Abstract
In lactobacilli, CcpA is known to modulate the expression of genes involved in sugar metabolism, stress response and aerobic adaptation. This study aimed to evaluate a ccpA mutant of Lacticaseibacillus casei BL23 to increase lactic acid production using cheese whey. The ccpA derivative (BL71) showed better growth than the L. casei wild-type in the whey medium. In a stirred tank reactor, at 48 h, lactate production by BL71 was eightfold higher than that by BL23. In batch fermentations, the final values reached were 44.23 g L−1 for BL71 and 27.58 g L−1 for BL23. Due to a decrease in the delay of lactate production in the mutant, lactate productivity increased from 0.17 g (L.h)−1 with BL23 to 0.80 g (L.h)−1 with BL71. We found that CcpA would play additional roles in nitrogen metabolism by the regulation of the proteolytic system. BL71 displayed higher activity of the PepX, PepQ and PrtP enzymes than BL23. Analysis of prtP expression confirmed this deregulation in BL71. Promoter analysis of the prtP gene revealed CcpA binding sites with high identity to the cre consensus sequence and the interaction of CcpA with this promoter was confirmed in vitro. We postulate that deregulation of the proteolytic system in BL71 allows a better exploitation of nitrogen resources in cheese whey, resulting in enhanced fermentation capacity. Therefore, the ccpA gene could be a good target for future technological developments aimed at effective and inexpensive lactate production from dairy industrial wastes.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lactic acid (2-hydroxypropionic acid) is an organic compound with applications in the pharmaceutical, chemical and textile industries, widely used as an acidulant/flavoring, pH buffering agent or inhibitor of bacterial spoilage in a wide variety of processed foods (Abdel-Rahman et al. 2013). This acid has also received great attention as a precursor of the biodegradable polylactic acid polymer (Narayanan et al. 2004; John et al. 2007; Sahoo and Jayaraman 2019). Great amounts of lactate are produced through carbohydrates fermentation by lactic acid bacteria (LAB), such as those belonging to the former genus Lactobacillus, which has been recently split into more than twenty new genera (Zheng et al. 2020), which are GRAS (generally recognized as safe) microorganisms. The metabolism of sugars by lactobacilli (which refers to all microorganisms that were classified as Lactobacillaceae until the recent taxonomical modifications (Zheng et al. 2020)) is characterized by the production of lactate through the action of the lactate dehydrogenase enzyme, as the main product of its strictly fermentative metabolism (Allievi et al. 2019). Contrarily to chemical synthesis, lactate production through fermentation allows obtaining optically pure D- and L-lactic acid, instead of a racemic mixture. Additional advantages of using microorganisms in lactate production reside in the fact that it can be produced from industrial wastes and other inexpensive substrates and by-products (Bernardo et al. 2016). The most expensive component of microbial growth substrates is usually the nitrogen source. In addition, some lactobacilli are extremely fastidious and require more than 10 essential amino acids for their growth. Among by-products from the cheese industry, whey contains significant amounts of lactose as a carbon source (approximately 5% w/v), and also carries proteins, vitamins, and minerals. In addition, it contains other essential nutrients necessary for the growth of LAB, like branched-chain amino acids. Thus, the reutilization of this agro-industrial by-product provides cost reductions and environmental sustainability (Rabaioli Rama et al. 2019; Zotta et al. 2020).
The efficient use of nutrients by microorganisms is associated with carbon catabolite repression (CCR), which allows microbes to select their preferred source from multiple offerings of carbon catabolites. Production from industrial waste often starts from mixed sugars and can therefore be hindered by the process of CCR, which becomes an important limiting factor (Fujita 2009). The catabolite control protein A (CcpA) is a mediator of CCR in low-G + C Gram-positive bacteria (Mahr et al. 2000; Viana et al. 2005; Monedero et al. 2007; Zomer et al. 2007; Deutscher 2008). In several Firmicutes, the ccpA gene is clustered with the pepQ gene, encoding an X-Pro aminopeptidase. CcpA-dependent activation of pepQ has been postulated for Lactobacillus delbrueckii subsp. lactis (Schick et al. 1999) and demonstrated in Lactococcus lactis (Zomer et al. 2007), a fact that indicates a link between the regulation of carbon and nitrogen metabolism. This link has also been described for Bacillus subtilis (Sonenshein 2007; Fujita 2009; Wünsche et al. 2012) and Staphylococcus aureus (Li et al. 2010), where CcpA controls genes responsible for nitrogen assimilation and amino acid metabolism.
LAB are likely to respond to changes in nitrogen availability by regulating the activity of the proteolytic system. It is known that the transcriptional regulator CodY negatively regulates the expression of several components of the proteolytic system in B. subtilis and Lc. lactis and responds to the intracellular pool of branched-chain amino acids (Guédon et al. 2001; Petranovic et al. 2004; Sonenshein 2005); however, CodY is absent in lactobacilli species, (Kleerebezem et al. 2003; Wakai and Yamamoto 2013; Alcántara et al. 2016). Recently, the promoters located in the intergenic region of the prsA-prtP genes, encoding the main cell-surface proteinase (prtP) and its maturase (prsA) in Lacticaseibacillus casei BL23, have been shown to be regulated by the repressor PrcR in peptide-rich growth media (Alcántara et al. 2016; Coll-Marqués et al. 2020). PrcR would directly regulate the metabolism of nitrogen, peptides and amino acids, since its inactivation has been found to result in a reduced growth rate in de Man, Rogosa and Sharpe (MRS) medium but in a rapid acidification of milk as a consequence of the high expression of PrtP, as the fast milk acidifying phenotype is related to the expression of cell-envelope proteinases (Dandoy et al. 2011). However, PrcR does not play a role exactly analogous to that of CodY in streptococci and lactococci, suggesting that, in L. casei BL23, other elements could be involved in the regulation of prtP and peptidases. Recent evidence has demonstrated the roles of the pleiotropic regulator CcpA in non-CCR processes such as stress resistance and aerobic metabolism in Lactiplantibacillus plantarum and Lactobacillus delbrueckii subsp. bulgaricus, but some molecular mechanisms remain unclear (Mazzeo et al. 2012; Zotta et al. 2012, 2017; Zhang et al. 2020). Also, it was reported that aerobic cultivation affects the expression of the peptidase system in L. casei (Siciliano et al. 2019). In this study, we aimed to evaluate the likely role of L. casei CcpA in the performance of this species during fermentation of a low-cost substrate (cheese whey) to produce lactic acid and to analyze putative molecular targets of the nitrogen metabolism that might be modulated by CcpA.
