Abstract
Food is a must for all life to survive. Global food production has increased owing to population augmentation, increasing the generation of food and agricultural residues. Agro-wastes are important sources of natural compounds such as biopolymers that can be used to develop value-added products contributing to replacing synthetic compounds. Biopolymers like pectin, starch, and chitin, have multiple functional properties, useful to improve the sensorial properties of foods, and provide a sustainable alternative to conventional materials in food processing and packaging. Researchers and the industrial sector have successfully converted agricultural residues into valuable edible biopolymers with prebiotic potential, antioxidant, and antimicrobial properties by using emerging technologies such as ultrasound, microwave-assisted extraction, enzymatic hydrolysis, fermentation, and/or bioconversion. The variation of the extraction conditions using emerging technologies allows the modification of the structural properties of the polymer giving a wide range for their application in the food sector. Besides, the incorporation of Industry 4.0 in these processes allows the optimization, automatization, and obtention of high yields of polymers of improved quality. This review highlights the potential of emerging technologies to convert agricultural waste into valuable biopolymers to promote a greener and more innovative food sector.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The constant growth of the global population promotes the increment in food processing demand, rising the amount generation of food processing residues [1]. Over the past 50 years, agriculture has become more productive than ever, producing 23.7 million tons of food per day, producing around 250 million tons each year of waste biomass [2]. Agricultural by-products are materials that are generated during agricultural production or processing but are not the primary target products. These materials are often considered waste or residues, but they can be valuable resources that can be utilized for various purposes [3,4,5]. The type of crops grown, the area, and the agricultural methods all affect the kind and amount of agro-waste generated annually [6]. Food and Agriculture Organization of the United Nations estimated that global agricultural production produces about 5 billion tons of crop residues per year [7]. However, the actual amount of agro-waste is difficult to quantify due to the absence of comprehensive data in many countries.
Agro-waste can have both beneficial and negative effects. On the plus side, agro-waste can be utilized as a source of biomass for the generation of energy, such as biogas or biofuels, as well as for the development of functional foods, functional or intelligent packaging, and organic fertilizer. Instead, if agro-wastes are not managed properly, they can lead to environmental problems such as soil degradation, water pollution, and greenhouse gas emissions. Agro-wastes can also serve as a breeding ground for pests and diseases, leading to the spread of plant pathogens [6, 8]. Concern over the disposal issues has sparked interest in the research into the valorization of waste streams. Valorization lessens the amount of waste produced while also having a positive environmental impact [1, 3]. By recycling agricultural wastes into economically viable goods with added value, society may not only encourage waste recovery but also increase its profits [1, 9].
In the last years, efforts have been made to find alternative materials that can be labeled as “green” to meet the growing demand for sustainable products. These contribute to the replacement of synthetic compounds or high-cost natural compounds. Green materials provide opportunities to valorize the agro-waste which is highly valuable, attending the sustainable production trend [3, 10]. A growing interest is being shown in the extraction and use of biopolymers because of their biodegradability/compostability and biocompatibility. Biocompatible polymers have the potential to address sustainability and safety concerns in the food industry, while also improving efficiency and functionality without introducing harmful substances into the food system [11, 12]. Additionally, biopolymers made from these wastes have a wide range of chemical and mechanical characteristics and can be applied in a variety of ways such as biodegradable packaging [13, 14], coatings [15], protect active compounds [16], and biocontrol agents [17], and to develop functional foods [18]. There are two basic categories into which biopolymers can be placed: (1) polymers produced by living organisms like bacteria, plants, and animals, and (2) polymers produced chemically as a result of the biological conversion of amino acids, sugars, natural fats, or oils [19]. These are available in high amounts in natural sources such as agro-waste. In addition to the aforementioned effects of improper waste management, the use of conventional solvent extraction also harms the environment. For these reasons, the food industry and scientific sector are in a constant search of solutions to the replacement of synthetic compounds and the use of agro-wastes by green processes [20, 21]. However, it is complicated for the food industry sector to maintain a continuous supply of natural polymers obtained from agro-waste using emerging technologies due to the unmet demand and underdeveloped commercialization of these sustainable materials. While there is growing interest in eco-friendly alternatives to traditional polymers, the widespread adoption of biopolymers from agro-wastes is hindered by various factors. These include limited awareness among consumers and industry stakeholders, technical challenges in optimizing extraction and processing methods, and the need for standardized quality control and regulatory frameworks [22, 23]. Addressing these gaps through comprehensive research and collaboration between academia, industry, and regulatory bodies is essential to bridge the divide between the current state of knowledge and the practical implementation of agro-waste-derived biopolymers in the food sector [24].
Following this idea, every alternative implemented for biodegradable polymer production can improve sustainability and contributes to circular economy models. This review serves as a comprehensive source of insights into the transformative potential of emerging technologies for converting the vast amount of agricultural residues into valuable biopolymers. The scientific advances, challenges, and opportunities associated with this process were highlighted, providing a deeper understanding of how biopolymers from agricultural waste can give a better opportunity to integrate them into the food industry. This knowledge enables researchers, policymakers, and industry stakeholders to take informed decisions, promoting research and development efforts, and driving the adoption of innovative, environmentally friendly materials in the food sector. In essence, this review points the way to a future where the sustainable use of agricultural waste plays a central role in improving the resilience and efficiency of the food industry, minimizing its environmental footprint into the circular economy concept.
2 Agricultural by-products
Agro-wastes are plentiful in nature and high in carbon content, making them desirable renewable substrates for the synthesis of biopolymers for a variety of technological applications. Agricultural wastes include lignocellulosic biomass, agro-industrial effluents, and residues from bioconversion processes [10]. These by-products can be classified into several categories based on their origin and characteristics. Some common categories of agricultural residues include (i) plant wastes which are generated after harvesting or pruning crops, such as straws, stalks, leaves, husks, and shells. These residues can be processed to extract cellulose, lignin, and hemicellulose; (ii) animal by-products are generated during the transformation of animal products, such as meat, dairy, and leather, including bones, skin, blood, feathers, and fat, which are a source of collagen, gelatin, and fatty acids; (iii) food processing wastes are produced during the processing of agriculture products, such as fruits, vegetables, grains, and oilseeds. They can be processed to obtain pectin, fiber, and oils; and (iv) waste from agro-industrial processing, which is generated during the processing of agricultural products into value-added products, such as biofuels, bioplastics, and biochemicals. Other agricultural by-products that do not fit into the above categories, such as weeds, invasive species, and residues from bioenergy production are used for animal feed, fertilizer, or fuel and the extraction of natural dyes, antioxidants, and bioactive compounds [6, 8, 10, 25]. Most of these are used for animal feed and biofuel production. However, proper structural modifications of these residues can lead to obtaining biopolymers with improved properties [19, 26, 27].
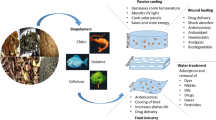
The accessibility of starting materials/precursors, which should be reasonably priced and easily accessible in significant quantities, is necessary for the production of bio-based polymers from agro-wastes. Due to their massive production capacities and share of global output, the two largest economies in the world, China and India, could take the lead in the manufacture of fruit- and vegetable-based biopolymers [4]. The general process to obtain and apply a polymer in the food industry follows the next steps, identification of the source, a clear idea of the properties of the polymer, selection of the extraction technique and conditions, assessment of methods for their incorporation in a food process (Fig. 1).
3 Methods biopolymer for extraction: influence on structure and properties
The potential for using agro-industrial wastes as primary or secondary feedstocks for the fermentation or extraction of biopolymers is immense [3]. Biopolymers created from agricultural waste have a wide range of chemical and mechanical properties, making them ideal for several applications [26, 28]. To recover biomass polymers, numerous alternative processing methods have been carefully designed. Biopolymers are often created in one of three ways: directly from biomass, such as certain polysaccharides and proteins; by microorganisms; or from genetically engineered bacteria [29]. Some conventional and novel methods used to extract polymers from agro-waste are reviewed in more detail in the following sections.
3.1 Traditional methods to extract polymers from agro-wastes
Acids, bases, and surfactants are used in chemical extraction. The most popular chemical process for obtaining biopolymers is alkaline extraction using sodium hydroxide. Additionally, it has been proven to be a successful approach for the breakdown of chemical bonds to solubilize hydrophobic proteins [29]. The nature and selectivity of interactions with the cell wall are determined by the used solvent, efficiency disruption is highly dependent on it. Due to the incomplete knowledge of the affinities of each solvent to various macromolecules, it is difficult to predict the mechanism involved in a chemical extraction [30]. Pre-treatment of biomass is essential for its use; it is important to consider the relationship between the structure and function of the biopolymer or other polymers that are derived from the same source, such as lignin and cellulose [31]. Source influences polymer extraction more significantly than polymer type. For instance, a demineralization process is needed to remove calcium carbonate, which makes up 30 to 50% of crab shells, to extract chitosan from the shells. The extraction requires large amounts of chemicals, including acids and alkalis (in concentrations ranging from 30 to 50%, w/v), high processing temperatures (> 100 °C), and extended processing durations. While no demineralization procedure is necessary if the source is a fungal cell wall because fungal mycelia have a lower concentration of inorganic elements. As a result, extraction needs for mildly alkaline and acidic (0.1–0.2 N) conditions as well as temperatures between 100 and 120 °C for a brief time (1 h) are required for extraction [12, 32].
Since solvent extraction yields positive outcomes and more sustainable procedures are being developed, new and alternative solvents with ecologically friendly profiles are continually needed. Deep eutectic solvents (DESs) have become more popular in the extraction industry as a result. These are a new class of compounds known as DESs, which can comprise a wide range of anionic and/or cationic species and are composed of Lewis or Brønsted acids and bases, which are distinguished by large depressions in their melting temperatures relative to their neat constituent components [33, 34]. Some DESs are already safe because they are made of gentle ingredients. However, several amides and polyols with minimal inherent toxicity, including urea, glycerol, ethylene glycol, fructose, and erythritol, can make various formulations of type III eutectics. Metal salts with their innate toxicity are present in all types I, II, and IV eutectics. Fractions rich in cellulose and lignin were obtained from chestnut residues using a mixture of chlorine chloride-oxalic acid dihydrate. Compositional analyses show that the contents of cellulose/hemicellulose and lignin are consistent with reports in the literature. The scientists concluded that another method for valuing these leftovers is to fully use chestnut shells by employing safe and environmentally friendly solvents, such as DES [33].
