Abstract
The European rabbit plays a major role in seed dispersal in its native environment. We evaluated its ecological interactions with plants through the dispersal of seeds by endozoochory in two invaded arid ecotones of Argentina. We found 855 whole seeds in 1283 fecal pellets, belonging to one non-native (Sesuvium portulacastrum) and five native plants (Arjona sp., Fabiana denudata, Frankenia juniperoides, Lycium chilensis, Poa sp.). Our results indicate that the European rabbit is a legitimate disperser of F. juniperoides, L. chilensis, and S. portulacastrum by the consumption and dissemination of viable seeds. Contrastingly, the rabbit is an illegitimate disperser of Arjona sp., F. denudata, and Poa sp. Our study identifies new interactions between an invasive herbivore and sympatric plants in the arid ecosystems of Argentina. We put forth that the mutualistic interaction between the European rabbit and both native and non-native plants highlights the complexity of trophic networks in invaded environments.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The presence of a new (exotic) species in an ecosystem can alter its ecological network directly, by establishing new interactions with the native species of the invaded community, or indirectly, by influencing their abundance, distribution, or behavior (McConkey et al. 2012; Blackburn et al. 2014). Although negative consequences of invasions are often emphasized, some studies show that mutualisms may occur between non-native and native species, or among non-native species themselves, thus facilitating the establishment of exotic species in invaded communities (Simberloff and Von Holle 1999; Traveset and Richardson 2006). Positive interactions frequently occur among non-native species, indicating that mutualisms may be important in varied invasion scenarios (Braga et al. 2018). Among the types of plant–animal mutualisms that facilitate invasions is seed dispersal by endozoochory (i.e., internal seed dispersal: Howe 1986; Herrera and Pellmyr 2002). This type of mutualism shows a lack of specificity in animal-dispersed plants and provides reciprocal benefits between them (Howe and Smallwood 1982). The consumption of seeds by herbivores can benefit plants because the dissemination of viable seeds carried in feces allows them to escape the often high mortality rates close to the parental plant (Janzen 1970), and also because it renders it possible to explore new vacant areas for recruitment, thus allowing the expansion of those plant species (Howe and Smallwood 1982; Jordano et al. 2011). Furthermore, under certain conditions, seed dispersal makes it possible to colonize novel sites suitable for germination and survival (Howe and Smallwood 1982). On the other hand, animal dispersers obtain a nutritional reward from the fruits and seeds they consume and transport (Jordano 1987).
Another important factor to consider is the effectiveness of seed dispersal (Schupp et al. 2010). That is, the contribution of each disperser to the reproduction of a given plant species (Reid 1989), as well as to the contribution in dispersal that a plant population receives from its dispersers (Schupp et al. 2010). Quantitative components (e.g., number of seeds dispersed by a dispersal agent, number of visits a dispersal agent makes multiplied by the number of seeds dispersed per visit) and qualitative ones (i.e., probability that a dispersed seed will produce a new adult) are to be considered when evaluating the effectiveness of the dispersal process (Schupp et al. 2010). Focusing on the qualitative component of the process, a given disperser may modify the viability and/or the germination of seeds when they are ingested and pass through its digestive tract (quality of treatment in the mouth and gut), as well as their survival depending on the quality of the sites where the seeds are deposited once laid in the feces (quality of deposition) (Schupp 1993). Now, considering the quality of the treatment, when an animal ingests seeds of a given species and disposes of them in viable conditions for their germination, it is considered a legitimate disperser of that species (Herrera 1995). On the contrary, when an animal delivers unviable seeds, it is considered an illegitimate seed disperser or a seed predator (Castro et al. 2008).