Material and methods
Microorganisms and culture media
The microorganisms used in the present work were Lacticaseibacillus casei BL23 (Acedo-Félix and Pérez Martínez 2003) and L. casei BL71 (BL23 ccpA::erm), gently provided by Dr. Pérez-Martínez (Monedero et al. 1997). In these strains, the ability to metabolize milk lactose is linked to the lactose phosphoenolpyruvate: phosphotransferase system and a 6-phospho-β-galactosidase (Mazé et al. 2010). Stock cultures (1 mL) were stored at −70 °C in MRS medium (tryptone 10 g L−1, yeast extract 4 g L−1, meat extract 8 g L−1, sodium acetate 5 g L−1, MgSO4·7H2O 0.2 g L−1, MnSO4·4H2O 0.05 g L−1, Tween 80 1 mL L−1 and glucose 20 g L−1, pH 6.5, Biokar, Beauvais, France) with 20% (v/v) glycerol. Strains were grown at 37 °C under static conditions in MRS or Chemically Defined Medium (CDM) (Piuri et al. 2003) ± meat peptone 0.1–0.5%. Erythromycin 5 µg mL−1 was added to BL71 cultures for all determinations and when the strain was used as inoculum for stirred tank fermentation.
Cheese whey is composed of 4.6–5.2% lactose, 0.6–0.8% soluble proteins and free amino acids, 0.3–0.4% lipids, 8–10% of dried extract of mineral salts. Additionally, it contains 0.02–0.05% of citric and lactic acids, non-protein nitrogenous components, such as urea and uric acid, vitamins of group B, and traces of galactose and glucose are found by the hydrolysis of lactose (Panesar et al. 2007; Carvalho et al. 2013).
Cheese whey medium was composed of 5% w/v of cheese whey powder (kindly provided by Lácteos Vidal; www.lacteosvidal.com.ar), 0.6 mM MgSO4, 0.3 mM MnSO4, 1 mL L−1 Tween 80, and 50 mM NaPO4 pH 7. Cheese whey medium was either supplemented or not with 0.5% yeast extract (Difco) (YE) or 1.5% corn steep liquor (CSL).
Bottle batch fermentation (uncontrolled conditions)
Fermentation assays were performed in MRS or cheese whey medium. 18 mL cultures of both strains were grown separately in bottles under microaerophilic conditions to an initial OD600 of 0.2 and were incubated for 65 h. Samples were collected every 2 h during the first 24 h and every 4 h between 24 and 65 h of culture. A Hanna pH meter model HI98103 was used for pH determinations. Viable bacteria counts (CFU mL−1) were determined from serial decimal dilutions in 0.85% NaCl.
Stirred tank batch fermentation (controlled conditions)
The cultures used as inoculum were grown in MRS medium and incubated at 37 °C and 50 rpm for 17 h. The volume of inoculum used was calculated to initiate the fermentation at an OD600 of 0.5. The batch fermentation was carried out in MRS and cheese whey supplemented with 0.5% YE (Difco) in a 5-L Biostat B plus bioreactor (Sartorius) in 5 L and 1 L working volumes respectively for both strains. The temperature was maintained at 37 ± 1 ºC and the pH at 6 by the addition of 1:2 (v/v) NH4OH solution, which also served as a nitrogen source. The %pO2 set point was < 10% maintained by N2 addition (1vvm). Fermentations in MRS were incubated for 24 h, while cheese whey fermentations with BL23 and BL71 continued for 72 and 55 h, respectively. Base consumption defined the end of these last ones. Samples were collected every 3 h. The pH was measured using a pH electrode (Mettler Toledo, Germany) and the oxygen concentration was determined with a polarographic probe (Mettler-Toledo). Foam formation was prevented through the addition of 5% (v/v) antifoam A (Sigma, St. Louis, MO, USA). The cultures were sampled throughout all the bioprocess phases to determine the number of CFU mL−1 by plating on MRS agar plates and for analytical determination.
Analytical determinations
The biomass formation was monitored by OD600, CFU mL−1 and dry weight (DW) during growth in MRS. Due to its intrinsic turbidity, bacterial growth in the cheese whey medium was only evaluated by determining the number of CFU mL−1. The dry mass was determined by taking variable volumes according to the OD600, and centrifugation at 4000 ×g for 10 min at 4 °C. The pellet was washed with 30 mL of distilled water, centrifuged again under the same conditions and resuspended in 2 mL of distilled water. This volume was placed on the aluminum tray of a moisture analyzer (Sartorius Moisture Analyzer series MA100) to determine DW. The remaining glucose concentration in MRS medium was determined using a colorimetric reaction (Glicemia Enzimática AA líquida Wiener Lab, Rosario, Argentina). Samples were taken every 15 min after reaching the exponential growth phase and sugar was measured according to the manufacturer´s instructions.
The enzymatic technique developed by Gutmann and Wahlefeld (1974) was followed for lactate determination. This protocol allows specific determination of the L- and D-lactate isomers. L/D-lactate was oxidized to pyruvate in the presence of NAD+ by the action of L(+)-lactate dehydrogenase or D(+)-lactate dehydrogenase. The reaction mixtures contained 0.5 M glycine, 0.4 M hydrazine pH 9, 40 mM NAD+ and 5 mg protein mL−1 of lactate dehydrogenase. The samples were incubated for 30 min at 37 °C and the presence of lactate was measured by the formation of NADH in a spectrophotometer at 340 nm. Lactic acid was measured in cheese whey fermentations both in uncontrolled conditions in bottle and in controlled conditions. In bottle fermentations, lactate determination was performed at 22 and 65 h of culture. In stirred tank fermentations, lactate measurements were performed every 3–4 h.
In order to estimate the kinetic parameters, samples were collected at each time point and OD600, DW or CFU mL−1 were determined (according to each culture condition). The cultures growth parameters were determined graphically during the exponential phase, by calculating µ values from the slope of the linear regression of Ln (CFU mL−1) vs time.