The use of proteolytic bacteria or enzymes provides a greener alternative to chemical extraction [35]. The most popular method for breaking down the cell walls is enzyme-assisted extraction. To choose the best enzyme to break down certain macromolecules using this technique, one should be aware of the complexity and composition of each source, which could reduce the process’s efficiency [29, 36]. The protein hydrolysates also contained bioactive peptides that might be employed in animal feed as emulsifiers and growth accelerators [37]. The yield and purity of biopolymer obtained by chemical extraction can be increased by including enzymes in the process. The rod-shaped cellulose nanocrystals obtained from sugarcane bagasse by acid hydrolysis (H2SO4 solution, 64% w/w; 1:10 g/mL cellulose: diluted H2SO4 at 45 °C for 60 min) had lengths between 250 and 480 nm and diameters between 20 and 60 nm. While X-ray diffraction and thermal examination found that cellulose nanocrystals had higher crystallinity (72.5%) than chemically pure cellulose (63.5%) but showed inferior thermal stability, the elemental analysis revealed 0.72% sulfur impurity. Lab-extracted cellulose has the potential to be nano-reinforced into bio-nanocomposite for industrial applications because of the high crystallinity (72.5%) [38].
Some advantage of the use of enzymes for chitin extraction is the obtention of polymers that have higher molecular weight compared to those chemically obtained [39]. By employing a demineralization process (1 M HCl, ratio 1:30, 75 min at room temperature) followed by deproteinization (3 M NaOH, ratio 1:30, 75 min at room temperature) and finally the decolorization step (50% NaOH, ratio 1:50, 90 °C for 50 min), α-chitosan was extracted from shrimp shells (Penaeus monodon), which significantly inhibit the growth of the human ovarian cancer cell line PA-1 [40]. α-Chitosan is less reactive than β-chitosan to cellulase hydrolysis due to its higher crystallinity and structural properties which are strongly affected by deacetylation degree (up to 60%) and molecular weight [41]. The proper selection enzyme is a key point in the properties of the polymer. Agree with this, jackfruit leaf protein hydrolysates obtained with pancreatin had a high content of essential amino and hydrophobic amino acids, due to a higher degree of hydrolysis. Besides, the lower β-sheet fractions and high β-turn contents improved the emulsifying properties in comparison to the hydrolysates obtained with pepsin [42]. As a result, enzymes are crucial in the extraction of biopolymers from a variety of sources, including biomass, bacteria, and marine species (Table 1). Biopolymers have intricate structures that can be broken down by enzymes into smaller, easier-to-manage molecules, making separation and purification easier. Additionally, they can be utilized to change the extracted biopolymers’ characteristics, expanding the range of applications. Finally, the extraction effectiveness can be increased by combining enzymes such as cellulase, xylanase, viscozyme, and lysozyme to degrade these structures. This results in better yields than chemical extraction because natural sources are complex and can be composed of several polymers.
3.2 Microwave-assisted extraction (MAE)
MAE is a promising technique for the extraction of biopolymers from various sources, which is based on microwave irradiation, which directly activates the majority of molecules with a dipole rotation or ionic conductivity. As a result of the material’s interactions with the electromagnetic field, the temperature will increase quickly [29, 51]. To facilitate mass transfer and the subsequent extraction of the desired chemicals, H-bonds are broken and dissolved ions move into the biomass, increasing solvent permeation into the biomass; resulting causes the biopolymers to expand and rupture, releasing more extractable material [29]. MAE has gained popularity as a potent method for quick and effective chemical synthesis [52] and extraction of natural compounds [53]. Because the reaction mass is heated in three dimensions using this technique, the process can be finished in a matter of minutes rather than hours or even days. [44, 51]. This indicates that, when compared to conventional extraction techniques, MAE uses less time and energy throughout the extraction process. For large-scale extraction operations, this can result in significant cost savings and lessen the process’s negative effects on the environment.
As in any extraction process, the used conditions during MAE will have an impact on the structure of the biopolymer [54]. The extraction process for pectin involves several physicochemical steps that are regulated by variables like pH, temperature, time, and solvents [55, 56]. Recently, conventional heat reflux extraction (HRE, temperature: 70, 80, or 90 °C, time: 90, 120, or 150 min; pH: 1.4, 1.5, and 1.6) and MAE (power: 470, 550, or 630 W; time: 4, 5 or 6 min; pH: 1.8, 1.8 or 2.4) were assessed for pectin extraction from tobacco. The obtained yield was 11.27 and 8.88% for HRE and MAE, respectively. FTIR and esterification tests showed that predominantly esterified pectin was obtained in both extraction techniques [56]. Pectin from leftover lime peel is extracted using various acids (hydrochloric or citric acid) using HRE or MAE. The results confirmed that pectin obtained by MAE had a larger equivalent weight and esterification degree than pectin prepared by conventional process, with a strong band of about 1730 cm−1 owing to methyl esterified uronic carboxyl groups [57]. The pectin produced by MAE can be categorized as high methoxyl pectin with a quick gel setting. With increasing solid concentration, the rheological characteristics of the pectin solution from both heating methods improved. These rheological properties and pectin’s capacity to absorb water may or may not be advantageous depending on the intended applications. The qualities of pectin are always lost when the frequency is excessive. By altering the extraction procedures as well as the extraction parameters, particularly the purifying methods, it is possible to increase the end product’s quality and purity. Even so, MAE is a substitute to shorten the time required for pectin extraction with appropriate polymer characteristics [45, 56, 57]. Therefore, MAE offers several advantages over traditional extraction methods, including higher extraction rates, reduced extraction time and energy consumption, and the ability to extract biopolymers from a wide range of sources.
3.3 Pressurized fluid-assisted extraction
3.3.1 Supercritical fluid extraction (SFE)
A substance that exhibits a high density (akin to that of liquids), low viscosity (like a gas), and zero surface tension is known as a supercritical fluid. It exists above its critical temperature (Tc) and pressure (Pc), providing the possibility of being employed for several purposes. The main factors that affect the SFE include pressure, time, feed, CO2 flow rate, and the use of co-solvent [58, 59]. Depending on the matrix and the target components, there may be different optimal extraction conditions. Consequently, it is common practice to optimize the extraction parameters utilizing experimental designs [58]. High-temperature, high-pressure water is used to extract natural substances below its critical point (Tc = 374.15 °C, Pc = 22.1 MPa) utilizing subcritical water, an environmentally beneficial process. Subcritical water’s physicochemical characteristics change as the temperature rises. The diffusion coefficient increased as the water temperature rose, but the dielectric constant, viscosity, and surface tension all gradually decreased. As a result, subcritical water can act like methanol or ethanol [60]. The fact that water is harmless makes it better suited for the extraction of compounds that can be safely consumed by people or other animals. This is the main benefit of subcritical water extraction from natural sources. Additionally, the liquid waste produced after extraction does not need to be disposed of if organic modifiers are not utilized. However, the greatest drawback of subcritical water extraction is the possibility of analyte deterioration due to the high temperature [60, 61]. Subcritical water extraction of sodium alginate recovered from Himanthalia elongata, produced a polymer of low content of β-D-mannuronic acid and as a consequence a low β-d-mannuronic acid/α-l-guluronic acid ratio resulting in intermediate strength gelling matrices without compromising thermal or viscoelastic stability [62].
When cells are exposed to supercritical carbon dioxide (SC-CO2) their pH drops, their membrane is altered, and internal components are released [63]. Carbon dioxide has several advantages over other substances, including the fact that its critical temperatures (31 °C) and pressure (72.8 bar) are straightforward to obtain [64, 65]. In addition to this, SC-CO2 enables the extraction of the polymer without affecting its structure and properties, as occurs in the extraction with solvents, which interact with the H-bonds of the molecule separating the polymer chains. The extraction of cellulose by SC-CO2 followed by mild acid hydrolysis has been proposed as an effective technique for the obtention of these polymers from plant biomass in the nearby future [59]. Pectin extracted from cacao pod husk by HRE with citric acid or subcritical water, either with or without pre-treatment with SC-CO2 extraction of phenols. A higher yield (10.9%) was achieved under subcritical conditions in a period that was three times shorter than that of conventional extraction (8%); besides, the pectin showed a higher molecular weight (750 kDa). Methyl esterification degree and galacturonic acid were comparable (36 and 55%, respectively) in both methods. Furthermore, compared to subcritical water extraction, pectin recovered by citric acid showed a 2-fold higher amount of impurities. Pectin composition and structure barely showed any signs of previous supercritical treatment, demonstrating the treatment’s effectiveness. Hence, SFE with CO2 and water can be successfully used for the extraction of phenols and pectin from cacao husk [66]. SC-CO2 breaks H-bonds between hemicelluloses and cellulose promoting cell wall porosity and improving its exhibition for successive pectin extraction, without damaging monosaccharides such as galactose which has been related to anticancer activity [64, 67].
