Abstract
Recent years have witnessed rise in the production of microbial fermentation of metabolites and proteins for industrial applications. The innovative method aims to improve the conventional batch fermentation and the separation. Furthermore, the idea of downstream processing of the target product enhances the efficiency of the overall process and makes it cost-effective and eco-friendly. Even though being cost-effective and eco-friendly, the process faces challenges in commercial applications. When the concentration of the final product/by-product reaches a certain level, feedback inhibition is a common problem encountered in the fermentation process. Excessive accumulation of end products/by-products in culture also inhibits cell growth and secretion of target metabolites. The fermentation results in the production of antibiotics, fungal metabolites, and amino acids, which come with serious inhibition problems. Hence, to mitigate such problems and enhance fermentation performance, extractive fermentation via in situ ion exchange adsorptive technique can be incorporated. The present review highlights the advances and suggests the strategies to resolve the inhibition problems using extractive fermentation via internal, external, and dispersed resin systems. Furthermore, methods for synthesis of resins are discussed and adsorption mechanism of ion exchanges is also elaborated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The insights from the World Bank [1] demonstrate that the world yearly food waste generation was around 1.3 billion tons in 2012. Landfilling of food waste causes greenhouse gas emissions and environmental degradation, creating ozone depletion substances, and in addition, restricted landfilled space is likewise an issue associated with it. In order to overcome the environmental issues and assign value to the waste, resource recovery from food waste is necessary. The different bioprocesses,such as fermentation, can be used in order convert the waste into fuel and valuable products [2]. However, high concentrations of ethanol in fermentation broth limit the yield of ethanol, which is a major problem encountered during fermentation processes and is referred to as end-product inhibition.
Extractive fermentation is one of the technique from different techniques proposed by various researchers for the continuous production of ethanol [3]. The motivation behind this process is continuous removal of produced ethanol while minimizing the product inhibition and maintaining high growth rates of ethanol-producing microorganisms. By replacing traditional azeotropic distillation, extraction is also beneficial for the recovery of ethanol from diluted fermentation broth [4]. There are different techniques that can be employed to minimize the end-product inhibition such as liquid–liquid extraction, adsorbents, absorbents, organic solvents, and ionic liquids. The end-product inhibition minimization efficiency depends on the relative affinity of solution [5,6,7,8,9]. Adsorption appears to be an ideal method because it has the advantage of being a simple procedure and having low labor intensity and easy process development. Macroporous resins are durable polar and non-polar polymers with possible adsorption molecule recovery, high adsorption capacity, easy regeneration, and relatively low cost [10]. The molecule recovery includes but not limited to licorice flavonoids, glycyrrhizin [11], scutellarin [12], madecassoside and asiaticoside [13], rosavin [14], lycopene [15], chlorogenic acid [16], genistein, apigenin etc. Most ion exchange resins contain functionalized cross-linked polystyrene–divinylbenzene co-polymers, which fall into two broad categories [17]. The cation exchange resin and anion exchange resin can be classified based on strong acid, weak acid, strong base, and weak base, respectively. Other ion exchange materials include acrylics [18] and perfluorinated chain polymers bearing sulfonic acid heads, like Nafion® [19] and Aquivion [20]. In general, 0.5 to 20% of cross-linking agents use low cross-linked and reticulated resins having a gel (micropore) and macroporous structure, respectively, to control the porosity of the resin [21]. Therefore, the gel-type resin is generally superior to the macroporous resin because the reaction position of the reactants in the solution is more easily obtained [22]. However, in the case of flow processes, internal mass transfer (diffusion) and pressure drop restrictions [23, 24] are usually lower for macroreticular resins [25, 26]. Batch processing has certain drawbacks regarding ethanol production [27, 28], which inhibit the fermentation process when glucose concentration is high [29]. The discontinuous fermentation produces a maximum ethanol concentration of 12% v/v since the microorganisms cannot tolerate high concentrations of ethanol [3]. Furthermore, the productivity of discontinuous fermentation is limited by the reduced ethanol production caused by high concentration in the fermentation broth.
This inhibition is a well-known feature of many fermentation processes and is often referred to product inhibition. Apart from batch processing, continuous fermentation resolves the problem inhibition which can increase ethanol production. However, this process cannot be done in high cell density, which reduces the concentration of ethanol and results in significant loss of residual substrate [30]. In this case, the recovery of the extraction product is of particular interest because it allows the in situ removal of the inhibitor product by solvent extraction [31]. Extractive fermentation could lower the usage of energy as compared to distillation processes [32]. Extractive fermentation can be achieved through the coupling of fermentation with liquid extraction. This review aims to highlight the applications of various ion exchange resins for extractive fermentation. Furthermore, the strategies to resolve the product inhibition problems using the mentioned technique via internal, external, and dispersed resin systems are discussed, commented, and critically analyzed. Methods of synthesis of resins are also discussed, and adsorption mechanisms of ion exchangers are elaborated.
2 Extractive fermentation
Extractive fermentation process is an in situ extraction process to remove the produced ethanol and other inhibitory compounds, thus eliminating the inhibitory effect caused by these compounds [33, 34]. In addition to the solvent selection criteria for conventional extraction (such as high partition coefficient and selectivity and low solubility), solvents suitable for extraction and fermentation should be non-toxic to microorganisms (i.e., biocompatibility). The density of the water phase is different from the density of the broth to ensure phase separation by gravity, low viscosity, high interfacial tension, and low emulsification tendency in the broth; high stability, low cost, etc., are some of the basic requirements for a suitable solvent [35, 36].
3 Ion exchange process
Ion exchange process takes place between two phases, resulting in exchange of their ions with the help of resins. In ion exchange process, resins which mainly cross-linked polymer networks are employed, which are electrostatically bound insoluble. In these polymers, exchange of ions happens when ions having the same charge-containing solution are used. The degree of the exchange or separation depends on the ion concentration of the solution and the insoluble phase of the ions. According to reports, the use of Diaion HP-20 polyaromatic resin can improve the production of antibiotics in the fermentation of actinomycetes [37]. The production medium containing the adsorbent resin can also be used to reduce the autotoxicity of fungal metabolites [38]. The extraction and fermentation of Burkholderia E264 has also been reported to increase the yield of thailandepsin A, a natural product with strong inhibitory activity of histone deacetylases and promising anticancer activity [39]. By adding Diaion HP-20 polycyclic aromatic adsorption resin to the culture, the feedback inhibition of Thai protease can be effectively reduced, thereby significantly increasing the potency of the target product. Removal of the acetate formed by the anion exchange resin has been used successfully to improve the production of recombinant interferon-α2b in Escherichia coli [40, 41]. Due to the presence of anion exchange resin in the culture, the physiology of E. coli does not change or does change during the entire cultivation process. The adsorption capacity of the resin for acetic acid depends on the characteristics of the resin and the matrix. The weakly basic anionic exchange resins have exhibited higher adsorption capacity and acetic affinity compared to strongly basic resins [42].