PepQ, PepX and PrtP enzymatic activities were assayed as described in Piuri et al. (2003) from cultures grown to stationary phase in CDM. This medium has been previously used in species of the genera Lacticaseibacillus and Lactococcus for the study of components of the proteolytic system, evaluating the addition of different nitrogenous supplements, peptides and amino acids (Morishita et al. 1974; Guédon et al. 2001; Piuri et al. 2003; Alcántara et al. 2016). The activities of PepQ, PepX and PrtP are, respectively expressed as nmol of Leu and Pro released per minute per milligram of protein, nmol of nitroanilide released per minute per milligram of protein, and fluorescence units per minute per 10 mg of protein. The data were statistically analyzed using the Prism 5.0 software (GraphPad Inc.). The experiments were performed in triplicate and are presented as the mean ± standard deviation (error bars). The Student's test was applied to establish whether the differences could be considered significant at p < 0.05.
Protein purification of His6-CcpA
Escherichia coli FT1 carrying pET15b-ccpAL.casei was grown in Luria–Bertani (LB) broth to an OD600 of 0.6 at 37 °C and recombinant protein expression was induced for 4 h with 0.5 mM IPTG. The cells were lysed by sonication in a buffer containing 50 mM Tris–HCl (pH 8.0), 300 mM NaCl, 5% glycerol, and 0.1 mM dithiothreitol, and the clarified supernatants were loaded onto Ni–NTA columns (Qiagen, Hilden, Germany). The columns were washed with 30 mM and 50 mM imidazole, and the proteins eluted with 120 mM imidazole. His6-CcpA-containing fractions were pooled, dialyzed at 4 °C against 50 mM Tris–HCl pH 7, 40% glycerol, 0.1 mM phenylmethylsulfonyl fluoride and stored in aliquots at –70 °C.
Electrophoretic mobility shift assay
An electrophoretic mobility shift assay (EMSA) was performed with biotinylated oligonucleotide probes. To amplify the promoter region of the prtP gene, a PCR was performed using GoTaq DNA polymerase (Promega, Madison, WI, USA) by following the manufacturer's instructions. The probe was made using primers Promp1: 5′-TTGGATCCTTTATTCTAGCGTTGGC-3′ and prt2bio: Biotin-TEG-5′-GATGATACCTTAGTTTGCTGCGAGA-3′. The unlabeled probe was made under the same conditions but using an unbiotinylated oligonucleotide (5′-GATGATACCTTAGTTTGCTGCGAGA-3′) as reverse primer. The amplification program was: 95 °C, 5 min; 25 cycles of: 1 min at 94 °C, 1 min at 54 °C, 1 min at 72 °C; and 10 min elongation at 72 °C. Chromosomal DNA from L. casei BL23 was used as a template. The reaction product was purified from a 1% agarose gel in 1X TAE buffer using GFX® PCR DNA and the Gel Band Purification Kit (Pharmacia Biosciences, NJ, USA), and used as probe in the EMSA. Purified His6-CcpA protein (0.35; 1.33, 2.66 and 5.32 μM final concentration) was incubated with 1.5 ng of probe in a buffer containing 10 mM Tris–HCl, pH 7.4, 50 mM KCl, 5 mM MgCl2, 1 mM dithiothreitol, 10% glycerol, and 3 μg mL−1 sheared salmon sperm DNA in 20 μL at 37 °C for 30 min. When required, 3 ng of unlabeled probe was used to compete with the labeled probe. Samples were resolved by electrophoresis in 7.5% polyacrylamide gels. The gels were electro-transferred to nylon membranes and probes were detected by chemiluminescence with alkaline phosphatase-conjugated streptavidin and CDP-Star (GE-Biosciences, Chicago, IL, USA). Chemiluminescent images were taken using a Fuji LAS1000 equipment and Image Gauge 3.122 software (Fuji Film).
Supershift assay
Antibody supershift assays were performed using 375-bp and 420-bp probes synthesized by PCR with the oligonucleotide pairs Promp1/prt2bio and PrtM: 5′- GCTACTTTCAGTCACCTTGC-3′/PromP2: 5`-TTCTGCAGAGAACCAAATCAAACCC-3′, respectively, according to Ghosh et al. (2006). After pre-incubation of the purified protein with or without anti-CcpA antiserum (Küster et al. 1996) for 30 min, an EMSA was carried out as described previously.
Regulator fishing assay
A “regulator fishing” approach was used as described in Rey et al. (2003), using streptavidin-coated M-PVA Magnetic Beads (Chemagen Technical, GmbH, Rodgau, Germany). Biotinylated probes which include catabolite responsive element (cre) sequences were used (positions −211 and +10 for the 375-bp probe and −211 for the 420-bp probe; see above and Supplementary Figure 1. DNA fragments were immobilized with streptavidin-coated magnetic particles, as recommended by the manufacturer, using DNA-binding buffer A (50 mM Tris pH 7.5, 0.5 mM EDTA, 1 M NaCl). After this, magnetic particles were resuspended in protein-binding buffer B (20 mM Tris pH 8.0, 1 mM EDTA, 10% glycerol, 1 mM dithiothreitol, 100 mM NaCl, 0.05% Triton ×100) and incubated with crude cell extracts (0.5 mg of protein) of L. casei BL23 for 20 min at room temperature. Subsequently, the magnetic particles were washed three times with buffer B. Bound proteins were eluted with elution buffer C (20 mM Tris pH 8.0, 1 mM EDTA, 10% glycerol, 1 mM dithiothreitol, 1 M NaCl, 0.05% Triton X-100). Eluted proteins were then analyzed by SDS-PAGE and Western blot with anti-CcpA antibody.
Western blot
Samples were electrotransferred to PVDF membranes according to the manufacturer’s instructions (Macherey–Nagel, Düren, Germany). Polyclonal antibody against CcpA (Küster et al. 1996) was used at a 1:2000 dilution. Detection was performed with HRP-conjugated anti-rabbit IgG (Santa Cruz Biotechnology, Dallas, TX, USA) and chemiluminescence using ECL (Sigma, St. Louis, MO, USA).