3.3.2 High-pressure homogenization (HPH)
HPH has the potential to be a successful technology because it can operate in wet conditions without the requirement for prior drying. By using pumps to speed up the liquid flow and create stresses (such as cavitation and shear forces) as the liquid passes through the homogenization valve, cell walls are disrupted [68]. However, high rates of cell lysis are, difficult to obtain with high-shear homogenization devices, and a significant amount of the energy absorbed is transferred to heat, which is undesirable for heat-sensitive extracts like proteins. Additionally, because of the method’s high energy input requirements, it is primarily recommended for recovering items with high economic value [69, 70]. HPH promotes hydrolysis, esterification, etherification, and polymerization in UAE [69]. The okara soybean was subjected to HPH at various intensities to extract the protein and soluble fiber. The breakdown of the structure, which is accompanied by a gradual pH reduction due to the release of organic acids and polyphenols formerly contained in cotyledon cells, causes the solubility to rise as the intensity rises [71]. Potato peel wastes were subjected to HPH, and the polymer obtained displayed an increment in the content of galacturonic acid while decreased esterification degree, (galacturonic acid + arabinose)/rhamnose ratio, and molecular weight in comparison to unpressurized samples. The pectin that had been treated with HPH showed better emulsifying properties and higher viscosity. The morphological characteristics demonstrated the degradation of pectin side chains brought on by pressure treatment. The findings indicate that HPH is a successful method for converting potato peel waste pectin into a thickening or stabilizing ingredient [50]. High-pressure mechanical stresses caused the pectin’s branched chains to break down, weakening the bond between the molecules [70, 72]. In agreement, HHP has been compared with the enzymatic process to de-esterified commercial pectin. At 400 MPa, 45 °C/15 min esterification degree was reduced from 65.32 ± 0.64 to 28.08 ± 1.39%, whereas 120 min was necessary by the enzymatic process to reach a similar result (26.64 ± 1.00%). Pectin modified by HHP had greater viscosity and homogenous gelation, resulting in gels with improved viscoelastic properties. The viscosity-average and molecular weight showed that HHP does not affect the molecular weight, suggesting that this technique is efficient for producing low methoxyl pectin [73].
3.4 Ultrasound-assisted extraction (UAE)
The extraction using UAE or ultrasonication is based on the generation of intense shock waves that create microbubbles in the liquid medium. Cavitation phenomena promote the expansion and violent collapse violently of the microbubbles, generating shock waves with high energy that increase the temperature and pressure resulting in cell wall disruption. When all these elements come together, cells are effectively disrupted, but not specifically, and intracellular chemicals are subsequently released as a result [74, 75]. Besides, certain reactive hydroxyl radicals like H+ and OH- may be produced during the process and may interact with biomolecules. Thus, some molecules should be supplied to the medium (such as nitrogen) to avoid damage caused by reactive free radicals [76]. Ultrasound (US) has been demonstrated to be a technique that allows obtaining chitin and chitosan of higher quality than the obtained by chemical method [77]. In the case of pectin, several sources have been tested using ultrasound. The use of this technique resulted in a 20% higher yield of extracted pectin and an enhanced molecular weight average when compared to the chemical extraction of pectin from grape pomace under the same conditions (75 °C for 60 min and pH 2.0) [59]. The US at different times (15 min) at 60 °C demonstrated to provide a higher yield (~ 5%) than the conventional technique (1440 min) in the obtention of pectin from tomato wastes. Both techniques provide esterified pectin, but the conventional extraction gave lower pectin quality factors such as methoxyl, and anhydrobiotic acid contents and degree of esterification. Which are important parameters in the gelling properties of pectin. Additionally, the extracted pectin increased in the L parameter, indicating that the lightness samples may have a favorable sensory impact on food applications [46].
The US can be used to modify the structure of these natural polymers by breaking down their chemical bonds, thereby improving their solubility, reactivity, and functionality [49, 78]. This technique can be used to modify the chemical groups of polymers derived from agro-wastes in several ways, including (i) esterification of natural polymers, such as cellulose and hemicellulose. This process introduces new chemical groups, such as carboxyl or acetyl groups, which can improve the solubility, reactivity, and functionality of the polymers [78]; (ii) etherification is the introduction of chemical groups, such as methyl or phenyl groups, which can improve the solubility and reactivity of the polymers. US-assisted etherification of lignin with methyl iodide can produce methylated lignin, which has improved solubility and can be used as a dispersant in various applications, such as coatings, adhesives, and composites [79]; (iii) hydrolysis, US can break down the polymers into smaller molecules, such as monosaccharides, which can be further processed into various chemicals, such as biofuels and platform chemicals. US hydrolysis of hemicellulose from sugarcane bagasse with dilute sulfuric acid can produce xylose, a five-carbon sugar that can be used as a feedstock for the production of xylitol, a low-calorie sweetener [80]; and (iv) polymerization of natural monomers, such as lignin-based monomers, into polymers with desired properties. This process can produce novel polymers that have improved properties, such as thermal stability and mechanical strength. For example, US-assisted polymerization of lignin-based monomers with acrylic acid can produce lignin-acrylic acid copolymers, which have good thermal stability and can be used as coatings and adhesives [81].
Owing UAE uses a lot of energy and is insufficient on its own to completely extract proteins, this approach is not practical for use on a wide scale [82]. For this, it can be combined with other extraction techniques to polymer obtention from agro-wastes [47]. US and maceration followed by pancreatin enzyme for 180 min were tested to determine their effect on the leaf jackfruit hydrolysates. US hydrolysates showed a higher amount of essential amino acids; instead, maceration hydrolysates showed a higher content of hydrophobic amino acids. The high amount of polar groups improves the solubility, emulsifying, and foaming properties of hydrolysates obtained by the US [83]. Rice straws were heated using the US-reflux technique to extract the cellulose fibers, the yield was 1.3 more with this method in comparison with alkali extraction and just required 1.5 h. Additionally, while applying this technique, cellulose fibers showed a somewhat higher degree of purification compared to alkali extraction while retaining a comparable degree of crystallinity and aspect ratio, as well as a higher level of hydrophilicity and a lesser inclination to clump. The US heating method enables the production of active extracts that are rich in antioxidant chemicals and have a variety of uses in the food business. As a result, it is possible to fully value agricultural waste [48]. In addition to extraction, US can be used to modify the chemical groups of natural polymers derived from agro-wastes, thereby improving their properties and expanding their applications in various industries. In general, the use of green alternatives for the extraction of polymers, enables the obtention of a biopolymer of higher quality and purity, hence, with better properties than the obtained by the traditional reflux or heat system (Fig. 2).
3.5 Microbial activity
Microbial transformation is one of the most studied alternatives for the obtention of polymers from agro-wastes. This is possible because the high amount of carbon allows microbial growth. Agro-wastes by themselves or in combination with other polymers or chemical compounds can be employed as a substrate for the development of a variety of value-added goods through several microbial pathways [84, 85]. A possible method for producing high-value goods like nutraceutical foods is microbial fermentation. In this regard, the most popular whey valorization agents are yeasts and lactic acid bacteria (LAB) [86, 87], because of their great proliferative potential in acidic conditions and high proteolytic activity [86, 88]. Whey proteins are hydrolyzed by LAB cell membrane proteases to produce extracellular oligopeptides that may have biological effects when consumed, adding value to fermented meals. Purified peptides produced by Pediococcus acidilactici SDL1414 from whey might be utilized to create functional meals that are antihypertensive or as nutraceuticals or supplements to treat hypertension because these peptides were able to inhibit 84.7 ± 0.67% of the activity of the angiotensin-converting enzyme [88]. Proteolytic LAB and yeast cultures can ferment whey to produce probiotic-enhanced functional meals that are rich in bioactive peptides or they can serve as a trustworthy and efficient substitute for animal sources of protein in human diets. In addition to making polyalkenoates and peptides, bacteria are crucial for the synthesis of cellulose. It has lately been employed in the manufacture of bio-paper enhanced with lysozyme. The final solution gives the enzyme a wider range of operating temperatures and greater thermal stability, retaining its function for up to 80 days without any additional storage. When S. aureus and E. coli were exposed to bio-paper, their growth was inhibited by 82 and 68%, respectively [89]. Microbial fermentation is a popular method for the production of biopolymers with a wide range of applications in various industries. This technique can be used to produce multiple biopolymers sequentially and can be made more sustainable by using industrial waste as a feedstock. Empirical modeling of batch fermentation kinetics can help understand the production process and optimize yield. In general, the microbial production of biopolymers has the potential to provide a sustainable and eco-friendly alternative to petroleum-based plastics.
4 Importance of polymers from agro-waste into the food industry
Biopolymers, such as xanthan gum and chitosan, can be used as thickeners and emulsifiers in the food industry, being a sustainable alternative to synthetic thickeners and emulsifiers. They are effective at improving the texture and stability of food products by preventing ingredient separation and controlling viscosity [90]. Xanthan gum is a polysaccharide, produced by the bacterium Xanthomonas campestris during sugar fermentation, with great thickening properties widely used in a range of food products, including sauces, salad dressings, dairy products, and baked goods. When added to these products, xanthan gum forms a gel-like structure that helps to maintain the desired texture and viscosity. It is also an effective emulsifier, helping to stabilize oil-in-water emulsions [91]. Otherwise, chitosan is a natural thickener and emulsifier in the food industry for its ability to absorb water and form a gel-like structure. Chitosan can be used to enhance texture and stability in items including dairy, drinks, dressings, and sauces. Additionally, it is prized for its antioxidant and antibacterial qualities, which can increase the shelf life of food goods [92]. In the food sector, the use of biopolymers as thickeners and emulsifiers offers a sustainable and healthier substitute for synthetic additives. [90].
Otherwise, natural polymers also can be successfully used to increase the durability of thermoplastic and thermosetting polymer matrices and help replace glass fibers in the creation of natural films, coatings, and packaging [93, 94]. The use of food packaging-based biopolymers such as starch is extensive in the food industry because they do not change the organoleptic properties of the coated food [95, 96]. Additionally, the cross-linking capabilities of polyvalent cations are frequently used to enhance the cohesiveness, stiffness, and mechanical resistance of natural films and coatings, notably, those composed of alginate [97, 98]. Food packaging or edible coatings made of alginate has an excellent gas barrier, which slows the pace of enzymatic browning [99], whereas chitosan allows the obtention of films with great control of gas exchange (O2 and CO2) and inhibitory activity against several pathogenic and spoilage microorganisms. These properties make this polysaccharide very important to the extent of the shelf life of fresh and minimally processed foods [100, 101]. Concerning to collagen and gelatin films have good transparency, adherence to the food products, and mechanical, and barrier properties against O2 and CO2, being possible their manufacture by extrusion or dipping processes. The hydration degree increases the flexibility of this kind of film, for example, gelatin can absorb water up to 10× increase in volume, producing film instability [102]. These coatings serve as good encapsulating matrices for chemical substances or probiotic microorganisms or transporters of active compounds [103]. Biopolymers are one of the most important study areas in the development of natural polymers, due to providing a reliable alternative for the replacement of synthetic compounds. However, the development of a formulation that can be applied to several foods is complicated. Because of their mechanical and gelling characteristics, biopolymers’ stability and reactivity to chemical and physical conditions are highly dependent on the source and extraction technique [23, 104].