Non-native herbivorous mammals can act as important legitimate dispersers of both non-native (Holmgren 2002; Dacar et al. 2019) and native plant species (Campos et al. 2008; Calviño-Cancela 2011; Muñoz-Gallego et al. 2019) and in many cases provide plants with the same services as native dispersers (Westcott and Fletcher 2011). Alternatively, they can also act as illegitimate dispersers (Foster et al. 2014; Dénes et al. 2018). In particular, lagomorphs (including hares and rabbits) are dispersers of viable grass and herb seeds accidentally consumed while feeding on foliage and fruits (Schupp et al. 1997; Henríquez et al. 2014). Among the seed-dispersing lagomorphs is the European rabbit (Oryctolagus cuniculus), an herbivore introduced to several regions around the world and considered as one of the 100 most harmful invasive species due to its impact on biological diversity and human activities (Lowe et al. 2000; Long 2003). The European rabbit generates changes not only in plant community structure through herbivory but also in vertebrate community structure through competition or via its role as a keystone prey species (Courchamp et al. 2003; Davey et al. 2006; Barbar et al. 2018; Correa-Cuadros et al. 2022; Gubelin et al. 2023). Particularly in their role as endozoochorous seed dispersers, rabbits facilitate the establishment of non-native plants in areas where they colonize (Fernández and Saiz 2007; Bobadilla et al. 2020). These positive interactions among exotic species may potentially cause an invasional meltdown (Simberloff and Von Holle 1999), leading to an accelerated increase in the number of invading species and their impact on native communities. On the other hand, rabbits yield positive ecosystem services as seed-dispersing agents for some native plants (Castro et al. 2008; Jaksic and Castro 2021).
The European rabbit was first recorded in the arid ecosystems of Argentina in the 1970s (Jaksic et al. 2002; Bonino and Borrelli 2006; Bobadilla et al. 2021). The diverse ecological mosaic in this region constitutes an important scenario for the evolution of its biota and supports more species and endemic genera than other macrohabitats or biomes (Ojeda and Tabeni 2009). These arid ecosystems are undergoing rapid habitat conversion due to human activities (agriculture, grazing, and lumber and firewood extraction), desertification, salinization, and changes caused by shifting climate and expansion of invasive non-native species (Ojeda and Mares 1982), including the European rabbit (Bonino and Amaya 1985; Cuevas et al. 2011; Bobadilla et al. 2021). In the arid ecosystems of Argentina, the colonization and dispersal of European rabbit populations are known to be associated with riverbanks, streams, or moist areas that provide them with feeding and sheltering sites (Bonino and Soriguer 2009; Cuevas et al. 2011; Bobadilla et al. 2022). Nonetheless, there are no specific studies about the rabbit–plant interactions established in these recently invaded ecosystems.
Characterizing the attributes of the European rabbit’s role as a seed dispersal agent should enable understanding not only its effects on the plant communities but also if such interactions facilitate its colonization and expansion in the arid ecosystems of Argentina. We hypothesize that the European rabbit is a legitimate disperser of native and non-native plant species by consuming and disseminating whole seeds, so it could be expected that their feces will yield viable seeds of both types of plants. Therefore, we aim to evaluate the European rabbit’s ecological interactions with sympatric plants in its expansion range in the arid landscapes of Argentina. Specifically, (a) we identify, quantify, and characterize (fruit type and seed size) the seeds of native and non-native species found in European rabbit feces at two sites with different establishment times of rabbit populations; (b) we evaluate and compare the germination pattern (percentage and rate of germination and viability) of whole seeds found in feces with those collected directly from parental plants.