Transcriptional analysis of prtP
As mentioned in the evaluation of enzymatic activities (see Analytical determinations), to evaluate the differences in the components of the proteolytic system, it is necessary to use cells grown in CDM medium with or without meat peptone. RNA was extracted using the hot phenol method (Spatafora et al. 1995). Cells of logarithmic growth phase were harvested and washed at 4 °C in 50 mM EDTA pH 8, centrifuged for 5 min (20,000 xg) and resuspended in 10 mM Tris–HCl pH 8, 2 mM EDTA, 20 mM ammonium acetate pH 4 in an ice bath to a final OD600 of 15. Then, 0.5 mL of acid phenol pH 4.7 (Ambion, Austin, TX, USA) warmed to 65 °C was added and mixed by vortexing. After heating at 65 °C for 5 min, an extraction with chloroform was performed. The RNA was precipitated from the aqueous phase using a 1/10 volume of 5 M NaCl and 0.6 volume of isopropanol. The samples were treated with RQ1-RNAse free DNAse (Promega, Madison, WI, USA) and another extraction with chloroform was performed. After centrifugation, RNA was resuspended in DEPC water (Ambion, Austin, TX, USA). The samples were kept at − 20 °C for at least 1 h. DNA absence was confirmed by PCR. The nucleic acid concentration was measured using a Nanodrop2000 spectrophotometer (Thermo Scientific, Waltham, MA, USA). RT-qPCR was performed as described in Palomino et al. (2016), using the following primers: forward primer prtp7 5′-TCGGCGAAATCCAAGCAAAGG-3′ and reverse primer prtp8 5′-GCTGCGGTTGTGTCAGTGG-3′ for the prtP gene; forward primer 16srt1 5′-GCGAAGGCGGCTGTCTGG-3′ and reverse primer 16srt2 5′-GGCACTGAAGGGCGGAAACC-3′ for the 16S rRNA gene (used as reference). cDNA was mixed in the presence of Sybr Green as fluorophore in multiwell plates covered with film in the IQTM 5 Optical System Software (Bio-Rad) equipment. RT-qPCR reactions were performed under the following cycling conditions: for the prtP gene: 5 min at 95 °C, 30 cycles of 20 s at 90 °C, 20 s at 58 °C, and 20 s at 72 °C; and for the 16S rRNA gene: 5 min at 95 °C, 30 cycles of 20 s at 90 °C, 20 s at 54 °C, and 20 s at 72 °C. The relative change in the expression of individual genes was analyzed following the standard curve method (Larionov et al. 2005). The Student's test was applied to establish whether the differences could be considered significant at p < 0.05.
Results
Evaluation of the ccpA mutant in MRS or cheese whey batch fermentation
Our first objective was to find the best nitrogen supplements in the use of cheese whey as a substrate to produce lactic acid. We used wild-type L. casei (BL23) and its ccpA derivative mutant (BL71) in cheese whey batch fermentation under uncontrolled conditions. We evaluated cheese whey with or without different nitrogen sources (YE or CSL) for lactic acid production in bottle batch fermentations with both strains. A 40-h lag phase was observed for acidification in the absence of nitrogen (YE or CSL) supplementation (Fig. 1a). YE or CSL supplementation resulted in a three-fold decrease in the lag phase duration (15 h). Results of the number of CFU mL−1 were consistent with the acidification profiles (Fig. 1b), and a higher rate of acidification was observed for BL71 in all conditions.
Lactate concentration (Table 1) was determined for both isomers in the cheese whey experiments. The D/L-lactate proportions were similar for the wild type and the ccpA mutant in all the growth media assayed and higher levels of lactate were observed for the mutant strain in all conditions. When cheese whey medium was supplemented with YE, an almost two-fold increase in lactate production early in the growth was observed for BL71 compared to BL23 (Table 1).
After choosing the best nitrogen source, the following step was to scale-up the fermentation in a bioreactor under controlled conditions. We compared the fermentative characteristics of BL23 and BL71 in MRS batch fermentations in stirred tank reactors under controlled pH and low oxygen conditions (pO2 < 10%). The growth rates (µ), measured through the increase in dry weight (DW) and CFU mL−1, for the BL71 strain (ccpA mutant) (DW 0.42 h−1 and CFU mL−1 0.47 h−1) were consistently higher than those for the BL23 strain (DW 0.24 h−1 and CFU mL−1 0.28 h−1). Glucose consumption, which was measured to determine the yield of the process, showed a more efficient use of substrate for BL71 [Y(x/s) BL71 = 0.27 g L−1] than for BL23 [Y(x/s) BL23 = 0.12 g L−1].
After characterizing the kinetic parameters of both strains in MRS (without nitrogen restriction) in a bioreactor, fermentations were carried out in cheese whey plus 0.5% YE in a stirred tank under controlled pH and oxygen conditions (pO2 < 10%). Lactate production was determined and the obtained kinetic parameters are shown in the Supplementary Table 1 Figures 2a and 2b show the growth patterns obtained. In Fig. 2a, BL23 shows a pattern compatible with a diauxic growth, which is not observed in the mutant strain lacking the CcpA regulator. Under these conditions, the ccpA mutant strain showed a shorter interval of time for lactate production compared to BL23. Thus, BL23 shows a delay in production of more than 20 h compared to BL71. The 48-h cumulative base consumption, which was 16.1 and 0.3 g L−1 of NH4OH for BL71 and BL23, respectively, was used as an indicator of the ability to produce acidic compounds. According to this, the 48-h cumulative lactic acid production was 2.42 ± 0.52 g L−1 for BL23 and 19.33 ± 1.11 g L−1 for BL71, i.e. an eight-fold increase in production compared to BL23 strain (Table 2). This parameter correlates with the higher value of CFU mL−1 (8.9 × 108 for BL23 and 4.36 × 109 for BL71). The cumulative production of lactate at the end of the fermentation, defined by base consumption (72 h for BL23 and 55 h for BL71), was higher for BL71 (44.23 g L−1) than for BL23 (27.58 g L−1). Thus, under the culture conditions used, the ccpA mutation increased the productivity of lactate from 0.17 to 0.80 g (L.h)−1 at 55 h of culture (the peak of lactate production for BL71). This result shows that BL71 achieves a maximum value of production of lactate 17 h before BL23. Not only the final amount of lactate produced was higher but also the specific productivity was 0.38 g (L.h)−1 for BL23 and 0.8 g (L.h)−1 for BL71. These results show that the ccpA mutation favors utilization of the different carbon and nitrogen sources of the culture medium.