4 Methods of resin synthesis
Since the late 1950s, ion exchange resins have been used to help the production of pharmaceuticals. These resins proved to be safe and active excipients and are now used in many commercial applications around the world [43]. Resins have wide application in different areas which could make the process easier for removal inhibition compound during fermentation. Resin synthesis could be done through different methods, which provide solution for extraction of inhibitory compounds from fermentation process. Ion exchange resins contain acidic or basic functional groups, which are insoluble polymers and have the ability to exchange counterions in the aqueous solution. Periodic mesoporous organosilica (PMO) organic materials have been reported as resins and have recently been synthesized in various compositions [44, 45]. The PMOs allow chemical modification of the framework through the bridge of the conversion of organic groups. Therefore, materials containing organic functional groups in these main chains have many potential applications. It is possible to use PMO materials as adsorbents for metal or organic compounds, once organic bridges are functionalized. For example, polysilsesquioxane materials having porous arylene groups and ethylene bridges adsorb phenolic compounds [46]. Synthesis of various PMOs is reported in the literature, for example bipyridinium-containing MCM-41 structure as an electron acceptor [47]. Synthesis of thioether-functionalized, organic–inorganic-ordered mesoporous materials for selective Hg2+ adsorbents by co-condensation of (1,4)-bis(triethoxysilyl)propane tetrasulfide with TEOS composites is also reported [48]. Anionic organic–inorganic hybrid exchange resin has been reported to be synthesized by the gradual functionalization of cerium oxide and anion exchange ligand or the joint assembly of the anion exchange ligand and the cerium dioxide precursor [49]. PMO anion exchange resins can be synthesized by a simple one-vessel synthesis using organic cerium dioxide precursors with functionalized bridges. To synthesize anion exchange resin PMO, some new organic precursors of cerium dioxide N-(3-trietoxymethyl decylpropyl), N(3)-(3-trimethoxy) base methyl nonylpropyl-4,5-dihydroimidazolium iodide (1), cetyltrimethylammonium chloride (2), and 1-hexadecyl-3-methylimidazolium bromide (3) have also been used [50]. Figure 1 presents the synthesis of periodic mesoporous organosilicate anion exchange resins.
Synthetic procedure for the synthesis of periodic mesoporous organosilicate anion exchange resins [51]
The resins having chloride ions have a high electronegativity compared to lactate ions. Therefore, chlorine-containing resins use hydroxide ions instead of lactate [9, 12]. This can be achieved using a packed, resin-packed column (RPC) of NaOH solution. Saturation involves rinsing of the packed resin column thoroughly with distilled water, NaOH treatment column washing with distilled water to remove the alkali, and sterilization before connecting to the fermenter [52].
5 Adsorption mechanism of fermentation process
Adsorption is an energy-efficient technique used to separate different compounds from fermentation broth. This technique involves adsorption of the products onto the surface of adsorbents followed by desorption using temperature, pressure, or displacer to generate a concentrated solution. For selecting adsorbent, various factors could be taken into account, with the adsorption capacity, the adsorption speed, the ease of desorption, the cost of the adsorbent, and the selectivity of the desired product. The contact time between the adsorbate and adsorbent is directly influenced by kinetics of the adsorption process. The fast kinetics is ideal because the fermentation broth can circulate faster, and its concentration is below the inhibition range, whereas it requires a huge amount of adsorbent quantity and surface in order to achieve the product within the desired time [53]. Depending on the type of solid material, electron stability, and concrete adsorbent material used easily, active carbon and polymer resin are commonly used as bowl adsorbents for model solution and yeast vessels. Ruthenium dioxide molecular sieves have a very high ratio of SiO2/Al2O3 and a hydrophobic zeolite structure which makes them suitable for the selective adsorption of small organic compounds (C1–C5) in diluted solutions. Though activated carbon has shown good adsorption capacity, its recovery and regeneration have been complicated and need further investigations [54,54,55,56,58]. When the final product concentration reaches a certain level, feedback inhibition is a common problem in the fermentation process. Excessive accumulation of by-products in culture can also inhibit cell growth and inhibit the secretion of target metabolites. Integral fermentation and separation of fermentation products or by-products are possible methods used industrially to reduce products or inhibit by-products to improve fermentation performance. On the other hand, some metabolites produced by microorganisms such as lactic acid bacteria and recombinant bacteria are inhibited by by-products. Recently, proteins and metabolites are being produced commercially for various industrial applications and this is on the rise. There is a need to develop innovative fermentation methods that can replace the traditional ones and are also cost-effective. To overcome the problems associated with feedback inhibition and unwanted by-product accumulation in culture, many strategies have been proposed, such as genetic modification, application of fed-batch fermentations, adsorption membranes, electrodialysis, and macroporous ion exchange resins. Combining effective fermentation with macroporous adsorptive resins in culture can be used as an effective method to reduce feedback inhibition or to reduce the accumulation of inhibitory by-products. This, in turn, may increase the output of the product. It has been reported to improve the production of antibiotics in actinomycete fermentation by using polyaromatic resin Diaion HP-20 [59]. Furthermore, adsorption resin can be included in the medium to reduce the self-toxicity of fungal metabolites [60]. Extraction of Burkholderia thailandensis E264 has also been reported to enhance the production of the native product thailandepsin A with potent histone deacetylase inhibitory activity and promising anticancer activity [61]. Diaion HP-20, a polyaromatic adsorption resin, has been added to the culture to effectively reduce the feedback inhibition of thailandepsin A, which, in turn, significantly enhances the potency of the target product. The removal of acetate formed from anion exchange resins has been successfully used to enhance the production of recombinant interferon-α2b by E. coli [62]. In the presence of anion exchange resin in the medium, the physiology of E. coli cells does not change or does change throughout the culture. The adsorption capacity of the resins for acetic acid depends on the nature of the resin and the matrix. Compared to strongly basic anion exchange resins, weak base anion exchange resins have higher acetic acid absorption capacity and affinity. Compared with the fermentation without resin addition, the successful application in the 2-L stirred tank bioreactor increased the yield of recombinant interferon-α2b by about 1.8-fold. Since anion exchange resins are reusable, in order to efficiently remove acetic acid, the development of bioreactor systems with the in situ addition of anion exchange resins can be used to effectively culture recombinant E. coli for the production of biotechnological products. The above given literature shows the promising results achieved by using adsorption resins to tackle and resolve inhibition feedback problems. For better understanding, the research and development of this fermentation method should be extended to various fermentation processes. The ability of the product or resin by-product to absorb the resin depends on the properties of the resin and the matrix. The important factors for the expansion of the process approach should be determined. The kinetic models of the process should also be developed, which can be used as a reference for the development of large-scale extraction fermentations for improving the production of metabolites that are inhibited by products or by-products [63]. Solvent extraction techniques are not preferred because it can lead to some physical, chemical, and biochemical problems of cellular catalytic activity, such as interference with aseptic conditions and contamination of the fermentation system [64]. Membrane extraction has the apparent advantages over conventional extraction and can be used for separation, purification, removal of contaminants, and recovery [65].