4.1 Electrohydrodynamic processes as an alternative to the use of natural polymers in the food industry
The electrohydrodynamic atomization technique produces continuous micro- or nanoscale materials without the need for mechanical or thermal energy. Electrospray and electrospinning are the two techniques in the electrohydrodynamic process that produce fibers and particles, respectively [105]. The main difference between electrospray and electrospinning’s fundamental setups and principles is how the emitted jet moves while traveling through the external electrical field. The rheological characteristics (viscosity, surface tension, and chain entanglement behaviors) of the precursor are primarily influenced by the molecular weight and concentration of the polymer, which may cause a transition between electrospinning and electrospray upon the addition of polymers to the precursors [106]. Depending on the rheological properties of the solution and the processing parameters, the cross-sectional size and shape may fluctuate. Five different types of structures, including hollow, porous, aligned, core-shell, and multilayer coaxial fibers, are made using electrospinning [107, 108]. Micro- and nanostructures with sizes ranging from 2 nm to several micrometers may be created using a variety of polymers [94, 109, 110].
The electrospinning procedure may often be broken down into four sequential steps: (i) pressurizing a pressurized container or a syringe pump to charge the liquid droplet, which will give enough electrohydrodynamic resistance [111]. One the droplet is formed it is exposed to the electric field. A liquid structure called a Taylor cone is produced when the surface of the droplet becomes charged as a result of the electrostatic field’s influence and/or the injection of charges from an electrode [112]; (ii) charged jet extension along a straight path; electrical bending instability development and jet thinning in the presence of an electric field; and solidification and collection of the jet as solid fibers on a grounded collector [113, 114]. In addition, since this process is ongoing, longer fibers are produced compared to those produced using conventional chemical or physical techniques [115]. Adjusting the process settings and/or developing unique electrospinning equipment, tunable morphologies, and structures can result in the required qualities. The preparation of organic nanofibers using food grade solvents, the limited variety of polymers used in this technique, the lack of thorough research on the structure and functionality of nanofibers, and the high cost of manufacturing are all barriers to the electrospinning process’s use in the food industry [116, 117]. Additionally, it is still difficult to obtain homogeneous electrospun nanofibers with diameters less than 10 nm [117]. Despite this, electrospun micro- and nanostructures are appealing for novel applications in drug delivery, agriculture, food packaging, biomedical engineering, air filtration, energy production and storage, environmental protection, and photonic and electronic devices due to the unique properties of electrohydrodynamic processes [117,118,119].
Due to its capacity to create materials with enhanced structural and functional qualities, electrohydrodynamic processing is given high importance in the development of active food packaging (Table 2). One of the main advantages of these techniques is the possibility of applying the nanostructures added of additives or active compounds directly on the surface of the food [119, 126]. The characteristics of electrospun fibers can be modified to give functional capabilities, such as antioxidant and antibacterial, increasing the shelf life quality and nutritional content of perishable foods [109, 110]. Electrospun fibers are flexible for their application in the food business. In contrast to conventional methods (casting, bushing, and atomization), electrospun fibers offer advantages in the production of active packing and coating systems, including the improvement, stabilization, and control of the release of active compounds as well as extremely sensitive, quick, and dependable responsiveness under real-time changes [115]. A multilayer bio-paper made of two electrospun layers of poly(3-hydroxybutyrate-co-3-hydroxy valerate) impregnated with cellulose nanocrystals on the inner surface of one of the layers has recently been developed. Only by impregnating the material with nanocrystals did the oxygen barrier qualities dramatically increase, indicating that this material is a promising substitute for the creation of environmentally friendly food packaging [127]. Despite their advantages, active and intelligent electrospun fibers for perishable foods are currently being researched and are not frequently used in commercial products. The commercialization of electrospinning lab-scale innovations may be accelerated by their industrial validation [115].
5 Challenges and opportunities in the use of biopolymers from agro-waste in the food industry
Biopolymers from agro-waste have numerous opportunities in the food industry, including the development of sustainable and biodegradable packaging materials, functional food ingredients, and thickeners and emulsifiers. However, their use also poses several challenges such as ensuring their purity, yield, and functionality. The extraction of biopolymers from agro-waste can be complex, and variations in the composition and processing of agro-waste can affect the quality and performance of the biopolymers. Factors such as the season, soil type, processing method, and storage conditions can have an impact on yield and purity. Additionally, the functionality of biopolymers from agro-waste can be affected by factors such as temperature, pH, and ionic strength [31, 62]. These factors can influence the molecular conformation of the biopolymers, which in turn affects their rheological properties and performance as thickeners and emulsifiers. Improvements in extraction methods and quality control measures can help overcome these challenges. This includes the development of standardized extraction protocols and the use of analytical techniques to assess purity and functionality. Additionally, there is a need for research to understand how variations in agro-waste composition and processing affect the quality and performance of biopolymers [23, 128]. The conjunction of these factors is one of the main key points that limit the use of biopolymers in the food industry. Another challenge is consumer acceptance and market demand. Biopolymers from agro-waste are still relatively new and unfamiliar to many consumers, and there is a need to educate and raise awareness about their benefits and potential applications. Product development and marketing efforts can help increase demand for biopolymer-based products [23, 129]. Despite these challenges, biopolymers from agro-waste offer several opportunities in the food industry. Biopolymers are sustainable and biodegradable, making them an environmentally friendly alternative to traditional synthetic additives. They also offer functional benefits such as improving the texture and stability of food products and can be used in the development of sustainable food packaging materials. Advancements in extraction methods, product development, and sustainability efforts can help overcome these obstacles and promote the widespread adoption of biopolymers from agro-waste.
In recent years, the field of biopolymer extraction from agro-waste has been propelled into a new era of efficiency, precision, and sustainability, thanks to the integration of the concepts belonging to Industry 4.0. Industry 4.0 is the realization of digital transformation based on the integration of digital technologies, automation, and data exchange, bringing real-time decision-making, improved productivity, flexibility, and agility. Some of the concepts included in this revolution are the Internet of Things (IoT), Big Data Analyses, 3-D printing, cloud computing, machine learning, artificial intelligence (AI), robotics, and sustainable manufacturing [10, 130].
The use of these technologies enables the analyses of the intricate relationships between extraction parameters, extraction methods, source, and desirable polymer characteristics. The harnessing of the computational power of Industry 4.0 tools allows analyzing the vast datasets generated by different studies around the world to identify patterns and pinpoint optimal extraction conditions [10]. Employing big data analyses, matching learning, AI, IoT, and robotics, it is possible to establish the optimal conditions for biopolymers extraction, considering all the associated factors (extraction techniques, optimal solvent choices, and suitable agro-waste sources) and minimizing the waste. Besides, these tools facilitate the creation of predictive models that offer insights into potential outcomes based on varying input parameters; serving as invaluable decision-support tools, enabling researchers to anticipate biopolymer yields under different conditions [131]. Data analyses by statistical tools have been widely used to optimize the extraction and predict the behavior of natural polymers. The tensile of fiber based on strength kenaf fibers, basalt fiber, and nanographene was optimized considering the best response to this property reducing the amount of polymers. The optimal conditions were established at 1.626 g consisting of 15% kenaf fiber and 1.5% of graphene nanoparticles that produced fibers with an elastic modulus of 3.5126 GPa [132]. The integration of Industry 4.0 into the process of biopolymer production accelerates innovation and fosters the discovery of novel biopolymers with a wide application range.
Besides, the incorporation of sensors and monitoring systems into the extraction process enables continuous data collection and analysis in real time, ensuring that the extraction process proceeds according to plan. Facilitating the identification of any deviation or anomaly trigger immediate adjustments, maintaining consistent quality and yield [130]. As the automatization level increases, the level of human intervention is reduced, which can contribute to minimizing errors, and guarantees a reliable and efficient extraction workflow. Besides, the capability to analyze chemical compositions, molecular structures, and physical properties that provide these tools ensures consistent quality control of extracted biopolymers. Sensors, data collection, and data analyses in real-time enable the optimization and monitoring of processes like solid fermentation employing Aspergillus niger, which was utilized to boost the antioxidant activity of bioactive compounds derived from Citrus reticulata peels, which can be used for the development of functional foods [133]. On the other hand, the use of artificial intelligence and machine learning has allowed the development of several apps (Cheaf, EatChaFood, LeftoverSwap, and Fridge Pal) to manage the supply chain of foods and leftovers reducing and contributing to reducing waste [10]. The integration of the concepts of Industry 4.0 into the biopolymer extraction process from agro-waste represents a paradigm shift in sustainable material production contributing to a more sustainable and ecologically balanced future.
Agro-wastes are crucial to the circular economy, a type of economy that encourages resource reuse and recycling while attempting to reduce waste. Agro-waste can be utilized in the circular economy for a variety of purposes, such as the extraction of high-value compounds, production of biomass energy, biodegradable materials, and animal products [134, 135]. Concerning this, the worldwide bioplastics market was worth USD 10.2 billion in 2021, and it is predicted to increase at a compound annual growth rate (CAGR) of 17.1% between 2022 and 2030. A rise in health-related awareness, along with expanding of emerging economies such as Asia Pacific, leads to higher demand for bioplastics throughout the forecast period [136]. Whereas for the same period, it was expected that the global functional foods market expand at a CAGR of 8.5%; its estimated value in 2021 was USD 280.7 billion. The market is anticipated to develop due to the rising demand for food additives that are nourishing and fortifying. The COVID-19 pandemic has had little impact on this industry [137]. Otherwise, the best way to achieve agro-waste valorization is by the implementation of biorefinery. This possibility of the obtention of products to several industrial sectors reduces production costs [138].