a Growth curve (CFU mL−1) of the L. casei wild-type strain BL23 and the ccpA mutant strain BL71 and lactic acid production on cheese whey-based medium supplemented with 0.5% YE under pH controlled conditions. b Comparison of NH4OH consumption (in g L−1) in L. casei BL23 and BL71 on cheese whey-based medium with 0.5% YE
Involvement of CcpA in the regulation of the proteolytic system
To assess the likely role of CcpA in the regulation not only of CCR but also of the provision of nitrogen sources and the proteolytic system in L. casei, we analyzed growth in a CDM (Morishita et al. 1974; Piuri et al. 2003) with addition of meat peptone (80% peptides and 20% free amino-acids) as an extra nitrogen source. CDM can be used to assess differences triggered by increased provision of nitrogen sources that could not be studied in complex media like MRS or cheese whey (Morishita et al. 1974; Guédon et al. 2001; Piuri et al. 2003; Alcántara et al. 2016). The supplementation of CDM (a medium with nutrient restrictions) with meat peptone induced a most notorious effect on growth compared to YE supplementation (data not shown). As shown in Fig. 3, growth in CDM was similar for both strains, while addition of meat peptone resulted in a higher stimulation of growth in BL71. This strain was presumably able to use the nitrogen provision immediately, whereas BL23 showed a lag phase of 4 h (Fig. 3). The activities of the cell-wall proteinase PrtP and the peptidases PepX and PepQ were evaluated in bacterial cells grown in CDM. The three activities showed a significant increase in the BL71 strain (Fig. 4), which would account for a de-repression effect.
Proteolytic specific activities in L. casei BL23 and BL71. Activities for PepQ, PepX and PrtP are respectively expressed as nmol of Leu and Pro released per minute per milligram of protein, nmol of nitroanilide released per minute per milligram of protein, and fluorescence units per minute per 10 mg of protein. *p < 0.01, **p < 0.001
Molecular analysis of the CcpA mode of action in Lacticaseibacillus casei
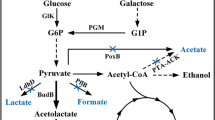
In view of the results on the proteolytic and peptidase activities, we next searched for consensus cre sequences, involved in the specific CcpA recognition, within the genomic regions of the genes encoding PrtP, PepX and PepQ (NCBI Reference Sequence: NC_010999.1; Mazé et al. 2010), using the consensus sequence described by Francke et al. (2008). The in silico analysis revealed two putative cre sites at positions −211 and +10 relative to the translation initiation site of prtP. Additional sites were observed at position +23 in the pepX gene, with high score alignment with a cre site identified in the L. casei BL23 phosphofructokinase gene (pfk), known to be recognized by CcpA (Viana et al. 2005). In L. casei, the pepQ gene is transcribed divergently to ccpA and three putative cre sites were observed at positions -123 in the orientation ccpA-pepQ and at -47 and -2 in the orientation pepQ-ccpA (Supplementary Figure 1). Depending on the relative position of the cre sites within CcpA-responsive promoters, CcpA may act either as a repressor or as an activator (Marciniak et al. 2012). Activation of transcription has been reported when putative cre sites were located upstream of the hexameric −35 sequence, whereas repression has been reported when the cre site was found in or downstream of putative −35 and −10 sequences (Fujita 2009). The location of cre sites in pepX and prtP (+23 and +10, respectively) was compatible with a repressive effect of CcpA, whereas the location of three cre sites in the pepQ-ccpA intergenic region would account for both effects, being the −2 position responsible for a repressive effect.
To further investigate the role of CcpA in the regulation of the L. casei proteolytic system, we evaluated the expression level of the prtP gene by RT-qPCR analysis, which showed a 2.2-fold de-repression of prtP in BL71 compared to BL23 when bacteria were grown in CDM supplemented with meat peptone (Fig. 5a). To investigate the nature of the relationship between CcpA and PrtP, we assessed the ability of CcpA to bind to the prtP promoter region by using two different approaches: the “regulator fishing” assay (Rey et al. 2003) and an EMSA. DNA probes that included cre sites at positions −211 and +10 (375 bp probe) and only −211 (420 bp probe) were designed (Fig. 5b) and incubated with crude cell extracts of L. casei BL23. As shown in Fig. 5c, CcpA binding to both probes was detected with an anti-CcpA polyclonal antibody (Küster et al. 1996); however, only high affinity interaction was observed when the +10 cre site was present in the probe, since high ionic strength was needed to release the protein from the 375-bp probe (Fig. 5c). No band was observed when extracts of BL71 were used (data not shown). EMSA experiments were also carried out with the 375-bp probe and purified His6-CcpA from L. casei. This promoter region has the particularity of generating a secondary structure that was evidenced in the absence of any added protein, probably due to the presence of palindromic runs of A and T sequences (Fig. 5d). Increasing amounts of His6-CcpA showed a concentration-dependent shift (Fig. 5d). This CcpA-triggered shift was corroborated by a supershift analysis using anti-CcpA antibodies and the 375-bp and 420-bp probes. Only when the +10 cre site was present in the probe, a supershift was observed, showing CcpA binding to the prtP promoter region (Supplementary Figure 2). Altogether, these results indicate that CcpA binds to the prtP promoter at the cre site in position +10, compatible with a repressive effect.
