision was being performed, if they had lower limb fractures, or if they had no radiographic follow-up. A total of ninety-four patients met the inclusion and exclusion criteria and were included in the study. Patients were classified into two groups according to whether the surgery had been performed using ACB (Nanoblock, Praxim, Grenoble, France) or CCB (DePuy Sigma®, DePuy Orthopaedics, Inc., Warsaw, IN). There were sixty-four patients (68%) in the CCB group and 30 patients (32%) in the ACB group. Both groups had similar demographic composition (Table 1). Postoperative long-leg radiographs were available for fifty patients (78%) in the CCB group and twenty-three patients (77%) in the ACB group. Lateral views were available for sixty patients (94%) in the CCB group and for twenty-two (87%) patients in the ACB group.
Surgical technique
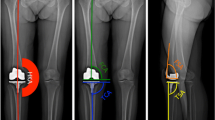
A standard total knee exposure using a midline parapatellar approach followed by a medial arthrotomy was performed on all cases. The navigation procedure in the CCB group was performed using either the VectorVision system (BrainLab, Feldkirchen, Germany) (43%) or the NanoStation Total Knee Surgetics system (Praxim, Grenoble, France) (57%). All of the ACB cases were done using the NanoStation system. The tourniquet was inflated after placement of the tibial tracker pins and just prior to making the skin incision. It was deflated after prosthesis cementation, just prior to wound closure. Two 3.2-mm tracker pins were used for fixation of the tibial reference array in the diaphysis. One 4-mm tracker pin and one 3.2-mm tracker pin were placed bicortically in a medial-to-lateral direction in the femoral metaphysis, within the incision, for fixation of the femoral array. Navigation was used throughout the procedure to verify the component and mechanical alignment before and after cementing, as well as for placement of the cutting block (Fig. 1).
Clinical assessment
Charts were reviewed to obtain the following data: age, gender, body mass index (BMI), tourniquet time, and operated side. Pre- and postoperative standing full-leg radiographs were reviewed to assess mechanical alignment [21], as well as tibial and femoral alignment in the coronal plane [2]. Lateral knee radiographs were used to measure sagittal tibial component alignment [2]. All measurements were made on digital radiographic images stored in our institution’s PACS system. Two examiners performed all the measurements, and interobserver agreement was assessed using intraclass correlation (ICC) [1, 2]. The mean of the two measurements was used for the final analysis.
Statistical analysis
Through an a priori power analysis based on the results of our previous cadaveric experiment, we determined that a minimum of 21 patients in the ACB group and 43 patients in the CCB group would be needed to detect a 0.5° difference in standard deviation (SD) of alignment between groups at the 95% confidence level and with 80% power. After testing for normality using the Shapiro–Wilk test, F tests were used to detect differences in variance for component alignment and mechanical alignment between the ACB group and the CCB group. We evaluated the differences in tourniquet time using stepwise multiple linear regression analysis. Our independent variables were as follows: cutting block type, BMI, age, gender, and type of navigation system used [20]. The selection of independent variables in the final models was based on their clinical significance and association with the dependent variable. We also identified cases of component or mechanical malalignment, defined as a difference greater than 3° from neutral [5], within each group. ICC was calculated using an online calculator [23]. Other statistical analyses were performed using Stata/IC 10.1 (StataCorp LP, College Station, TX). Results are presented as mean, SD, and 95% confidence interval (95% CI), when appropriate. Significance was set at α = 0.05. All P values quoted are for two-tailed tests.
Results
Component alignment
Mean coronal femoral alignment for the CCB group was 0.8° varus (SD = 1.95°), and for the ACB group, it was 1.1° varus (SD = 1.5°) (n.s.) (Fig. 2a). 5 out of 49 CCB cases (10%) and 2 out of 22 ACB cases (9%) were outside the ±3° limits of neutral alignment.
Mean coronal tibial alignment for the CCB group was 0.1° valgus (SD = 1.3°), and for the ACB group, it was 0.5° varus (SD = 1.01) (n.s.). One CCB case (2%) was outside the neutral alignment limits. No ACB cases were malaligned (Fig. 2b).