The industrialization of agro-wastes has contributed to circular economy development, which promotes the efficient use of leftover biomass and by-products for the creation of products with additional value, with a focus on recycling and reusing materials to protect environmental viability. Reduced greenhouse gas emissions, use of renewable resources, and valorization of waste biomass from agriculture, forestry, fishing, and aquaculture are some of the advantages of a circular economy [22, 139]. The utilization of agro-industrial wastes as the main raw material for the production of bioproducts is an ideal choice to attain the ultimate objective of a circular economy and Sustainable Development Goals (Fig. 3). A closed-loop waste management system is built using the 7Rs (redesign, reduce, reuse, repair, renew, recover, and recycle) in a circular economy [139, 140]. However, polymer extraction by emerging technologies generates leftovers, which can be used in fermentative process in the bioenergy, biofertilizer, and biochar production to continue with the generation of added value products into a circular economy scheme in fermentative processes, in the bioenergy generation, as biofertilizers, and in the biochar production to continue with the generation of added value products into a circular economy scheme [139, 141].
Despite the efforts of scientists and businesses, managing agricultural residues is a significant environmental and financial problem. Because there are not enough prediction tools, it is challenging to provide policymakers and end users with precise instructions. The Life Cycle Assessment (LCA) method is used to determine how products and services affect the environment [142]. According to LCA, similar waste valorization could provide significantly different outcomes depending on the particulars of the planned procedures. Additionally, certain waste-based products are competitive with and often even superior to traditional procedures [143]. However, its applicability has a data constraint due to inventory statistics for agricultural waste being inexistent or difficult to obtain, while the second obstacle is the low effectiveness of conversion methods for agricultural residue. Finally, the lack of regulatory laws on this topic and in the management of the products generates during agro-waste management represents the third issue in this area [6, 23]. With the constant technology evolution linked to research, these challenges should be effectively resolved. Furthermore, additional difficulties such as transportation, agro-waste costs, and so on may be efficiently addressed by applying comprehensive supply chain management together with supporting analyses. While the establishment of policies gradually reduce the obstacles connected with political, economic, and target market [6]. Agro-wastes can be integrated into the circular economy through various ways of reducing waste and environmental impact while promoting sustainable development.
6 Conclusions
Agro-industrial wastes or residues are rich in polymers and bioactive compounds consequently; they can be considered “raw material” for other industrial processes. Biopolymers including proteins and carbohydrates are one of the main products that can be obtained from agro-wastes and provide and added value to food products. However, these compounds are available by themselves but can be extracted by conventional processes and emerging technologies. Following the concept of green process and achieving sustainable manufacturing are necessary to use technologies that have a low impact on the environment, such as HPE, SFE, MAE, enzymes, green solvents, and so on that allow to maintain or improvement the properties of the compounds, contributing to the replacement of petroleum-based compounds. Optimum extraction conditions should be selected based on mass yield and according to the desired structural and functional features for the specific applications of the polymers. Natural polymers are widely used in the food sector to extend the shelf life of products and protect the bioactive compounds. In line with this, electrohydrodynamic processes are used to facilitate the use of natural polymers in the food industry through the development of smart packaging, edible coatings, and encapsulating active compounds for the formulation of new functional foods. The conjunction of all these factors contributes to the development of the circular economy. Proper agro-waste management can help to minimize negative impacts while maximizing its potential as a valuable resource. However, to achieve this effectively, it is important the implementation of regulatory policies and the generation of data to facilitate the analyses and real estimation of the real impact of the obtention of added value products from agro-wastes.
Data availability
Not applicable.
References
Vilariño MV, Franco C, Quarrington C (2017) Food loss and waste reduction as an integral part of a circular economy. Front Environ Sci 5:21. https://doi.org/10.3389/fenvs.2017.00021
Duque-Acevedo M, Belmonte-Ureña LJ, Cortés-García FJ, Camacho-Ferre F (2020) Agricultural waste: review of the evolution, approaches and perspectives on alternative uses. Glob Ecol Conserv 22. https://doi.org/10.1016/j.gecco.2020.e00902
Ranganathan S, Dutta S, Moses JA, Anandharamakrishnan C (2020) Utilization of food waste streams for the production of biopolymers. Heliyon 6:e04891. https://doi.org/10.1016/j.heliyon.2020.e04891
Maraveas C (2020) Production of sustainable and biodegradable polymers from agricultural waste. Polymers 12:1127. https://doi.org/10.3390/POLYM12051127
Bala S, Garg D, Sridhar K et al (2023) Transformation of agro-waste into value-added bioproducts and bioactive compounds: Micro/nano formulations and application in the agri-food-pharma sector. Bioengineering 10:152
Dey T, Bhattacharjee T, Nag P et al (2021) Valorization of agro-waste into value added products for sustainable development. Bioresour Technol Rep 16:100834
FAO (2022) World Food and Agriculture – Statistical Yearbook 2022. Italy, Rome
Varghese SA, Pulikkalparambil H, Promhuad K et al (2023) Renovation of agro-waste for sustainable food packaging: a review. Polymers 15:648. https://doi.org/10.3390/polym15030648
Rashid MI, Shahzad K (2021) Food waste recycling for compost production and its economic and environmental assessment as circular economy indicators of solid waste management. J Clean Prod 317:128467. https://doi.org/10.1016/j.jclepro.2021.128467
Taneja A, Sharma R, Khetrapal S et al (2023) Value addition employing waste bio-materials in environmental remedies and food sector. Metabolites 13:624. https://doi.org/10.3390/metabo13050624
Mahato RP, Kumar S, Singh P (2023) Production of polyhydroxyalkanoates from renewable resources: a review on prospects, challenges and applications. Springer, Berlin Heidelberg
El Knidri H, Belaabed R, Addaou A et al (2018) Extraction, chemical modification and characterization of chitin and chitosan. Int J Biol Macromol 120:1181–1189. https://doi.org/10.1016/j.ijbiomac.2018.08.139
Souza VGL, Rodrigues C, Valente S et al (2020) Eco-friendly ZnO/chitosan bionanocomposites films for packaging of fresh poultry meat. Coatings 10:110
Ya WL, Hai PH, Yu LC et al (2023) Effect of chitosan-ascorbic acid composite coating on postharvest quality of Custard apple (Annona squamosa). Process Biochem 129:76–85. https://doi.org/10.1016/j.procbio.2023.03.013
Iñiguez-Moreno M, Ragazzo-Sánchez JA, Barros-Castillo JC et al (2021) Characterization of sodium alginate coatings with Meyerozyma caribbica and impact on quality properties of avocado fruit. LWT - Food Sci Technol 152:112346. https://doi.org/10.1016/j.lwt.2021.112346
Calderón-Santoyo M, Iñiguez-Moreno M, Barros-Castillo JC et al (2022) Microencapsulation of citral with Arabic gum and sodium alginate for the control of Fusarium pseudocircinatum in bananas. Iran Polym J 1–12. https://doi.org/10.1007/s13726-022-01033-z
López-Cruz R, Ragazzo-Sánchez JA, Calderón-Santoyo M (2020) Microencapsulation of Meyerozyma guilliermondii by spray drying using sodium alginate and soy protein isolate as wall materials: a biocontrol formulation for anthracnose disease of mango. Biocontrol Sci Technol 30:1116–1132. https://doi.org/10.1080/09583157.2020.1793910
Carmona JC, Robert P, Vergara C, Sáenz C (2021) Microparticles of yellow-orange cactus pear pulp (Opuntia ficus-indica) with cladode mucilage and maltodextrin as a food coloring in yogurt. LWT - Food Sci Technol 138:110672. https://doi.org/10.1016/j.lwt.2020.110672
Puglia D, Pezzolla D, Gigliotti G et al (2021) The opportunity of valorizing agricultural waste, through its conversion into biostimulants, biofertilizers, and biopolymers. Sustainability 13:2710. https://doi.org/10.3390/su13052710
Satari B, Karimi K (2018) Citrus processing wastes: environmental impacts, recent advances, and future perspectives in total valorization. Resour Conserv Recycl 129:153–167. https://doi.org/10.1016/j.resconrec.2017.10.032
Arpit Singh T, Sharma M, Sharma M et al (2022) Valorization of agro-industrial residues for production of commercial biorefinery products. Fuel 322:124284. https://doi.org/10.1016/j.fuel.2022.124284
Kardung M, Cingiz K, Costenoble O et al (2021) Development of the circular bioeconomy: drivers and indicators. Sustain 13:413. https://doi.org/10.3390/su13010413