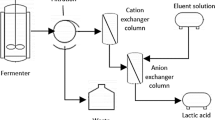
Ion exchange processes happen through the addition of ion exchange resins, which may be anion exchange resins or cation exchange resins depending upon the process. In extractive fermentation, ion exchange method which uses selected resins to separate components from fermentation broth was used. Figure 2 presents the ion exchange extractive method for the fermentation process.
6 Ion exchange adsorption in extractive fermentation process
In recent years, adsorbent resins have gained huge attention due to their in situ product recovery from fermentation processes. Selection of adsorbent depends on the type of extractant, product capacity, selectivity, cost, its stability, and kinetics. In addition to different adsorption mechanisms of the sorbents, different aspects of the sorbent distinguish them from each other. Due to the presence of other solutes competing for a limited number of surface adsorption sites, the performance of the adsorbent during fermentation is generally lower than that of the model solution [66, 67]. The presence of large surface area on the macroporous resins provides a pathway for biofilm formation and cell scaling [66, 68].
Neutral adsorbents are interacted with target molecules such as carbon, passive resins, and zeolites, due to relatively adverse effects of hydrophilicity and low rate between the steady and adsorbent stages. Apart from being relatively hydrophobic, other adsorbent properties can be used to provide selectivity. For example, a recent study used three hydrophobic zeolites with varying pore sizes to eliminate hydrolyzed components using size exclusion, allowing the selective elimination of various valuable by-products such as hydroxymethylfurfural and vanillin [67]. This selectivity in several small molecules is based on pore size of the resin along with the molecular size, which also plays a vital role in the recovery of fermentation products. Organic acid extraction through fermentations is of interest to increase the process profitability, but carboxylic acids generally lower the fermentation pH due to their low pKa values and do not interact in hydrophobic interactions. It is used to recover the functionality of hydrophobic resin products. Ion exchange resins can also be made with ion exchange resins. Ion exchange resins interact in ionic bonds but must be pre-equilibrated before and after the salt solution is used. This can cause waste problems [69].
The perillic acid produced by the oxidation of limonene is a suitable process for the exchange of ions. Compared to the alternative source of carbon, the glycerol carbon source is in a position where it is no longer adsorbed, and the ionic carbon source is expected to have a similar affinity in the direction of the resin. It is proposed that nutritional restrictions must be introduced. It is reported that out of the seven anion exchange resins screened, only two have no effect on the pH of the medium, and the affinity of the product is the criterion for determining the remaining resin. However, the hydrophobic terfopene substrate was once adsorbed significantly with the help of all the resins, which means that the performance of the substrate was lost and a fed-batch method fed with saturated air circulation of limonene and saturated resin needed limonene [66]. Another study reports that strongly basic functional groups provide excellent affinity for acid products, while gel-like structures provide greater selectivity than porous structures [70]. Given the nature of the polymer structures associated with affinity, these ideas can be used to ameliorate the selectivity and affinity of the adsorbent in future research.
The use of ion exchange adsorbents and resins for the repair of products is advantageous for hydrophobic compounds and target molecules that increase the cost due to the pH of the fermentation, and these structures are well represented in Table 1, which indicates their direct implementation. However, the recovery of strong invoices often requires warm or concentrated salt solution to reproduce the antigens, which introduce the essential energy and waste flow requirements. The literature points out that, despite this, trial and error is still widely used to symbolize the performance of adsorbents, which compromises some decision criteria, which are often necessary [69].
Chandel et al. [83] reported the detoxing of sugarcane bagasse hydrolys ate to enhance ethanol manufacturing with the help of Candida shehatae NCIM 3501. In their investigation, different cleansing techniques had been used and results consisting of neutralization, overliming, activated charcoal, ion alternate resins (IERs), and enzymatic cleansing with the use of laccase were reported. It was found that ion change cure was most efficient in casting off furans (63.4%), whole phenolic (75.8%), and acetic acid (85.2%), followed by using activated charcoal adsorption and overliming.
7 Application of resins in extractive fermentation
Extractive fermentation adsorption of the target product/by-product by ion exchange is a technique which uses either a bioreactor (internal system) or an external adsorbent-packed column with circulating fermentation broth (external system). Furthermore, in the bioreactor, the resins can be trapped inside the reactor compartment or can disperse freely. Fermentation is usually carried out in a medium containing an adsorbent resin to reduce the product’s toxicity and increase the stability of the product discharged during the fermentation. Various Streptomyces strains have been fermented in a medium with adsorbent resins [71, 72, 73,74,75,76,77,78,79, 84], but such a method is used less often to improve secondary metabolites in fungal fermentations [80,81,82]. Adsorption resins are widely used for solid-phase extraction, separation, and purification of various pharmaceutical products [85, 86]. Some of the resins used for different product formations couple with different microorganism. Table 1 shows a typical enlist of various types of adsorption resins for production of different substances. One of the main applications of ion exchange resins is the elimination of several acids from the extractive fermentation process. As shown in Table 2, suitable adsorbents for carboxylic acid and carboxylate extractions can be classified more thoroughly according to their electronic properties in ionic and non-ionic materials, as well as their functional and support morphology. Weak anion exchangers are charged at a limited pH; otherwise, they cannot exchange anions, while strong anion exchangers exchange anions over a wide pH range [103].
The recovery of in situ fermentation products can lower the metabolic inhibition and can subsequently reduce/minimize the degradation of product as well as wastewater treatment. This increases productivity and product yields and results in reduction in subsequent processing costs; however, process complexity and equipment costs may surge. Although most studies on in situ recovery of the product (except vacuum fermentation) use batch or semi-batch systems, the advantages of on-site recycling should be the most obvious for continuous processes. However, there is still a need to better estimate the capacity, limitations, and costs of in situ product recovery in continuous processes [104].