a Expression of the prtP gene determined by RT-qPCR. Relative transcription is plotted. Data represent the level of expression for the prtP gene normalized to that of rRNA 16S for each condition. C: CDM; CDMP: CDM with meat peptone. Bars show standard errors from three independent experiments, *p < 0.01. b prtP promoter sequence. The − 35/− 10 regions of the putative promoters are in bold italics and indicated by boxes and potential cre sequences are underlined and aligned with consensus sequences described in Supplementary Figure 1. Translation-initiation codons and putative ribosome-binding sites are indicated by boxes. Promoters were predicted using the Softberry software BPROM (www.softberry.com). Primers to amplify the 375-bp probe are displayed in open boxes, whereas primers to amplify the 420-bp probe are displayed in gray boxes. c Western blot analysis of “regulator fishing” experiments. Eluted proteins were detected with an anti-CcpA antibody. “Low” and “high” indicate the ionic strength of the elution buffer used in the “regulator fishing” assay, corresponding to buffers B and C described in materials and methods, respectively. His6-CcpA is the recombinant purified L. casei CcpA protein (Viana et al. 2005). d His6-CcpA EMSA with the 375-bp prtP promoter probe. Increasing amounts of His6-CcpA purified protein (0.35 µM to 5.32 µM) were incubated with a 375-bp DNA probe that includes the prtP promoter and cre sites at positions − 211 and +10. The arrows indicate the positions of free probe, secondary structure and mobility shift. *shows lanes where shift was visualized
Discussion
Strategies to improve lactic acid production by metabolic engineering approaches include the use of ccpA mutants that, for instance, show reduction of CCR of the galactose (gal) utilization operon in Lc. lactis (Singh et al. 2006). Our results indicate that inactivation of ccpA in L. casei can be a strategy for enhanced lactate production from cheese whey, an important food industry by-product. The substrate is one of the highest production costs for lactate synthesis, and the use of refined sugar (glucose for example) makes the process even more expensive. Since food-waste materials are a good alternative as substrates because of their high content of carbohydrates, many efforts have been made to use such substrates to produce lactate by Lacticaseibacillus. Wang et al. (2015), for example, chose soybean straw as the substrate to produce lactic acid by L. casei because of its high protein content, and found a productivity of 0.61 g (L.h)−1. In the present study, we found that lactic acid productivity in cheese whey-based medium with the L. casei ccpA mutant BL71 strain was 0.80 g (L.h)−1. This productivity achieved for BL71 in batch fermentations was in the order of that reported by others using cheese whey and Lacticaseibacillus rhamnosus (Alvarez et al. 2010; Rabaioli Rama et al. 2019). In batch fermentations using cheese whey-based medium, BL71 produced higher amounts of lactic acid and the production was significantly faster, indicative of a more efficient use of the substrate compared to the wild-type strain.
When comparing controlled to uncontrolled conditions, although at initial points the lactate production in the bottle culture was higher, at the end of the fermentation, the lactate production in the uncontrolled system was 9.39 g L−1 and in controlled bioreactor 44.23 g L−1, that was almost 5 times higher. The differences observed between both conditions could be explained by a difference in oxygen tension which is more marked in the early phases of fermentation. As previously reported in L. casei growth in cheese whey or milk media, different oxygen tension conditions can influence lactate production by mainly affecting LDH activity (Ricciardi et al. 2019; Siciliano et al. 2019). On the other hand, in bottle culture the final product is limited by pH, while in the bioreactor system this limitation is lower since the pH is controlled by adding base.
All these results suggested a possible improvement on the use of carbon and energy sources in rich medium due to the mutation of the global regulator CcpA.
Since most LAB rely on peptides rather than on amino acids for efficient nitrogen assimilation, the absence of efficient proteolytic and peptidase systems may limit their development in cheese whey medium. Thus, the fast milk acidification phenotype has been linked to the presence in LAB of surface proteases able to efficiently degrade milk casein (Dandoy et al. 2011). In agreement with the reports of other authors (Ricciardi et al. 2019), the growth of BL23 in cheese whey (Fig. 2a) showed a diauxic curve. This behavior was not observed in BL71. Diauxie was probably the consequence of the presence of other residual sugars in cheese whey, in addition to lactose. As expected, the BL71 mutant, unregulated for the CCR process, did not show this pattern. Although ccpA mutants from lactobacilli generally show a diminished growth rate in laboratory media (Monedero et al. 1997), this mutation allowed faster development of L. casei BL23 in cheese whey, reducing the lag time and increasing lactic acid productivity. Similarly, Alcántara et al. (2016) found that a mutant from L. casei BL23 deficient in the transcriptional regulator PrcR, which displays a slower growth in MRS than the wild type, showed faster milk acidification as a result of a deregulated expression of the cell-envelope proteinase PrtP. In agreement with these results, in the present study we showed that the BL71 strain expressed higher levels of the PepQ and PepX peptidases and the proteinase PrtP and a direct control of prtP expression was evidenced through CcpA binding to its promoter. Deregulation of the proteolytic system upon a ccpA mutation is thus the likely cause of the better performance displayed by the BL71 mutant strain during cheese whey fermentation.
Our results contrast with data obtained in Lc. lactis, where the pepQ gene in a ccpA mutant has been described to be downregulated by a factor of two (Zomer et al. 2007). In analogy, while CcpA activates the transcription of the pfk and pyk glycolytic genes in Lc. lactis (Luesink et al. 1999), it represses them in L. casei (Viana et al. 2005). An important difference between these two LAB is the absence of the pleiotropic nitrogen regulator CodY in lactobacilli, which, in Lc. lactis, controls the expression of components of the proteolytic system (Guédon et al. 2001). Therefore, the regulatory mechanisms controlling glycolysis and nitrogen metabolism in lactobacilli and lactococci are different. In Lc. lactis, coordinated action of CodY and CcpA would link carbon and nitrogen metabolism (Petranovic et al. 2004; Zomer et al. 2007), whereas in Lacticaseibacillus, CcpA could act together with PrcR in this task. However, regulation of proteolysis may differ among different lactobacilli and include new participants. In Lactobacillus delbrueckii subsp. lactis, for example, a distinct regulator (YebC) has been shown to control the expression of the surface proteinase PrtL and the oligopeptide transporters OppA1 and OptS by binding to their gene promoters (Brown et al. 2017). Our results point to CcpA as a valuable target for strain modification in lactobacilli to improve fermentative processes in which industrial waste products with high nitrogen content, such as cheese whey, are used. This gene target must be evaluated in different lactobacilli species and substrates for enhanced production of lactic acid.