Poonia A, Dhewa T (2022) Edible food packaging: applications, innovations and sustainability. Springer, Singapore
Kumar V, Lakkaboyana SK, Tsouko E et al (2023) Commercialization potential of agro-based polyhydroxyalkanoates biorefinery: a technical perspective on advances and critical barriers. Int J Biol Macromol 234:123733. https://doi.org/10.1016/j.ijbiomac.2023.123733
Rusková M, Opálková Šišková A, Mosnáčková K et al (2023) Biodegradable active packaging enriched with essential oils for enhancing the shelf life of strawberries. Antioxidants 12:755
Rai P, Mehrotra S, Priya S et al (2021) Recent advances in the sustainable design and applications of biodegradable polymers. Bioresour Technol 325:124739. https://doi.org/10.1016/j.biortech.2021.124739
Colla G, Cardarelli M, Bonini P, Rouphael Y (2017) Foliar applications of protein hydrolysate, plant and seaweed extracts increase yield but differentially modulate fruit quality of greenhouse tomato. HortScience 52:1214–1220. https://doi.org/10.21273/HORTSCI12200-17
Sadh PK, Duhan S, Duhan JS (2018) Agro-industrial wastes and their utilization using solid state fermentation: a review. Bioresour Bioprocess 5:1–15. https://doi.org/10.1186/s40643-017-0187-z
Cermeño M, Kleekayai T, Amigo-Benavent M et al (2020) Current knowledge on the extraction, purification, identification, and validation of bioactive peptides from seaweed. Electrophoresis 41:1694–1717. https://doi.org/10.1002/elps.202000153
Proença S, Escher BI, Fischer FC et al (2021) Effective exposure of chemicals in vitro cell systems: a review of chemical distribution models. Toxicol Vitr 73:105133. https://doi.org/10.1016/j.tiv.2021.105133
Jha A, Kumar A (2019) Biobased technologies for the efficient extraction of biopolymers from waste biomass. Bioprocess Biosyst Eng 42:1893–1901. https://doi.org/10.1007/s00449-019-02199-2
Al-Manhel AJ, Al-Hilphy ARS, Niamah AK (2018) Extraction of chitosan, characterisation and its use for water purification. J Saudi Soc Agric Sci 17:186–190. https://doi.org/10.1016/j.jssas.2016.04.001
Husanu E, Mero A, Rivera JG et al (2020) Exploiting deep eutectic solvents and ionic liquids for the valorization of chestnut shell waste. ACS Sustain Chem Eng 8:18386–18399. https://doi.org/10.1021/acssuschemeng.0c04945
Hansen BB, Spittle S, Chen B et al (2021) Deep eutectic solvents: a review of fundamentals and applications. Chem Rev 121:1232–1285. https://doi.org/10.1021/acs.chemrev.0c00385
Catone MV, Palomino MM, Legisa DM et al (2021) Lactic acid production using cheese whey based medium in a stirred tank reactor by a ccpA mutant of Lacticaseibacillus casei. World J Microbiol Biotechnol 37:61. https://doi.org/10.1007/s11274-021-03028-z
Jagtap S, Deshmukh RA, Menon S, Das S (2017) Xylooligosaccharides production by crude microbial enzymes from agricultural waste without prior treatment and their potential application as nutraceuticals. Bioresour Technol 245:283–288. https://doi.org/10.1016/j.biortech.2017.08.174
Etemadian Y, Ghaemi V, Shaviklo AR et al (2021) Development of animal/ plant-based protein hydrolysate and its application in food, feed and nutraceutical industries: State of the art. J Clean Prod 278:123219. https://doi.org/10.1016/j.jclepro.2020.123219
Kumar A, Singh Negi Y, Choudhary V, Kant Bhardwaj N (2020) Characterization of cellulose nanocrystals produced by acid-hydrolysis from sugarcane bagasse as agro-waste. J Mater Phys Chem 2:1–8. https://doi.org/10.12691/jmpc-2-1-1
Barcelos MCS, Ramos CL, Kuddus M et al (2020) Enzymatic potential for the valorization of agro-industrial by-products. Biotechnol Lett 42:1799–1827. https://doi.org/10.1007/s10529-020-02957-3
Srinivasan H, Kanayairam V, Ravichandran R (2018) Chitin and chitosan preparation from shrimp shells Penaeus monodon and its human ovarian cancer cell line, PA-1. Int J Biol Macromol 107:662–667. https://doi.org/10.1016/j.ijbiomac.2017.09.035
Jung J, Zhao Y (2013) Impact of the structural differences between α- And β-chitosan on their depolymerizing reaction and antibacterial activity. J Agric Food Chem 61:8783–8789. https://doi.org/10.1021/jf4018965
Calderón-Chiu C, Calderón-Santoyo M, Barros-Castillo JC et al (2022) Structural modification of jackfruit leaf protein concentrate by enzymatic hydrolysis and their effect on the emulsifier properties. Colloids Interfaces 6:52. https://doi.org/10.3390/colloids6040052
Kamal H, Jafar S, Mudgil P et al (2022) Camel whey protein with enhanced antioxidative and antimicrobial properties upon simulated gastro-intestinal digestion. Nutr Health 1–9. https://doi.org/10.1177/02601060221122213
Passos CP, Rudnitskaya A, Neves JMMGC et al (2019) Structural features of spent coffee grounds water-soluble polysaccharides: towards tailor-made microwave assisted extractions. Carbohydr Polym 214:53–61. https://doi.org/10.1016/j.carbpol.2019.02.094
Golbargi F, Gharibzahedi SMT, Zoghi A et al (2021) Microwave-assisted extraction of arabinan-rich pectic polysaccharides from melon peels: optimization, purification, bioactivity, and techno-functionality. Carbohydr Polym 256:117522. https://doi.org/10.1016/j.carbpol.2020.117522
Grassino AN, Brnčić M, Vikić-Topić D et al (2016) Ultrasound assisted extraction and characterization of pectin from tomato waste. Food Chem 198:93–100. https://doi.org/10.1016/j.foodchem.2015.11.095
Yang Y, Wang Z, Hu D et al (2018) Efficient extraction of pectin from sisal waste by combined enzymatic and ultrasonic process. Food Hydrocoll 79:189–196. https://doi.org/10.1016/j.foodhyd.2017.11.051
Freitas PAV, González-Martínez C, Chiralt A (2022) Applying ultrasound-assisted processing to obtain cellulose fibres from rice straw to be used as reinforcing agents. Innov Food Sci Emerg Technol 76:102932. https://doi.org/10.1016/j.ifset.2022.102932
Xu SY, Liu JP, Huang X et al (2018) Ultrasonic-microwave assisted extraction, characterization and biological activity of pectin from jackfruit peel. LWT - Food Sci Technol 90:577–582. https://doi.org/10.1016/j.lwt.2018.01.007
Xie F, Zhang W, Lan X et al (2018) Effects of high hydrostatic pressure and high pressure homogenization processing on characteristics of potato peel waste pectin. Carbohydr Polym 196:474–482. https://doi.org/10.1016/j.carbpol.2018.05.061
Gala S, Sumarno S, Mahfud M (2020) Comparison of microwave and conventional extraction methods for natural dyes in wood waste of mahogany (Swietenia mahagoni). J Appl Eng Sci 18:618–623. https://doi.org/10.5937/jaes0-23695
Sameut A, Zanndouche S, Boumaza C et al (2020) Chemical synthesis and hemi-synthesis of novel benzimidazoles derivatives using microwave-assisted process: Chemical characterization, bioactivities and molecular docking. Chem Proc 3:71. https://doi.org/10.3390/ecsoc-24-08306
Espada-Bellido E, Ferreiro-González M, Carrera C et al (2019) Extraction of antioxidants from blackberry (Rubus ulmifolius L.): comparison between ultrasound- and microwave-assisted extraction techniques. Agronomy 9:745. https://doi.org/10.3390/agronomy9110745
Jampafuang Y, Tongta A, Waiprib Y (2019) Impact of crystalline structural differences between α- and β-chitosan on their nanoparticle formation. Polymers 11:2010
Mellinas C, Ramos M, Jiménez A, Garrigós MC (2020) Recent trends in the use of pectin from agro-waste residues as a natural-based biopolymer for food packaging applications. Materials 13:673. https://doi.org/10.3390/ma13030673
Zhang M, Zeng G, Pan Y, Qi N (2018) Difference research of pectins extracted from tobacco waste by heat reflux extraction and microwave-assisted extraction. Biocatal Agric Biotechnol 15:359–363. https://doi.org/10.1016/j.bcab.2018.06.022
Rodsamran P, Sothornvit R (2019) Microwave heating extraction of pectin from lime peel: characterization and properties compared with the conventional heating method. Food Chem 278:364–372. https://doi.org/10.1016/j.foodchem.2018.11.067
Gallego R, Bueno M, Herrero M (2019) Sub- and supercritical fluid extraction of bioactive compounds from plants, food-by-products, seaweeds and microalgae – an update. TrAC - Trends Anal Chem 116:198–213. https://doi.org/10.1016/j.trac.2019.04.030
Atiqah MN, Gopakumar DA, Owolabi FAT et al (2019) Extraction of cellulose nanofibers via eco-friendly supercritical carbon dioxide treatment followed by mild acid hydrolysis and the fabrication of cellulose nanopapers. Polymers 11:1813. https://doi.org/10.3390/polym11111813
Cheng Y, Xue F, Yu S, Du S (2021) Subcritical water extraction of natural products. Molecules 26:4004
Švarc-gaji J, Brezo-borjan T, Gosselink RJA et al (2022) Optimization and potentials of kraft lignin hydrolysates obtained by subcritical water at moderate temperatures. Processes 10:2049
Flórez-Fernández N, Domínguez H, Torres M (2021) Functional features of alginates recovered from Himanthalia elongata using subcritical water extraction. Molecules 26:4726
Pavlova PL, Minakov AV, Platonov DV et al (2022) Supercritical fluid application in the oil and gas industry: a comprehensive review. Sustain 14:698. https://doi.org/10.3390/su14020698
Li R, Xia Z, Li B et al (2021) Advances in supercritical carbon dioxide extraction of bioactive substances from different parts of Ginkgo biloba L. Molecules 26:4011. https://doi.org/10.3390/molecules26134011