7.1 Internal adsorption column
The configuration of the internal adsorption column (in the bioreactor) has the advantage of avoiding the influence of the shear force of the wheel, which can adversely affect the resin. While the same benefits can be achieved using an external column system, the design has other advantages over external variables. Among the external variables, the environmental conditions are better, such as pH, temperature, dissolved oxygen tension (DOT), and aeration rate, and can reach high mass transfer rates [19]. The product recovery during fermentation (in situ product recovery) can be completed in a bioreactor, which not only improves overall productivity but also enables simpler operations. Bae et al. [79] reported the development of a new type of internal column system for pigment recovery, i.e., prodigiosin from fermented broth of Serratia sp. (KH-95). The bioreactor is designed with a second compartment, which is composed of a 316 stainless steel internal filter and a porous HP-20 adsorbent. Table 2 summarizes the performance of the internally dispersed column system and the resin-free (control) system compared to the external column.
It should be noted that the new system provides 1.8 times higher pigment yield than the external column bioreactor system. It has been reported that in the dispersion and outer resin system, the phenomenon of cell adsorption on the resin is encountered because the pigment is still attached to the cell wall. It is also emphasized that the conditions in the outer column and the internal media of the bioreactor (such as pH, DOT, and substrate concentration) may vary. Therefore, as the battery is recycled throughout the system, it can also cause a decrease in the performance of the external resin system.
7.2 External adsorption column
In the dispersed resin system, the shear force of the impeller and shaft seal results in resin wear, which is a major disadvantage associated with this system [19]. This effect is obvious in aerobic fermentation, which usually requires stirring. Hence, it is preferable to use an external adsorption system to reduce the shear force on the cells after the resin collides. Usually, during external extractive fermentation, a separation column containing resin is attached with external bioreactor and the broth is passed through a chromatographic column to capture products/substances, then the broth is sent back to the bioreactor for reuse. In addition, the reuse of the broth also provides other advantages, namely higher nutrient/substrate conversion rate and reduced input water requirements [20].
7.3 Integrated bioreactor-packed bed column
A method to recover citric acid in situ in the fermentation of Aspergillus niger W1-2 through an anion exchange resin has been previously developed [20]. It connects the packed bed chromatography column filled with resin to the bioreactor system. After the provided time of fermentation, the broth is passed through the column, where the resin is not attracted to the nutrients except KH2PO4. Compared with conventional fermentation, the integrated system can reduce fermentation time and increase productivity and sugar conversion rate. To overcome the inhibition of the final product in the Serratia fermentation process, KH-95, an ion exchange resin, is used to produce a red pigment like prodigiosin [5]. To confirm the product inhibition, the resins (in HP-20, SP-850, and XAD-16) were used according to the adsorption performance of the resin in the fixed-bed column mode. In the process, the pigmented culture was loaded from the top of the column. Although SP-850 has the highest adsorption capacity (approximately 11.8% and 38.1% more than HP-20 and XAD-16, respectively), its desorption capacity is the lowest (approximately 15% and 20% lower than HP-20 and XAD-16, respectively). Therefore, HP-20 was selected and used for follow-up research. Moreover, the impact of resin time on fermentation performance was also investigated in the shake flask fermentation. In general, it has been found that once the resin is added, cell growth is inhibited. It is important to note that the resin addition during seeding process resulted in only 75% cell growth of the control. However, after 10 h of resin addition, its growth steadily increased up to 95%. This phenomenon is attributed to the reduction in the casein content in the culture (reduction of about 23.5 to 47.1% compared to the control), which is due to the unwanted adsorption on the added resin and consumption of amino acids in the middle.
7.4 Integrated bioreactor-expanded bed adsorption
An integrated stirred tank bioreactor-expanded bed adsorption (STB-EBA) system was designed to remove acetate to enhance the production of PrIFN-α2b in E. coli [21]. Compared with packed bed chromatography columns, the expanded bed adsorption (EBA) system can directly process the fermentation broth loaded with biomass without any intermediate clarification. Due to the bed expansion characteristics of EBA, a large void volume is generated in it, so the cells/particles will pass through the chromatographic column and the target compound will be captured by the adsorbent in the chromatographic column. The influence of EBA parameters—resin loading (0–12 g/L) (hence, the height of the sedimentation bed, H0 from 0 to 150 mm), column surface linear velocity (240 to 900 cm/h), the culture, and influence of liquid viscosity (3.2–113.9 mPa·s) on fermentation performance—were studied. It was found that at a sedimentation bed height of 150 mm (resin concentration of 12 g/L), the dry cell weight was the highest (14.97 g/L), and the total acetate adsorption capacity was 5.45 g/L. As the resin expands to the top of the tower overflows, which restricts the further increase in the height of the sedimentation bed, it was found that after the bed expansion, when the viscosity was high (113.9 mPa·s), the resin tightly accumulated on the upper adapter net, causing the chromatographic column to block. However, it was determined that the viscosity of the fermentation broth was much lower than this point (15.75 mPa·s), so there was no problem. At the same time, for the dispersed resin system, it is found that the mixing time will increase at low stirring speeds (100–200 rpm). In addition, at high stirring speeds (>400 rpm), compared to the systems with and without resin, no significant difference in mixing time was observed in STB-EBA–integrated system. Table 2 summarizes the performance of the three systems: resin-free system, dispersion system, and STB-EBA–integrated system in the fermentation of PrIFN-α2b, and there were 42 new trends in ion exchange research performance comparison of recombinant E. coli. Among the dispersion systems, the STB-EBA system showed the highest concentration of PrIFN-α2b, specific yield and volumetric productivity, and a shorter growth cycle and time to reach the maximum concentration of PrIFN-α2b. In addition, the simplified culture solution clarification reduces the overall length of downstream processing. It is worth noting that through the integrated system, the output of PrIFN-α2b is 3 times and 1.4 times higher than that of resin-free and dispersed systems, respectively. Other reports on the implementation of STB-EBA devices can also be found in the literature, despite the use of different types of resins [22,23,24,25]. In addition to the bioreactor, the external device EBA column is also used in combination with the extraction tank to recover active compounds from herbal medicines [26, 27].