Sagittal tibial alignment was a mean 0.5° of anterior slope (SD = 2.9°) for the CCB group and 0.7° anterior slope (SD = 2.5°) for the ACB group (n.s.). 15 out of 60 CCB cases were malaligned (25%), compared to 4 out of 26 ACB cases (15%) (Fig. 2c).
Mechanical leg alignment
Preoperatively, the CCB group had a mean mechanical alignment of 1.8° varus (SD = 9.6°), while the ACB group had a mean 1.8° varus (SD = 9.37°) (n.s.).
After surgery, mechanical leg alignment variability, as reflected by the SD, improved for the CCB (mean = 0.7° varus; SD = 2.7°; P < 0.0001) and the ACB group (mean = 1.8° varus; SD = 1.7°; P < 0.0001). However, there was significantly less variability in postoperative mechanical alignment in the ACB group compared to the CCB group (P = 0.0091) (Fig. 3). 12 out of 50 patients in the CCB group (24%) and 4 out of 23 patients in the ACB group (17%) had postoperative mechanical malalignment.
Tourniquet time
In our multiple linear regression model, the use of an adjustable cutting block reduced tourniquet time by 14.8 min (P = 0.008), from 91 min (SD = 17.7 min) to 76 min (SD = 16.7 min), compared to using CCB (Fig. 4). Tourniquet time was 8.1 min less for women than for men (P = 0.051). No significant effects of BMI, age, operated side, and type of navigation system used were found (n.s.).
Interobserver agreement
There was high interobserver reliability for all radiographic measurements. The ICC for coronal femoral alignment was 0.88. For coronal tibial alignment, the ICC was 0.86. For sagittal tibial alignment, the ICC was 0.85. Finally, the ICC for preoperative mechanical alignment measurements was 0.99, while for postoperative measurements, it was 0.98.
Discussion
The most important finding of this study was that the use of an adjustable cutting block significantly reduced tourniquet time and postoperative mechanical alignment variability. Furthermore, although statistically nonsignificant, the adjustable cutting block group showed less variability of coronal femoral and tibial component alignment than the conventional group and a lower percentage of malaligned tibial components.
Computer navigation improves implant positioning and mechanical alignment [5, 13, 15, 19]. However, a perceived drawback is the increase in surgical time often associated with the technology [5], which limits its cost-effectiveness. Manzotti et al. [14] showed that surgeon experience with navigation could help reduce surgical time. Improved instrumentation, better suited to navigated procedures, could also help decrease the time required to perform navigated surgeries. Klima et al. [9] first showed an improvement in the time required for the fixation of an adjustable cutting guide of 3.5 min over conventional blocks (2.9 vs. 6.4 min). Our previous cadaver study demonstrated a 4.5-min improvement over conventional guides (2.2 vs. 6.6 min) by reducing the time required for guide positioning [12]. This may be due primarily to an easier fixation and alignment process, as the initial fixation of the guide to the bone does not have to precisely match the desired target alignment for the cut. Rather, it can be approximated and then fine-tuned by turning the adjustment screws on the guide with the aid of the navigation system’s interface. Although we did not record the time required for each surgical step, we have shown in the current study that the use of the adjustable cutting block reduced tourniquet time by an estimated 14.8 min compared to the conventional jigs. This is especially important considering that early release of the tourniquet results in a reduction in postoperative pain and earlier straight-leg raising [1] and that prolonged operating time is associated with a higher rate of infection [24]. Furthermore, considering the current overhead costs of a surgical center, this is a significant reduction that may increase the cost-effectiveness of navigated TKA [16]. Moreover, as the alignment of the cutting guide can be adjusted after fixation to the bone, the target alignment of the cut can be adjusted and achieved with more precision. This was confirmed by the results of the current study. We should also note that, although our measurement of sagittal tibial slope showed a slightly anterior positioning of the component, this is most likely due to the radiographic measurement technique used. Since as previous research has shown that using the posterior cortex as reference can lead to a difference of about 3° when compared to the anatomic axis of the tibia, it is likely that the true position of the tibial component is closer to our target of 3º of posterior slope.