Arumugham T, Rambabu K, Hasan SW et al (2021) Supercritical carbon dioxide extraction of plant phytochemicals for biological and environmental applications – a review. Chemosphere 271:129525. https://doi.org/10.1016/j.chemosphere.2020.129525
Muñoz-Almagro N, Valadez-Carmona L, Mendiola JA et al (2019) Structural characterisation of pectin obtained from cacao pod husk. Comparison of conventional and subcritical water extraction. Carbohydr Polym 217:69–78. https://doi.org/10.1016/j.carbpol.2019.04.040
Uwineza PA, Waśkiewicz A (2020) Recent advances in supercritical fluid extraction of natural bioactive compounds from natural plant materials. Molecules 25. https://doi.org/10.3390/molecules25173847
Lao F, Cheng H, Wang Q et al (2020) Enhanced water extraction with high-pressure carbon dioxide on purple sweet potato pigments: comparison to traditional aqueous and ethanolic extraction. J CO2 Util 40. https://doi.org/10.1016/j.jcou.2020.101188
Jadhav HB, Annapure US, Deshmukh RR (2021) Non-thermal technologies for food processing. Front Nutr 8:1–14. https://doi.org/10.3389/fnut.2021.657090
Levy R, Okun Z, Shpigelman A (2021) High-pressure homogenization: principles and applications beyond microbial inactivation. Food Eng Rev 13:490–508. https://doi.org/10.1007/s12393-020-09239-8
Fayaz G, Plazzotta S, Calligaris S et al (2019) Impact of high pressure homogenization on physical properties, extraction yield and biopolymer structure of soybean okara. LWT - Food Sci Technol 113:108324. https://doi.org/10.1016/j.lwt.2019.108324
Ju J, Xie Y, Guo Y et al (2019) Application of edible coating with essential oil in food preservation. Crit Rev Food Sci Nutr 59:2467–2480. https://doi.org/10.1080/10408398.2018.1456402
Zhao W, Guo X, Pang X et al (2015) Preparation and characterization of low methoxyl pectin by high hydrostatic pressure-assisted enzymatic treatment compared with enzymatic method under atmospheric pressure. Food Hydrocoll 50:44–53. https://doi.org/10.1016/j.foodhyd.2015.04.004
Costello KM, Velliou E, Gutierrez-Merino J et al (2021) The effect of ultrasound treatment in combination with nisin on the inactivation of Listeria innocua and Escherichia coli. Ultrason Sonochem 79:105776. https://doi.org/10.1016/j.ultsonch.2021.105776
Huu CN, Rai R, Yang X et al (2021) Synergistic inactivation of bacteria based on a combination of low frequency, low-intensity ultrasound and a food grade antioxidant. Ultrason Sonochem 74:105567. https://doi.org/10.1016/j.ultsonch.2021.105567
Zupanc M, Pandur Ž, Stepišnik Perdih T et al (2019) Effects of cavitation on different microorganisms: the current understanding of the mechanisms taking place behind the phenomenon. A review and proposals for further research. Ultrason Sonochem 57:147–165. https://doi.org/10.1016/j.ultsonch.2019.05.009
Vallejo-Domínguez D, Rubio-Rosas E, Aguila-Almanza E et al (2021) Ultrasound in the deproteinization process for chitin and chitosan production. Ultrason Sonochem 72:105417. https://doi.org/10.1016/j.ultsonch.2020.105417
Hoo DY, Low ZL, Low DYS et al (2022) Ultrasonic cavitation: an effective cleaner and greener intensification technology in the extraction and surface modification of nanocellulose. Ultrason Sonochem 90:106176. https://doi.org/10.1016/j.ultsonch.2022.106176
Wang B, Sun D, Wang HM et al (2019) Green and facile preparation of regular lignin nanoparticles with high yield and their natural broad-spectrum sunscreens. ACS Sustain Chem Eng 7:2658–2666. https://doi.org/10.1021/acssuschemeng.8b05735
Chen SJ, Chen X, Zhu MJ (2022) Xylose recovery and bioethanol production from sugarcane bagasse pretreated by mild two-stage ultrasonic assisted dilute acid. Bioresour Technol 345:126463. https://doi.org/10.1016/j.biortech.2021.126463
Chang X, Sun J, Xu Z et al (2019) A novel nano-lignin-based amphoteric copolymer as fluid-loss reducer in water-based drilling fluids. Colloids Surfaces A Physicochem Eng Asp 583:123979. https://doi.org/10.1016/j.colsurfa.2019.123979
Soto-Sierra L, Stoykova P, Nikolov ZL (2018) Extraction and fractionation of microalgae-based protein products. Algal Res 36:175–192. https://doi.org/10.1016/j.algal.2018.10.023
Vera-Salgado J, Calderón-Chiu C, Calderón-Santoyo M et al (2022) Ultrasound-assisted extraction of Artocarpus heterophyllus L. leaf protein concentrate: Solubility, foaming, emulsifying, and antioxidant properties of protein hydrolysates. Colloids and Interfaces 6:50. https://doi.org/10.3390/colloids6040050
Kaur R, Panwar D, Panesar PS (2020) Biotechnological approach for valorization of whey for value-added products. In: Kosseva MR, Webb C (eds) Food industry wastes, 2nd edn. Academic Press, Cambridge, pp 275–302
Chourasia R, Phukon LC, Abedin MM et al (2022) Whey valorization by microbial and enzymatic bioprocesses for the production of nutraceuticals and value-added products. Bioresour Technol Reports 19:101144. https://doi.org/10.1016/j.biteb.2022.101144
Chourasia R, Abedin MM, Chiring Phukon L et al (2021) Biotechnological approaches for the production of designer cheese with improved functionality. Compr Rev Food Sci Food Saf 20:960–979. https://doi.org/10.1111/1541-4337.12680
Chua JY, Lu Y, Liu SQ (2018) Evaluation of five commercial non-Saccharomyces yeasts in fermentation of soy (tofu) whey into an alcoholic beverage. Food Microbiol 76:533–542. https://doi.org/10.1016/j.fm.2018.07.016
Daliri EBM, Lee BH, Park BJ et al (2018) Antihypertensive peptides from whey proteins fermented by lactic acid bacteria. Food Sci Biotechnol 27:1781–1789. https://doi.org/10.1007/s10068-018-0423-0
Buruaga-Ramiro C, Valenzuela SV, Valls C et al (2020) Development of an antimicrobial bioactive paper made from bacterial cellulose. Int J Biol Macromol 158:587–594. https://doi.org/10.1016/j.ijbiomac.2020.04.234
Singhal S, Swami Hulle NR (2022) Citrus pectins: structural properties, extraction methods, modifications and applications in food systems – a review. Appl Food Res 2:100215. https://doi.org/10.1016/j.afres.2022.100215
Dzionek A, Wojcieszyńska D, Guzik U (2022) Use of xanthan gum for whole cell immobilization and its impact in bioremediation - a review. Bioresour Technol 351:126918. https://doi.org/10.1016/j.biortech.2022.126918
Chaudhary S, Kumar S, Kumar V, Sharma R (2020) Chitosan nanoemulsions as advanced edible coatings for fruits and vegetables: composition, fabrication and developments in last decade. Int J Biol Macromol 152:154–170. https://doi.org/10.1016/j.ijbiomac.2020.02.276
Suvarna V, Nair A, Mallya R et al (2022) Antimicrobial nanomaterials for food packaging. Antibiotics 11:729. https://doi.org/10.3390/antibiotics11060729
Liu Y, Wang S, Lan W, Qin W (2019) Fabrication of polylactic acid/carbon nanotubes/chitosan composite fibers by electrospinning for strawberry preservation. Int J Biol Macromol 121:1329–1336. https://doi.org/10.1016/j.ijbiomac.2018.09.042
Chi K, Wang H, Catchmark JM (2020) Sustainable starch-based barrier coatings for packaging applications. Food Hydrocoll 103:105696. https://doi.org/10.1016/j.foodhyd.2020.105696
Pinzon MI, Sanchez LT, Garcia OR et al (2020) Increasing shelf life of strawberries (Fragaria ssp) by using a banana starch-chitosan-Aloe vera gel composite edible coating. Int J Food Sci Technol 55:92–98. https://doi.org/10.1111/ijfs.14254
Mahajan PV, Lee DS (2023) Modified atmosphere and moisture condensation in packaged fresh produce: scientific efforts and commercial success. Postharvest Biol Technol 198:112235. https://doi.org/10.1016/j.postharvbio.2022.112235
Rodrigues FJ, Cedran MF, Garcia S (2018) Influence of linseed mucilage incorporated into an alginate-base edible coating containing probiotic bacteria on shelf-life of fresh-cut yacon (Smallanthus sonchifolius). Food Bioprocess Technol 11:1605–1614. https://doi.org/10.1007/s11947-018-2128-z
Pilon L, Tetelboim MC, Gallo CR et al (2016) Physicochemical and microbiological changes in freshcut pineapple coated with wheat gluten and alginate. Acta Hortic 1111:221–226. https://doi.org/10.17660/ActaHortic 2016.1111.32
Obianom C, Romanazzi G, Sivakumar D (2019) Effects of chitosan treatment on avocado postharvest diseases and expression of phenylalanine ammonia-lyase, chitinase and lipoxygenase genes. Postharvest Biol Technol 147:214–221. https://doi.org/10.1016/j.postharvbio.2018.10.004
Yousuf B, Srivastava AK, Ahmad S (2020) Application of natural fruit extract and hydrocolloid-based coating to retain quality of fresh-cut melon. J Food Sci Technol 57:3647–3658. https://doi.org/10.1007/s13197-020-04397-3
Hassan B, Chatha SAS, Hussain AI et al (2018) Recent advances on polysaccharides, lipids and protein based edible films and coatings: a review. Int J Biol Macromol 109:1095–1107. https://doi.org/10.1016/j.ijbiomac.2017.11.097
Scartazzini L, Tosati JV, Cortez DHC et al (2019) Gelatin edible coatings with mint essential oil (Mentha arvensis): film characterization and antifungal properties. J Food Sci Technol 56:4045–4056. https://doi.org/10.1007/s13197-019-03873-9
Murrieta-Martínez C, Soto-Valdez H, Pacheco-Aguilar R et al (2019) Effect of different polyalcohols as plasticizers on the functional properties of squid protein film (Dosidicus Gigas). Coatings 9:77. https://doi.org/10.3390/coatings9020077
Charles APR, Jin TZ, Mu R, Wu Y (2021) Electrohydrodynamic processing of natural polymers for active food packaging: a comprehensive review. Compr Rev Food Sci Food Saf 20:6027–6056. https://doi.org/10.1111/1541-4337.12827