8 Discussion
The adsorption resins can be a promising solution for feedback inhibition problem caused by the accumulation of products/by-products in the culture. The adsorption capacity of the resins for products or by-products depends on the characteristics of the resin and the matrix. Ion exchange resins exhibit a finite amount of functional groups and thus sites where ions can be exchanged. The ion exchange capacity of the resin is given by the number of ion exchange sites per unit weight or volume. Commercial ion exchange resins make the reagents convenient because the large beads are easily filtered and packed into columns. The molecule recovery includes but not limited to licorice flavonoids, glycyrrhizin [11], scutellarin [12], madecassoside and asiaticoside [13], rosavin [14], lycopene [15], chlorogenic acid [16], genistein, apigenin, etc. Ion exchange resins are classified into two major categories, i.e., cation exchange resins with acidic functional groups and anion exchange resins with basic groups. And according to the acidity and basicity of the functional groups, they are further classified into subcategories, such as strongly or weakly acidic cation exchange resins and strongly or weakly basic anion exchange resins [17]. The cation exchange resin and anion exchange resin can be classified based on strong acid, weak acid, strong base, and weak base, respectively. Other ion exchange materials include acrylics [18] and perfluorinated chain polymers bearing sulfonic acid heads, like Nafion® [19] and Aquivion [20]. In general, 0.5 to 20% of cross-linking agents use low cross-linked and reticulated resins having a gel (micropore) and macroporous structure, respectively, to control the porosity of the resin [21]. Therefore, the gel-type resin is generally superior to the macroporous resin because the reaction position of the reactants in the solution is more easily obtained [22]. However, in the case of flow processes, internal mass transfer (diffusion) and pressure drop restrictions [23, 24] are usually lower for macroreticular resins [25, 26]. Batch processing has certain drawbacks regarding ethanol production [27, 28], which inhibit the fermentation process when glucose concentration is high [29]. The discontinuous fermentation produces a maximum ethanol concentration of 12% v/v since the microorganisms cannot tolerate high concentrations of ethanol [3]. Furthermore, the productivity of discontinuous fermentation is limited by the reduced ethanol production caused by high concentration in the fermentation broth.
9 Perspectives and challenges of adsorptive ion exchange resin
Extractive fermentation by using resins for removal of product/by-product of fermentation is mainly employed to alleviate the problem of feedback inhibition. Research on the selectivity of adsorption and desorption is very important especially for high-value biologics and process cost. However, studies have not focused on the purity of the desorption product. In addition, it has been found that at high resin concentrations, degradation of fermentation performance (i.e., cell growth and metabolites) is observed. Use of new resins, such as resins that can operate with higher conductivity (resins with alternative support matrix (no need to dilute/percolate before adsorption)); disposable or disposable resins (to overcome cleaning in place (CIP) and verification trouble and improve the entire economic process); extraction; and fermentation technology should also be studied. In addition, the possibility of combining adsorption technology based on ion exchange resins with other separation technologies (such as electric field and membrane technology) can also be explored. Nutrients shear effect on cells/products under high resin load or reduce mixing efficiency. The interaction between the resins and the media components can be studied in detail to better understand the overall complexity of the process. Research on this fermentation approach can be extended to various fermentation processes for better understanding. The adsorption capacity of product or by-product by the resins depends on resins’ characteristics and matrix, in which some information on these can be generalized. The important factors in scaling up the process approach need to be identified. Kinetic models that can be used to describe the process are also required, which could be used for developing large-scale extractive fermentation.
10 Conclusion
-
Extractive fermentation using ion exchange resin for removal of product/by-product of fermentation is mainly employed to mitigate the problem of feedback inhibition. Current review covers ion exchange application for mitigating the problem of product inhibition.
-
Numerous strategies are proposed to overcome the inhibition and accumulation problems such as fed-batch fermentation, genetic modification, membrane adsorption, using macroporous ion exchange resins, and electrodialysis. In the culture, along with efficient fermentation, the macroporous adsorption resins can be effective in reducing the feedback inhibition and accumulation of by-products.
-
In addition, the feasibility of combining ion exchange resin-based adsorptive techniques with other separation technique such as electric field- and membrane-based techniques may also be scrutinized. The use of immobilized cell to protect them from shear stress and/or unwanted interaction with the adsorbent may also be studied. Ultimately, the operation of the ion exchange resin–based extractive fermentation in continuous mode may deserve special attention.
-
On a separate note, the factor of cost of resin and lifetime of resins should also be taken into account during the selection especially for large-scale operation. Finally, considering that there are abundant available experimental findings on adsorption of specific products/by-products and resins, the data should be generalized to assist the selection of resin in the future.
References
D. Hoornweg and P. Bhada-Tata. What a waste: a global review of solid waste management. 2012.