There are several possible limitations of this research. First, the retrospective nature of the study may introduce confounding factors to the results. When testing for differences in tourniquet time, we controlled for age, BMI, operated side, gender, and the type of navigation system used [20]. We found that men had higher tourniquet times than women. This is consistent with the findings by Kosashvili et al. [10] and may be due to differences in lean mass percentage, femoral morphology, soft tissue laxity, or other unexplored variables. Importantly, the gender composition of both groups was similar, with a slightly higher percentage of men in the adjustable cutting group (33%) than in the conventional group (24%) (n.s.). However, since the two groups overlap in time, we cannot rule out the occurrence of selection bias in deciding to use the adjustable block versus the conventional instrumentation. Second, as this study focused on the results of a single surgeon at a single hospital, we cannot state how generalizable the improvements in tourniquet time and alignment may be. However, these in vivo results are consistent with our previous cadaver study, in which the surgeries were performed by a different surgeon [12]. Third, the measurement of postoperative mechanical alignment and implant positioning was performed on plain radiographs. The accuracy of this method is always of concern, especially if malrotation is present. All radiographs evaluated in the current study were performed by the same department within the same institution using a standard technique. While we attempted to reduce measurement error by excluding radiographs if technique problems were identified and using the next available postoperative radiograph, the reader should be aware of this limitation. Fourth, we specifically evaluated tourniquet time and not operating room time. We acknowledge that a change in tourniquet time may not directly correlate to total operating room time. However, the surgical technique and the setup for the navigation system were exactly the same in both groups, except for the additional fixation of the NanoBlock in the ACB group. Since NanoBlock fixation was performed after the tourniquet was inflated, we believe that tourniquet time effectively conveys the time efficiency information necessary to compare both techniques. Fifth, surgeries in the CCB group were performed using two different navigation systems, which may confound the results. However, we have accounted for the type of navigation system used in our regression model, and we found this variable not to have a significant association with the outcome variable, in this model. Sixth, since the start of the study period, new technology has become available. Robotic cutting blocks [11] and custom jigs are [6] now being increasingly used. However, we believe that this only further underscores the current interest in increasing the productivity of TKA while improving outcomes.
The findings of this study may be of special interest to surgeons who are currently performing computer-navigated TKA and who wish to further reduce surgical time without compromising the quality of the procedure.
Conclusion
ACB for TKA significantly reduced postoperative mechanical alignment variability and tourniquet time compared to conventional navigated instrumentation, while providing equal or better component alignment.
References
Barwell J, Anderson G, Hassan A, Rawlings I (1997) The effects of early tourniquet release during total knee arthroplasty: a prospective randomized double-blind study. J Bone Joint Surg Am 79(2):265–268
Bathis H, Perlick L, Tingart M, Luring C, Zurakowski D, Grifka J (2004) Alignment in total knee arthroplasty. A comparison of computer-assisted surgery with the conventional technique. J Bone Joint Surg Br 86(5):682–687
Bolognesi M, Hofmann A (2005) Computer navigation versus standard instrumentation for TKA: a single-surgeon experience. Clin Orthop Relat Res 440:162–169
Bozic KJ, Maselli J, Pekow PS, Lindenauer PK, Vail TP, Auerbach AD (2010) The influence of procedure volumes and standardization of care on quality and efficiency in total joint replacement surgery. J Bone Joint Surg Am 92(16):2643–2652