Chen L, Ru C, Zhang H et al (2022) Progress in electrohydrodynamic atomization preparation of energetic materials with controlled microstructures. Molecules 27:2374. https://doi.org/10.3390/molecules27072374
Cai X, Zhu P, Lu X et al (2017) Electrospinning of very long and highly aligned fibers. J Mater Sci 52:14004–14010. https://doi.org/10.1007/s10853-017-1529-0
Homaeigohar S, Davoudpour Y, Habibi Y, Elbahri M (2017) The electrospun ceramic hollow nanofibers. Nanomaterials 7:383. https://doi.org/10.3390/nano7110383
Yuan Y, Tian H, Huang R et al (2023) Fabrication and characterization of natural polyphenol and ZnO nanoparticles loaded protein-based biopolymer multifunction electrospun nanofiber films, and application in fruit preservation. Food Chem 418:135851. https://doi.org/10.1016/j.foodchem.2023.135851
Pan J, Ai F, Shao P et al (2019) Development of polyvinyl alcohol/β-cyclodextrin antimicrobial nanofibers for fresh mushroom packaging. Food Chem 300:125249. https://doi.org/10.1016/j.foodchem.2019.125249
Echegoyen Y, Fabra MJ, Castro-Mayorga JL et al (2017) High throughput electro-hydrodynamic processing in food encapsulation and food packaging applications: Viewpoint. Trends Food Sci Technol 60:71–79. https://doi.org/10.1016/j.tifs.2016.10.019
Rosell-Llompart J, Grifoll J, Loscertales IG (2018) Electrosprays in the cone-jet mode: from Taylor cone formation to spray development. J Aerosol Sci 125:2–31. https://doi.org/10.1016/j.jaerosci.2018.04.008
Xue F, Gu Y, Wang Y et al (2019) Encapsulation of essential oil in emulsion based edible films prepared by soy protein isolate-gum acacia conjugates. Food Hydrocoll 96:178–189. https://doi.org/10.1016/j.foodhyd.2019.05.014
Liao Y, Loh CH, Tian M et al (2018) Progress in electrospun polymeric nanofibrous membranes for water treatment: fabrication, modification and applications. Prog Polym Sci 77:69–94. https://doi.org/10.1016/j.progpolymsci.2017.10.003
Gagaoua M, Pinto VZ, Göksen G et al (2022) Electrospinning as a promising process to preserve the quality and safety of meat and meat products. Coatings 12:644. https://doi.org/10.3390/coatings12050644
Lv D, Zhu M, Jiang Z et al (2018) Green electrospun nanofibers and their application in air filtration. Macromol Mater Eng 303:1800336. https://doi.org/10.1002/mame.201800336
Vázquez-González Y, Prieto C, Stojanovic M et al (2022) Preparation and characterization of electrospun polysaccharide fucopol-based nanofiber systems. Nanomaterials 12:498. https://doi.org/10.3390/nano12030498
Cruz-Salas CN, Prieto C, Calderón-Santoyo M et al (2019) Micro-and nanostructures of agave fructans to stabilize compounds of high biological value via electrohydrodynamic processing. Nanomaterials 9:1659. https://doi.org/10.3390/nano9121659
Iñiguez-Moreno M, Calderón-Santoyo M, Barros-Castillo JC et al (2022) Nanofibers added with citral: characterization and their application to postharvest control of Fusarium pseudocircinatum in bananas. J Food Process Preserv:e17188. https://doi.org/10.1111/jfpp.17188
Gundewadi G, Rudra SG, Gogoi R et al (2021) Electrospun essential oil encapsulated nanofibers for the management of anthracnose disease in Sapota. Ind Crops Prod 170:113727. https://doi.org/10.1016/j.indcrop.2021.113727
Yue TT, Li X, Wang X-X et al (2018) Electrospinning of carboxymethyl chitosan/polyoxyethylene oxide nanofibers for fruit fresh-keeping. Nanoscale Res Lett 13:239. https://doi.org/10.1186/s11671-018-2642-y
Lin L, Gu Y, Cui H (2018) Novel electrospun gelatin-glycerin-ε-Poly-lysine nanofibers for controlling Listeria monocytogenes on beef. Food Packag Shelf Life 18:21–30. https://doi.org/10.1016/j.fpsl.2018.08.004
Lin L, Zhu Y, Cui H (2018) Electrospun thyme essential oil/gelatin nanofibers for active packaging against Campylobacter jejuni in chicken. LWT - Food Sci Technol 97:711–718. https://doi.org/10.1016/j.lwt.2018.08.015
Li T, Shen Y, Chen H et al (2021) Antibacterial properties of coaxial spinning membrane of methyl ferulate/zein and its preservation effect on sea bass. Foods 10:2385. https://doi.org/10.3390/foods10102385
Ceylan Z, Meral R, Cavidoglu I et al (2018) A new application on fatty acid stability of fish fillets: coating with probiotic bacteria-loaded polymer-based characterized nanofibers. J Food Saf 38:e12547. https://doi.org/10.1111/jfs.12547
Cakmak H, Kumcuoglu S, Tavman S (2019) Electrospray coating of minimally processed strawberries and evaluation of the shelf-life quality properties. J Food Process Eng 42:e13082. https://doi.org/10.1111/jfpe.13082
Melendez-Rodriguez B, Bengue M-SM, Torres-Giner S et al (2021) Barrier biopaper multilayers obtained by impregnation of electrospun poly (3-hydroxybutyrate-co-3-hydroxyvalerate) with protein and polysaccharide hydrocolloids. Carbohydr Polym Technol Appl 2:100150. https://doi.org/10.1016/j.carpta.2021.100150
Szalaty TJ, Klapiszewski Ł, Jesionowski T (2020) Recent developments in modification of lignin using ionic liquids for the fabrication of advanced materials–a review. J Mol Liq 301:112417. https://doi.org/10.1016/j.molliq.2019.112417
Koller M, Mukherjee A (2022) A new wave of industrialization of PHA biopolyesters. Bioengineering 9. https://doi.org/10.3390/bioengineering9020074
Liu Y, Ma X, Shu L et al (2021) From Industry 4.0 to Agriculture 4.0: current status, enabling technologies, and research challenges. IEEE Trans Ind Informatics 17:4322–4334. https://doi.org/10.1109/TII.2020.3003910
Belaud JP, Prioux N, Vialle C, Sablayrolles C (2019) Big data for agri-food 4.0: application to sustainability management for by-products supply chain. Comput Ind 111:41–50. https://doi.org/10.1016/j.compind.2019.06.006
Taghipoor H, Mirzaei J (2023) Statistical predicting and optimization of the tensile properties of natural fiber bio-composites. Polym Bull 1–25. https://doi.org/10.1007/s00289-023-04713-9
Mamy D, Huang Y, Akpabli-Tsigbe NDK et al (2022) Valorization of Citrus reticulata peels for flavonoids and antioxidant enhancement by solid-state fermentation using Aspergillus niger CGMCC 3.6189. Molecules 27. https://doi.org/10.3390/molecules27248949
Kozlowska J, Prus W, Stachowiak N (2019) Microparticles based on natural and synthetic polymers for cosmetic applications. Int J Biol Macromol 129:952–956. https://doi.org/10.1016/j.ijbiomac.2019.02.091
Avramescu SM, Butean C, Popa CV et al (2020) Edible and functionalized films/coatings-performances and perspectives. Coatings 10:687. https://doi.org/10.3390/coatings10070687
Grand View Research (2022) Bioplastics market size, share & trends analysis report by product (biodegradable, non-biodegradable), by application (packaging, agriculture, consumer goods), by region, and segment forecasts, 2022 - 2030. In: Gd. View Res. Inc. https://www.grandviewresearch.com/industry-analysis/bioplastics-industry. Accessed 26 Mar 2023
Grand View Research (2022) Functional foods market size, share & trends analysis report by ingredient (carotenoids, prebiotics & probiotics, fatty acids, dietary fibers), by product, by application, by region, and segment forecasts, 2022 - 2030. https://www.grandviewresearch.com/industry-analysis/functional-food-market. Accessed 26 Mar 2023
Kim IJ, Jeong D, Kim SR (2022) Upstream processes of citrus fruit waste biorefinery for complete valorization. Bioresour Technol 362:127776. https://doi.org/10.1016/j.biortech.2022.127776
Leong HY, Chang CK, Khoo KS et al (2021) Waste biorefinery towards a sustainable circular bioeconomy: a solution to global issues. Biotechnol Biofuels 14:1–15. https://doi.org/10.1186/s13068-021-01939-5
Dahiya D, Sharma H, Rai AK, Nigam PS (2022) Application of biological systems and processes employing microbes and algae to Reduce, Recycle, Reuse (3Rs) for the sustainability of circular bioeconomy. AIMS Microbiol 8:83–102. https://doi.org/10.3934/microbiol.2022008
Demissie H, Gedebo A, Agegnehu G (2023) Agronomic potential of avocado-seed biochar in comparison with other locally available biochar types: a first-hand report from Ethiopia. Appl Environ Soil Sci 2023. https://doi.org/10.1155/2023/7531228
Tian H, Wang X, Tong YW (2020) Sustainability assessment: focusing on different technologies recovering energy from waste. INC
Klai N, Yadav B, El Hachimi O et al (2021) Agro-industrial waste valorization for biopolymer production and life-cycle assessment toward circular bioeconomy. In: Biomass, Biofuels, Biochemicals. Elsevier Inc, pp 515–555
Author information
Authors and Affiliations
Contributions
Maricarmen Iñiguez-Moreno: writing, original draft, conceptualization, investigation, writing, visualization, review, and editing; Montserrat Calderón-Santoyo: review and editing, supervision; Gabriel Ascanio: review and editing, supervision, project administration; Frida Ragazzo-Calderón: review and editing; Roberto Parra-Saldívar: review and editing; Juan Arturo Ragazzo-Sánchez: writing, review and editing, supervision, project administration. All the authors reviewed and approved the final version.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Iñiguez-Moreno, M., Calderón-Santoyo, M., Ascanio, G. et al. Harnessing emerging technologies to obtain biopolymer from agro-waste: application into the food industry. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-04785-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-04785-7