Bastidas-Oyanedel J-R, Bonk F, Thomsen MH, Schmidt JE (2015) Dark fermentation biorefinery in the present and future (bio)chemical industry. Rev Environ Sci Biotechnol 14:473–498
Minier M, Goma G (1982) Ethanol production by extractive fermentation. Biotechnol Bioeng 24:1565–1579
Keim CR (1983) Technology and economics of fermentation alcohol—an update. Enzym Microb Technol 5:103–114
Börnsen KO, Mohr MD, Widmer HM (1995) Ion exchange and purification of carbohydrates on a Nafion® membrane as a new sample pretreatment for matrix-assisted laser desorption/ionization mass spectrometry. Rapid Commun Mass Spectrom 9:1031–1034
Wang D-G, Liu W-Y, Chen G-T (2013) A simple method for the isolation and purification of resveratrol from Polygonum cuspidatum. J Pharm Anal 3:241–247
Li M-q, Geng Y-h, Liu G-m, Zhang Q, Kang Y-f (2006) Application of aqueous two phase extraction technique in separation and purification of resveratrol. Nat Prod Res Dev 18:647
Liang M-T, Liang R-C, Shu-qi CY, Ri-an CY (2013) Separation of resveratrol and emodin by supercritical fluid-simulated moving bed chromatography. J Chromatogr Separation Tech 4; 3:1000175
Takagai Y, Kubota T, Kobayashi H, Tashiro T, Takahashi A, Igarashi S (2005) Adsorption and desorption properties of trans-resveratrol on cellulose cotton. Anal Sci 21:183–186
Scordino M, Di Mauro A, Passerini A, Maccarone E (2004) Adsorption of flavonoids on resins: cyanidin 3-glucoside. J Agric Food Chem 52:1965–1972
Fu B, Liu J, Li H, Li L, Lee FSC, Wang X (2005) The application of macroporous resins in the separation of licorice flavonoids and glycyrrhizic acid. J Chromatogr A 1089:18–24
Gao M, Huang W, Liu C-Z (2007) Separation of scutellarin from crude extracts of Erigeron breviscapus (vant.) Hand Mazz by macroporous resins. J Chromatogr B Analyt Technol Biomed Life Sci 858:22–26
Jia G, Lu X (2008) Enrichment and purification of madecassoside and asiaticoside from Centella asiatica extracts with macroporous resins. J Chromatogr A 1193:136–141
Ma C, Tao G, JianTang ZL, Wang H, Gu X et al (2009) Preparative separation and purification of rosavin in Rhodiola rosea by macroporous adsorption resins. Sep Purif Technol 69:22–28
Liu Y, Liu J, Chen X, Liu Y, Di D (2010) Preparative separation and purification of lycopene from tomato skins extracts by macroporous adsorption resins. Food Chem 123:1027–1034
Zhang B, Yang R, Zhao Y, Liu C-Z (2008) Separation of chlorogenic acid from honeysuckle crude extracts by macroporous resins. J Chromatogr B 867:253–258
Helfferich FG (1962) Ion exchange chromatography: McGraw-Hill
Patil Y, Alaaeddine A, Ono T, Ameduri B (2013) Novel method to assess the molecular weights of fluoropolymers by radical copolymerization of vinylidene fluoride with various fluorinated comonomers initiated by a persistent radical. Macromolecules 46:3092–3106
Seen AJ (2001) Nafion: an excellent support for metal-complex catalysts. J Mol Catal A Chem 177:105–112
Okay O (2000) Macroporous copolymer networks. Prog Polym Sci 25:711–779
Ahmed M, Malik MA, Pervez S, Raffiq M (2004) Effect of porosity on sulfonation of macroporous styrene-divinylbenzene beads. Eur Polym J 40:1609–1613
Corain B, Zecca M, Jeřábek K (2001) Catalysis and polymer networks—the role of morphology and molecular accessibility. J Mol Catal A Chem 177:3–20
Takahashi R, Sato S, Sodesawa T, Arai K, Yabuki M (2005) Effect of diffusion in catalytic dehydration of alcohol over silica–alumina with continuous macropores. J Catal 229:24–29
Kovačič S, Krajnc P (2009) Macroporous monolithic poly (4-vinylbenzyl chloride) columns for organic synthesis facilitation by in situ polymerization of high internal phase emulsions. J Polym Sci A Polym Chem 47:6726–6734
Nicolaou KC, Hanko R, Hartwig W (2002) Handbook of combinatorial chemistry: drugs, catalysts, materials. Wiley-VCH, Weinheim
Perego C, Peratello S (1999) Experimental methods in catalytic kinetics. Catal Today 52:133–145
Selke R, Häupke K, Krause H (1989) Asymmetric hydrogenation by heterogenized cationic rhodium phosphinite complexes. J Mol Catal 56:315–328
Samsonov GV, Pasechnik V (1969) Ion exchange and the swelling of ion-exchange resins. Russ Chem Rev 38:547
Cheng H-C, Wang F-S (2007) Trade-off optimal design of a biocompatible solvent for an extractive fermentation process. Chem Eng Sci 62:4316–4324
Cheng H-C, Wang F-S (2008) Optimal process/solvent design for ethanol extractive fermentation with cell recycling. Biochem Eng J 41:258–265
Daugulis A, Swaine D, Kollerup F, Groom C (1987) Extractive fermentation-integrated reaction and product recovery. Biotechnol Lett 9:425–430
Widjaja T, Altway A, Permanasari AR, Gunawan S (2014, pp. 300–305) Production of Ethanol as a Renewable Energy by Extractive Fermentation. In: Production of ethanol as a renewable energy by extractive fermentation. Applied mechanics and materials, In
Liu W, Yang J, Tian Y, Zhou X, Wang S, Zhu J et al (2021) An in situ extractive fermentation strategy for enhancing prodigiosin production from Serratia marcescens BWL1001 and its application to inhibiting the growth of Microcystis aeruginosa. Biochem Eng J 166:107836
Eker S, Erkul B (2018) Biohydrogen production by extracted fermentation from sugar beet. Int J Hydrog Energy 43:10645–10654
Farias D, Maugeri-Filho F (2021) Sequential fed batch extractive fermentation for enhanced bioethanol production using recycled Spathaspora passalidarum and mixed sugar composition. Fuel 288:119673
Ajit A, Sulaiman AZ, Chisti Y (2017) Production of bioethanol by Zymomonas mobilis in high-gravity extractive fermentations. Food Bioprod Process 102:123–135
Karekar SC, Srinivas K, Ahring BK (2020) Continuous in-situ extraction of acetic acid produced by Acetobacterium woodii during fermentation of hydrogen and carbon dioxide using Amberlite FPA53 ion exchange resins. Bioresource Technol Rep 12:100568
Öztürk Y, Ekmekçi Z (2020) Removal of sulfate ions from process water by ion exchange resins. Miner Eng 159:106613
Elshikh M, Funston S, Chebbi A, Ahmed S, Marchant R, Banat IM (2017) Rhamnolipids from non-pathogenic Burkholderia thailandensis E264: physicochemical characterization, antimicrobial and antibiofilm efficacy against oral hygiene related pathogens. New Biotechnol 36:26–36