References
Abdel-Rahman MA, Tashiro Y, Sonomoto K (2013) Recent advances in lactic acid production by microbial fermentation processes. Biotechnol Adv 31:877–902. https://doi.org/10.1016/j.biotechadv.2013.04.002
Acedo-Félix E, Pérez-Martínez G (2003) Significant differences between Lactobacillus casei subsp. casei ATCC 393T and a commonly used plasmid-cured derivative revealed by a polyphasic study. Int J Syst Evol Microbiol 53:67–75. https://doi.org/10.1099/ijs.0.02325-0
Alcántara C, Bäuerl C, Revilla-Guarinos A, Pérez-Martínez G, Monedero V, Zúñiga M (2016) Peptide and amino acid metabolism is controlled by an OmpR-family response regulator in Lactobacillus casei. Mol Microbiol 100(1):25–41
Allievi MC, Palomino MM, Ruzal SM (2019) Production of lactate using Lactobacillus. In: Ruzal SM (ed) Lactobacillus genomics and metabolic engineering caister. Academic Press, UK, pp 61–80. https://doi.org/10.21775/9781910190890.04
Alvarez MM, Aguirre-Ezkauriatza EJ, Ramírez-Medrano A, Rodríguez-Sánchez Á (2010) Kinetic analysis and mathematical modeling of growth and lactic acid production of Lactobacillus casei var. rhamnosus in milk whey. J Dairy Sci 93:5552–5560
Bernardo MP, Coelho LF, Sass DC, Contiero J (2016) L-(+)-lactic acid production by Lactobacillus rhamnosus B103 from dairy industry waste. Braz J Microbiol 47(3):640–646. https://doi.org/10.1016/j.bjm.2015.12.001
Brown L, Villegas JM, Elean M, Fadda S, Mozzi F, Saavedra L, Hebert EM (2017) YebC, a putative transcriptional factor involved in the regulation of the proteolytic system of Lactobacillus. Sci Rep 7(1):8579. https://doi.org/10.1038/s41598-017-09124-1
Carvalho F, Prazeres AR, Rivas J (2013) Cheese whey wastewater: characterization and treatment. Sci Total Environ 445–446:385–396. https://doi.org/10.1016/j.scitotenv.2012.12.038
Coll-Marqués JM, Bäuerl C, Zúñiga M, Pérez-Martínez G (2020) Differences in the expression of cell envelope proteinases (CEP) in two Lactobacillus paracasei probiotic strains. FEMS Microbiol Lett 367(13):fnaa102. https://doi.org/10.1093/femsle/fnaa102
Dandoy D, Fremaux C, Henry de Frahan M, Horvath P, Boyaval P, Hols P et al (2011) The fast milk acidifying phenotype of Streptococcus thermophilus can be acquired by natural transformation of the genomic island encoding the cell-envelope proteinase PrtS. Microb Cell Fact 10:S21
Deutscher J (2008) The mechanisms of carbon catabolite repression in bacteria. Curr Opinion Microbiol 11:87–93
Francke C, Kerkhoven R, Wels M, Siezen RJ (2008) A generic approach to identify transcription factor-specific operator motifs; Inferences for LacI-family mediated regulation in Lactobacillus plantarum WCFS1. BMC Genomics 9:145
Fujita Y (2009) Carbon catabolite control of the metabolic network in Bacillus subtilis. Biosci Biotech Biochem 73:245–259
Ghosh P, Wasil LR, Hatfull GF (2006) Control of phage Bxb1 excision by a novel recombination directionality factor. PLoS Biol 4(6):e186
Guédon E, Martin C, Gobert FX, Ehrlich DS, Renault P, Delorme C (2001) Réseau de régulation de la transcription des genes du systeme protéolytique de Lactococcus lactis. Lait 81:65–74
Gutmann I, Wahlefeld AW (1974) Lactate measurements. In: Bergmeyer HU (ed) Methods of Enzymatic Analysis. Verlag Chemie, Weinhein, Academic Press, New York, pp 1464–1468
John RP, Nampoothiri KM, Pandey A (2007) Fermentative production of lactic acid from biomass: an overview on process developments and future perspectives. Appl Microbiol Biotechnol 74(3):524–534
Kleerebezem M, Boekhorst J, van Kranenburg R, Molenaar D, Kuipers OP et al (2003) Complete genome sequence of Lactobacillus plantarum WCFS1. Proc Natl Acad Sci USA 100(4):1990–1995
Küster E, Luesink EJ, de Vos WM, Hillen W (1996) Immunological crossreactivity to the catabolite control protein CcpA from Bacillus megaterium is found in many Gram-positive bacteria. FEMS Microbiol Lett 139(2–3):109–115
Larionov A, Krause A, Miller W (2005) A standard curve based method for relative real time PCR data processing. BMC Bioinformatics 6(1):62
Li C, Sun F, Cho H, Yelavarthi V, Sohn C et al (2010) CcpA mediates proline auxotrophy and is required for Staphylococcus aureus pathogenesis. J Bacteriol 192(15):3883–3892
Luesink EJ, Beumer CM, Kuipers OP, de Vos WM (1999) Molecular characterization of the Lactococcus lactis ptsHI operon and analysis of the regulatory role of HPr. J Bacteriol 181:764–771
Mahr K, Hillen W, Titgemeyer F (2000) Carbon catabolite repression in Lactobacillus pentosus: analysis of the ccpA region. Appl Environ Microbiol 66:277–283
Marciniak BC, Pabijaniak M, de Jong A, Dűhring R, Seidel G, Hillen W, Kuipers OP (2012) High- and low-affinity cre boxes for CcpA binding in Bacillus subtilis revealed by genome-wide analysis. BMC Genomics 13:401. https://doi.org/10.1186/1471-2164-13-401
Mazé A et al (2010) Complete genome sequence of the probiotic Lactobacillus casei strain BL23. J Bacteriol 192:2647–2648
Mazzeo MF, Cacace G, Peluso A, Zotta T, Muscariello L, Vastano V, Parente E, Siciliano RA (2012) Effect of inactivation of ccpA and aerobic growth in Lactobacillus plantarum: a proteomic perspective. J Proteomics 75:4050–4061. https://doi.org/10.1016/j.jprot.2012.05.019
Monedero V, Gosalbes MJ, Pérez-Martínez G (1997) Catabolite repression in Lactobacillus casei ATCC 393 is mediated by CcpA. J Bacteriol 179:6657–6664
Monedero V, Mazé A, Boël G, Zúñiga M, Beaufils S, Hartke A, Deutscher J (2007) The phosphotransferase system of Lactobacillus casei: regulation of carbon metabolism and connection to cold shock response. J Mol Microbiol Biotechnol 12(1–2):20–32
Morishita T, Fukada T, Shirota M, Yura T (1974) Genetic basis of nutritional requirements in Lactobacillus casei. J Bacteriol 120(3):1078–1084
Narayanan N, Roychoudhury PK, Srivastava A (2004) L (+) lactic acid fermentation and its product polymerization. Electron J Biotechnol 7(2):167–178