Dutton AQ, Yeo SJ, Yang KY, Lo NN, Chia KU, Chong HC (2008) Computer-assisted minimally invasive total knee arthroplasty compared with standard total knee arthroplasty. A prospective, randomized study. J Bone Joint Surg Am 90(1):2–9
Hasegawa M, Yoshida K, Wakabayashi H, Sudo A (2011) Minimally invasive total knee arthroplasty: comparison of jig-based technique versus computer navigation for clinical and alignment outcome. Knee Surg Sports Traumatol Arthrosc 19(6):904–910
Katz JN, Barrett J, Mahomed NN, Baron JA, Wright RJ, Losina E (2004) Association between hospital and surgeon procedure volume and the outcomes of total knee replacement. J Bone Joint Surg Am 86-A(9):1909–1916
Kellgren JH, Lawrence JS (1957) Radiological assessment of osteo-arthrosis. Ann Rheum Dis 16(4):494–502
Klima S, Zeh A, Josten C (2008) Comparison of operative time and accuracy using conventional fixed navigation cutting blocks and adjustable Pivotal cutting blocks. Comput Aided Surg 13(4):225–232
Kosashvili Y, Mayne IP, Trajkovski T, Lackstein D, Safir O, Backstein D (2010) Influence of sex on surgical time in primary total knee arthroplasty. Can J Surg 53(4):256–260
Koulalis D, O’Loughlin PF, Plaskos C, Kendoff D, Cross MB, Pearle AD (2010) Sequential versus automated cutting guides in computer-assisted total knee arthroplasty. Knee. doi:10.1016/j.knee.2010.08.007
Koulalis D, O’Loughlin PF, Plaskos C, Kendoff D, Pearle AD (2010) Adjustable cutting blocks for computer-navigated total knee arthroplasty a cadaver study. J Arthroplasty 25(5):807–811
Luring C, Beckmann J, Haibock P, Perlick L, Grifka J, Tingart M (2008) Minimal invasive and computer assisted total knee replacement compared with the conventional technique: a prospective, randomised trial. Knee Surg Sports Traumatol Arthrosc 16(10):928–934
Manzotti A, Cerveri P, De Momi E, Pullen C, Confalonieri N (2010) Relationship between cutting errors and learning curve in computer-assisted total knee replacement. Int Orthop 34(5):655–662
Mason JB, Fehring TK, Estok R, Banel D, Fahrbach K (2007) Meta-analysis of alignment outcomes in computer-assisted total knee arthroplasty surgery. J Arthroplasty 22(8):1097–1106
Novak EJ, Silverstein MD, Bozic KJ (2007) The cost-effectiveness of computer-assisted navigation in total knee arthroplasty. J Bone Joint Surg Am 89(11):2389–2397
Ritter MA, Davis KE, Meding JB, Pierson JL, Berend ME, Malinzak RA (2011) The Effect of Alignment and BMI on Failure of Total Knee Replacement. J Bone Joint Surg Am 93(17):1588–1596
Ritter MA, Faris PM, Keating EM, Meding JB (1994) Postoperative alignment of total knee replacement. Its effect on survival. Clin Orthop Relat Res 299:153–156
Rosenberger RE, Hoser C, Quirbach S, Attal R, Hennerbichler A, Fink C (2008) Improved accuracy of component alignment with the implementation of image-free navigation in total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 16(3):249–257
Sampath SA, Voon SH, Sangster M, Davies H (2009) The statistical relationship between varus deformity, surgeon’s experience, BMI and tourniquet time for computer assisted total knee replacements. Knee 16(2):121–124
Sharma L, Song J, Felson DT, Cahue S, Shamiyeh E, Dunlop DD (2001) The role of knee alignment in disease progression and functional decline in knee osteoarthritis. JAMA 286(2):188–195
Slover JD, Tosteson AN, Bozic KJ, Rubash HE, Malchau H (2008) Impact of hospital volume on the economic value of computer navigation for total knee replacement. J Bone Joint Surg Am 90(7):1492–1500
The Chinese University of Hong Kong stats toolbox: intraclass correlation. http://departmentobgcuhkeduhk/researchsupport/IntraClass_correlationasp
Willis-Owen CA, Konyves A, Martin DK (2010) Factors affecting the incidence of infection in hip and knee replacement: an analysis of 5277 cases. J Bone Joint Surg Br 92(8):1128–1133
Conflict of interest
Dr. Plaskos is an employee of Praxim Inc., manufacturer of the navigation system used in this study. None of the other authors have any conflicts of interest to report.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Suero, E.M., Plaskos, C., Dixon, P.L. et al. Adjustable cutting blocks improve alignment and surgical time in computer-assisted total knee replacement. Knee Surg Sports Traumatol Arthrosc 20, 1736–1741 (2012). https://doi.org/10.1007/s00167-011-1752-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-011-1752-1