Cho SH, Dahnum D, Cheong S-H, Lee HW, Lee U, Ha J-M et al (2020) Facile one-pot synthesis of ZnBr2 immobilized ion exchange resin for the coupling reaction of CO2 with propylene oxide. J CO2 Util 42:101324
Wojtaszek M, Wasielewski R (2021) The use of waste ion exchange resins as components of the coal charge for the production of metallurgical coke. Fuel 286:119249
Altarawneh M, Waters D, Goh B-M, Jiang Z-T, El-Harbawi M, Yin C-Y (2020) Adsorptive interactions between metaldehyde and sulfonic functional group in ion exchange resin. J Mol Liq 313:113555
Hughes L (2004) Ion exchange resins unique solutions to formulation problems. Pharm Technol 28:20–25
Asefa T, MacLachlan MJ, Coombs N, Ozin GA (1999) Periodic mesoporous organosilicas with organic groups inside the channel walls. Nature 402:867–871
Yoshina-Ishii C, Asefa T, Coombs N, MacLachlan MJ, Ozin GA (1999) Periodic mesoporous organosilicas, PMOs: fusion of organic and inorganic chemistry ‘inside’ the channel walls of hexagonal mesoporous silica. Chem Commun 353:2539–2540
Burleigh MC, Markowitz MA, Spector MS, Gaber BP (2002) Porous polysilsesquioxanes for the adsorption of phenols. Environ Sci Technol 36:2515–2518
Álvaro M, Ferrer B, Fornés V, García H (2001) A periodic mesoporous organosilica containing electron acceptor viologen units. Chem Commun 24:2546–2547
Zhang L, Zhang W, Shi J, Hua Z, Li Y, Yan J (2003) A new thioether functionalized organic–inorganic mesoporous composite as a highly selective and capacious Hg2+ adsorbent. Chem Commun 30:210–211
Lee B, Bao LL, Im H-J, Dai S, Hagaman EW, Lin J (2003) Synthesis and characterization of organic–inorganic hybrid mesoporous anion-exchange resins for perrhenate (ReO4-) anion adsorption. Langmuir 19:4246–4252
Lee B, Im H-J, Luo H, Hagaman EW, Dai S (2005) Synthesis and characterization of periodic mesoporous organosilicas as anion exchange resins for perrhenate adsorption. Langmuir 21:5372–5376
Lam KS, Gustavson DR, Veitch JA, Forenza S (1993) The effect of cerulenin on the production of esperamicin A 1 by Actinomadura verrucosospora. J Ind Microbiol Biotechnol 12:99–102
Srivastava A, Roychoudhury PK, Sahai V (1992) Extractive lactic acid fermentation using ion-exchange resin. Biotechnol Bioeng 39:607–613
Yang X, Tsai G-J, Tsao GT (1994) Enhancement of in situ adsorption on the acetone-butanol fermentation by Clostridium acetobutylicum. Sep Technol 4:81–92
Regdon I, Kiraly Z, Dekany I, Lagaly G (1994) Adsorption of 1-butanol from water on modified silicate surfaces. Colloid Polym Sci 272:1129–1135
Regdon I, Dékány I, Lagaly G (1998) A new way for calculating the adsorption capacity from surface excess isotherms. Colloid Polym Sci 276:511–517
Takeuchi Y, Miyata N, Asano S, Harada M (1995) Adsorption of 1-butanol and p-xylene vapor and their mixtures with high silica zeolites. Sep Technol 5:23–34
Abdehagh N, Tezel F, Thibault J (2013) Adsorbent screening for biobutanol separation by adsorption: kinetics, isotherms and competitive effect of other compounds. Adsorption 19:1263–1272
Zheng Y-N, Li L-Z, Xian M, Ma Y-J, Yang J-M, Xu X, He DZ (2009) Problems with the microbial production of butanol. J Ind Microbiol Biotechnol 36:1127–1138
Lee J, Park H, Park D, Lee H, Kim Y, Kim C (2003) Improved production of teicoplanin using adsorbent resin in fermentations. Lett Appl Microbiol 37:196–200
Singh MP, Leighton MM, Barbieri LR, Roll DM, Urbance SE, Hoshan L, McDonald LA (2010) Fermentative production of self-toxic fungal secondary metabolites. J Ind Microbiol Biotechnol 37:335–340
Liu B, Hui J, Cheng Y-Q, Zhang X (2012) Extractive fermentation for enhanced production of thailandepsin A from Burkholderia thailandensis E264 using polyaromatic adsorbent resin Diaion HP-20. J Ind Microbiol Biotechnol 39:767–776
Tan JS, Ramanan RN, Ling TC, Shuhaimi M, Ariff AB (2011) Enhanced production of periplasmic interferon alpha-2b by Escherichia coli using ion-exchange resin for in situ removal of acetate in the culture. Biochem Eng J 58–59:124–132
Ariff A (2012) Extractive fermentation employing adsorbent resin to enhance production of metabolites subject to product or byproduct inhibition. Ferment Technol 1:e113
Wardell JM, King CJ (1978) Solvent equilibriums for extraction of carboxylic acids from water. J Chem Eng Data 23:144–148
Thang VH, Koschuh W, Kulbe KD, Kromus S, Krotscheck C, Novalin S (2004) Desalination of high salt content mixture by two-stage electrodialysis as the first step of separating valuable substances from grass silage. Desalination 162:343–353
Mirata MA, Heerd D, Schrader J (2009) Integrated bioprocess for the oxidation of limonene to perillic acid with Pseudomonas putida DSM 12264. Process Biochem 44:764–771
Ranjan R, Thust S, Gounaris CE, Woo M, Floudas CA, von Keitz M et al (2009) Adsorption of fermentation inhibitors from lignocellulosic biomass hydrolyzates for improved ethanol yield and value-added product recovery. Microporous Mesoporous Mater 122:143–148
Wang Z, Liang R, Xu J-H, Liu Y, Qi H (2010) A closed concept of extractive whole cell microbial transformation of benzaldehyde into L-phenylacetylcarbinol by Saccharomyces cerevisiae in novel polyethylene-glycol-induced cloud-point system. Appl Biochem Biotechnol 160:1865–1877
Dafoe JT, Daugulis AJ (2014) In situ product removal in fermentation systems: improved process performance and rational extractant selection. Biotechnol Lett 36:443–460
Xue C, Zhao J, Lu C, Yang ST, Bai F, Tang IC (2012) High-titer n-butanol production by clostridium acetobutylicum JB200 in fed-batch fermentation with intermittent gas stripping. Biotechnol Bioeng 109:2746–2756
Marshall VP, Little M, Johnson L (1981) A new process and organism for the fermentation production of volonomycin. J Antibiot 34:902–904
Marshall V, McWethy S, Sirotti J, Cialdella J (1990) The effect of neutral resins on the fermentation production of rubradirin. J Ind Microbiol 5:283–287
Lam K, Veitch J, Lowe S, Forenza S (1995) Effect of neutral resins on the production of dynemicins by Micromonospora chersina. J Ind Microbiol 15:453–456