Palomino MM, Waehner PM, Fina Martin J, Ojeda P, Malone L et al (2016) Influence of osmotic stress on the profile and gene expression of surface layer proteins in Lactobacillus acidophilus ATCC 4356. Appl Microbiol Biotechnol 100(19):8475–8484
Panesar PS, Kennedy JF, Gandhi DN, Bunko K (2007) Bioutilisation of whey for lactic acid production. Food Chem 105:1–14. https://doi.org/10.1016/j.foodchem.2007.03.035
Petranovic D, Guédon E, Sperandio B, Delorme C, Ehrlich D, Renault P (2004) Intracellular effectors regulating the activity of the Lactococcus lactis CodY pleiotropic transcription regulator. Mol Microbiol 53:613–621
Piuri M, Sanchez-Rivas C, Ruzal SM (2003) Adaptation to high salt in Lactobacillus: role of peptides and proteolytic enzymes. J Appl Microbiol 95:372–379
Rabaioli Rama G, Kuhn D, Beux S, Jachetti Maciel M, Volken de Souza CF (2019) Potential applications of dairy whey for the production of lactic acid bacteria cultures. Int Dairy J 98:25–37. https://doi.org/10.1016/j.idairyj.2019.06.012
Rey DA, Pühler A, Kalinowski J (2003) The putative transcriptional repressor McbR, member of the TetR-family, is involved in the regulation of the metabolic network directing the synthesis of sulfur containing amino acids in Corynebacterium glutamicum. J Biotechnol 103:51–65
Ricciardi A, Zotta T, Ianniello RG, Boscaino F, Matera A, Parente E (2019) Effect of respiratory growth on the metabolite production and stress robustness of Lactobacillus casei N87 cultivated in cheese whey permeate medium. Front Microbiol 10:851. https://doi.org/10.3389/fmicb.2019.00851
Sahoo TK, Jayaraman G (2019) Co-culture of Lactobacillus delbrueckii and engineered Lactococcus lactis enhances stoichiometric yield of d-lactic acid from whey permeate. Appl Microbiol Biotechnol 103(14):5653–5662
Schick J, Weber B, Klein JR, Henrich B (1999) PepR1, a CcpA-like transcription regulator of Lactobacillus delbrueckii subsp. lactis. Microbiology 145(11):3147–3154
Siciliano RA, Pannella G, Lippolis R, Ricciardi A, Mazzeo F, Zotta T (2019) Impact of aerobic and respirative life-style on Lactobacillus casei N87 proteome. Int J Food Microbiol 298:51–62
Singh SK, Ahmed SU, Pandey A (2006) Metabolic engineering approaches for lactic acid production. Proc Biochem 41:991–1000
Sonenshein AL (2005) CodY, a global regulator of stationary phase and virulence in Gram-positive bacteria. Curr Opin Microbiol 8:203–207
Sonenshein AL (2007) Control of key metabolic intersections in Bacillus subtilis. Nat Rev Microbiol 5(12):917–927
Spatafora G, Rohrer K, Barnard D, Michalek S (1995) A Streptococcus mutans mutant that synthesizes elevated levels of intracellular polysaccharide is hypercariogenic in vivo. Infect Immun 63:2556–2563
Viana R, Pérez-Martínez G, Deutscher J, Monedero V (2005) The glycolytic genes pfk and pyk from Lactobacillus casei are induced by sugars transported by the phosphoenolpyruvate: sugar phosphotransferase system and repressed by CcpA. Arch Microbiol 183(6):385–393. https://doi.org/10.1007/s00203-005-0003-6
Wang J, Wang Q, Xu Z, Zhang W, Xiang J (2015) Effect of fermentation conditions on L-lactic acid production from soybean straw hydrolysate. J Microbiol Biotechnol 25(1):26–32
Wakai T, Yamamoto N (2013) A Novel branched chain amino acids responsive transcriptional regulator, BCARR, negatively acts on the proteolytic system in Lactobacillus helveticus. PLoS ONE 8(10):e75976. https://doi.org/10.1371/journal.pone.0075976
Wünsche A, Hammer E, Bartholomae M, Völker U, Burkovski A, Seidel G, Hillen W (2012) CcpA forms complexes with CodY and RpoA in Bacillus subtilis. FEBS J 279(12):2201–2214. https://doi.org/10.1111/j.1742-4658.2012.08604.x
Zhang G, Li LL, C, (2020) Effects of ccpA gene deficiency in Lactobacillus delbrueckii subsp. bulgaricus under aerobic conditions as assessed by proteomic analysis. Microb Cell Fact 19(1):1–12. https://doi.org/10.1186/s12934-020-1278-7
Zheng J, Wittouck S, Salvetti E, Franz C, Harris HMB, Mattarelli P et al (2020) A taxonomic note on the genus Lactobacillus: description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int J Syst Evol Microbiol 70:2782–2858. https://doi.org/10.1099/ijsem.0.004107
Zomer AL, Buist G, Larsen R, Kok J, Kuipers OP (2007) Time-resolved determination of the CcpA regulon of Lactococcus lactis subsp. cremoris MG1363. J Bacteriol 189:1366–1381
Zotta T, Ricciardi A, Guidone A, Sacco M, Muscariello L, Mazzeo MF (2012) Inactivation of ccpA and aeration affect growth, metabolite production and stress tolerance of Lactobacillus plantarum WCFS1. Int J Food Microbiol 155:51–59. https://doi.org/10.1016/j.ijfoodmicro.2012.01.017
Zotta T, Parente E, Ricciardi A (2017) Aerobic metabolism in the genus Lactobacillus: impact on stress response and potential applications in the food industry. J Appl Microbiol 122:857–869. https://doi.org/10.1111/jam.13399
Zotta T, Solieri L, Iacumin L, Picozzi C, Gullo M (2020) Valorization of cheese whey using microbial fermentations. Appl Microbiol Biotechnol 104:2749–2764. https://doi.org/10.1007/s00253-020-10408-2
Funding
The present report was supported by grants from the Universidad de Buenos Aires (UBA) (UBACyT 20020170200329BA and 20020170100019BA) and Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina. JFM is a graduate fellow of CONICET; VMG is a member of CSIC (Spain); MVC and DML are members of INTI (Argentina) and MMP, SMR and MCA are members of CONICET (Argentina).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Catone, M.V., Palomino, M.M., Legisa, D.M. et al. Lactic acid production using cheese whey based medium in a stirred tank reactor by a ccpA mutant of Lacticaseibacillus casei. World J Microbiol Biotechnol 37, 61 (2021). https://doi.org/10.1007/s11274-021-03028-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-021-03028-z