Kara M, Asano K, Kawamoto I, Takiouchi T, Katsumata S, Takahashi K-I et al (1989) Leinamycin, a new antitumor antibiotic from Streptomyces; producing organism, fermentation and isolation. J Antibiot 42:1768–1774
Fujii N, Katsuyama T, Kobayashi E, Hara M, Nakano H (1995) The clecarmycins, new antitumor antibiotics produced by Streptomyces: fermentation, isolation and biological properties. J Antibiot 48:768–772
Nagata H, Ochiai K, Aotani Y, Ando K, Yoshida M, Takahashi I et al (1997) Lymphostin (LK6-A), a novel immunosuppressant from Streptomyces sp. KYI 1783: taxonomy of the producing organism, fermentation, isolation and biological activities. J Antibiot 50:537–542
He H, Ding W-D, Bernan VS, Richardson AD, Ireland CM, Greenstein M, Ellestad GA, Carter GT (2001) Lomaiviticins A and B, potent antitumor antibiotics from Micromonospora l omaivitiensis. J Am Chem Soc 123:5362–5363
Jia B, Jin Z-H, Lei Y-L, Mei L-H, Li N-H (2006) Improved production of pristinamycin coupled with an adsorbent resin in fermentation by Streptomyces pristinaespiralis. Biotechnol Lett 28:1811–1815
Bae J, Moon H, Oh K-K, Kim C-H, Lee DS, Kim S-W et al (2001) A novel bioreactor with an internal adsorbent for integrated fermentation and recovery of prodigiosin-like pigment produced from Serratia sp. KH-95. Biotechnol Lett 23:1315–1319
Jarvis BB, Armstrong CA, Zeng M (1990) Use of resins for trichothecene production in liquid cultures. J Antibiot 43:1502–1504
McDonald LA, Abbanat DR, Barbieri LR, Bernan VS, Discafani CM, Greenstein M et al (1999) Spiroxins, DNA cleaving antitumor antibiotics from a marine-derived fungus. Tetrahedron Lett 40:2489–2492
Lomascolo A, Lesage-Meessen L, Labat M, Navarro D, Delattre M, Asther M (1999) Enhanced benzaldehyde formation by a monokaryotic strain of Pycnoporus cinnabarinus using a selective solid adsorbent in the culture medium. Can J Microbiol 45:653–657
Chandel AK, da Silva SS, and Singh OV (2011) Detoxification of lignocellulosic hydrolysates for improved bioethanol production. In: Biofuel production: recent developments and prospects, ed: InTech
Lam KS, Gustavson DR, Veitch JA, Forenza S (1993) The effect of cerulenin on the production of esperamicin A1 by Actinomadura verrucosospora. J Ind Microbiol 12:99–102
Gailliot FP (1998) Initial extraction and product capture. Nat Prod Isolat 93:53–89
Dechow FJ (1989) Separation and purification techniques in biotechnology. Noyes Publications, Park Ridge
Tung LA, King CJ (1994) Sorption and extraction of lactic and succinic acids at pH > pKa1 I Factors governing equilibria. Ind Eng Chem Res 33:3217–3223
Jun Y-S, Huh YS, Park HS, Thomas A, Jeon SJ, Lee EZ, Won HJ, Hong WH, Lee SY, Hong YK (2007) Adsorption of pyruvic and succinic acid by amine-functionalized SBA-15 for the purification of succinic acid from fermentation broth. J Phys Chem C 111:13076–13086
Gluszcz P, Jamroz T, Sencio B, Ledakowicz S (2004) Equilibrium and dynamic investigations of organic acids adsorption onto ion-exchange resins. Bioprocess Biosyst Eng 26:185–190
Husson SM, King CJ (1999) Multiple-acid equilibria in adsorption of carboxylic acids from dilute aqueous solution. Ind Eng Chem Res 38:502–511
Dethe M, Marathe K, Gaikar V (2006) Adsorption of lactic acid on weak base polymeric resins. Sep Sci Technol 41:2947–2971
Cao N, Du J, Gong C, Tsao G (1996) Simultaneous production and recovery of fumaric acid from immobilized Rhizopus oryzae with a rotary biofilm contactor and an adsorption column. Appl Environ Microbiol 62:2926–2931
Li Q, Xing J, Li W, Liu Q, Su Z (2009) Separation of succinic acid from fermentation broth using weak alkaline anion exchange adsorbents. Ind Eng Chem Res 48:3595–3599
Leite JA, Fernandes BS, Pozzi E, Barboza M, Zaiat M (2008) Application of an anaerobic packed-bed bioreactor for the production of hydrogen and organic acids. Int J Hydrog Energy 33:579–586
Sosa AV, Córdoba PR, Perotti NI (2001) Fluidized bed design parameters affecting novel lactic acid downstream processing. Biotechnol Prog 17:1079–1083
Monteagudo JM, Aldavero M (1999) Production of L-lactic acid by Lactobacillus delbrueckii in chemostat culture using an ion exchange resins system. Journal of Chemical Technology & Biotechnology: International Research in Process. Environ Clean Technol 74:627–634
López-Garzón CS, Ottens M, van der Wielen LA, Straathof AJ (2012) Direct downstream catalysis: from succinate to its diethyl ester without intermediate acidification. Chem Eng J 200:637–644
Li Q, Li W-l, Wang D, Liu B-b, Tang H, Yang M-h, Liu QF, Xing JM, Su ZG (2010) pH neutralization while succinic acid adsorption onto anion-exchange resins. Appl Biochem Biotechnol 160:438–445
Davison BH, Nghiem NP, Richardson GL 2004 Succinic acid adsorption from fermentation broth and regeneration. In: Proceedings of the twenty-fifth symposium on biotechnology for fuels and chemicals held on May 4–7, 2003, in Breckenridge, pp. 653–669.
van der Wielen LUM, Straathof AJ (2010) High silica zeolites as an alternative to weak base adsorbents in succinic acid recovery. Ind Eng Chem Res 49:1837–1843
Gulicovski JJ, Čerović LS, Milonjić SK, Popović IG (2008) Adsorption of itaconic acid from aqueous solutions onto alumina. J Serb Chem Soc 73:825–834
Hwang YS, Lenhart JJ (2008) Adsorption of C4-dicarboxylic acids at the hematite/water interface. Langmuir 24:13934–13943
López-Garzón CS, Straathof AJ (2014) Recovery of carboxylic acids produced by fermentation. Biotechnol Adv 32:873–904
Roffler S, Blanch H, Wilke C (1984) In situ recovery of fermentation products. Trends Biotechnol 2:129–136
Fu Y, Zu Y, Liu W, Efferth T, Zhang N, Liu X et al (2006) Optimization of luteolin separation from pigeonpea [Cajanus cajan (L.) Millsp.] leaves by macroporous resins. J Chromatogr A 1137:145–152
Hossain KZ, Mercier L (2002) Intraframework metal ion adsorption in ligand-functionalized mesoporous silica. Adv Mater 14:1053–1056
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jatoi, A.S., Baloch, H.A., Mazari, S.A. et al. A review on extractive fermentation via ion exchange adsorption resins opportunities, challenges, and future prospects. Biomass Conv. Bioref. 13, 3543–3554 (2023). https://doi.org/10.1007/s13399-021-01417-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01417-w