Materials and methods
Study area
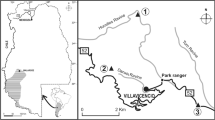
We selected 2 sampling sites with different times of settlement by European rabbit populations within the expansion range in the arid ecosystems of Argentina: (1) Bardas Blancas village (35° 52′ S—69° 48′ W, 1420 to 2800 m elevation), the core area of the rabbit’s expansion range (residence time over 45 y); and (2) Laguna de Llancanelo Provincial Reserve (35° 45´ S—69° 08´ W, 1270 to 1500 m elevation), the easternmost edge of the rabbit’s expansion range (residence time under 15 y), hereafter called Llancanelo Reserve (Fig. 1). These study sites lie in the arid ecotones of the Andean–Patagonia and the Monte–Patagonia ecoregions, respectively (hereafter simply called arid ecotones) (Méndez 2005, 2014). Bardas Blancas site is a dry steppe with an annual mean temperature of 11.9 °C, absolute maximum in December of 36.5 °C, and absolute minimum of − 14.6 °C in August (De Fina et al. 1964), and with a mean annual precipitation no greater than 300 mm (Norte 2000). The vegetation is a mosaic composed of grasslands dominated by Sporobolus, Pappostipa, and Poa; of shrublands, Chuquiraga, Neosparton, Larrea, and Prosopis; and of wetlands, called “vegas”, dominated by Cortaderia, Juncus, Baccharis, Tessaria, and Glycyrrhiza (Méndez 2014). Llancanelo Reserve is a Ramsar site encompassing approximately 90,000 ha with public and private land and includes one of the largest endorheic lagoons in the region, with permanent rivers and streams as well as temporary water inflows caused by fluctuations in nearby water bodies that flood the areas closest to the lagoon (Cabrera 1994; Palma Leotta et al. 2019). The Llancanelo Reserve constitutes the main natural wetland in the Monte–Patagonia arid ecotone of Argentina (Blendinger and Alvarez 2002). Here, the climate is arid, with a mean temperature of 19.5 °C in warmer months (December to February) and of 3 °C in cooler ones (June to August), with a mean annual rainfall ranging from 215 to 240 mm. The vegetation of the reserve contains 76% of the endemisms of the Mendoza province, and is characterized by five main environment types: (i) shrublands, dominated by Prosopis, Bougainvillea, and Chuquiraga; (ii) pichanal, dominated by Baccharis spartioides; (iii) sand dunes, characterized by Sporobolus, Suaeda, and Atriplex; (iv) wetlands, dominated by Distichlis, Frankenia, and Cortaderia; and (v) tamarindal, dominated by the invasive species Tamarix spp. (Méndez 2005).
Map of Argentina (left) and zoom in of the study area showing the 2 sampling sites with different settlement times by European rabbits within their expansion range (depicted by the contour area delineated in black lining) in arid ecotones of Argentina (based on Bonino and Amaya (1985), Bonino and Soriguer (2009), and Bobadilla et al. (2022))
Feces collection
Sampling was conducted during the wet season of 2018 (February–March) at the two study sites. We used a stratified random sampling model for 115 fixed strip transects of 1000 m2 (5 m × 200 m) laid out across the study areas on the basis of the habitat types recognized in each site (Bobadilla et al. 2022). We covered a total area of approximately 20 km2 in Bardas Blancas (45 transects) and 66 km2 in Llancanelo Reserve (70 transects). We collected fresh rabbit feces along those transects and checked them once during the wet season. The latrines were avoided because rabbits urinate in them, and this might affect seed germination (Dellafiore et al. 2009). Rabbit fecal pellets are easy to identify in the field by their distinctive size, color, shape, and rugosity (Salgado 2016). All feces collected in one transect were pooled into 1 sample (considered independent of the other transects) to be analyzed in the laboratory. In the middle of each study transect, we established one vegetation transect 50 m long, where we measured the specific composition of plants (Passera et al. 1986). We also collected mature fruits and seeds from plants present in each transect during same season and phenological stage as those obtained from feces. These were used to build a reference collection and for control treatment on germination assays.
Seeds extraction
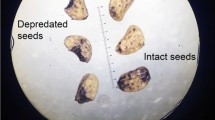
All feces in each sample were placed in test tubes with an aqueous solution for 30 min to facilitate pellet disintegration. Once the material had softened, the pellets were macerated and disintegrated without exerting pressure. Each sample was sieved to retain fragments on meshes of 1000 µm and then of 200 µm, washing the material with water several times. Each Petri dish was carefully observed under a binocular magnifying glass (10 ×) to separate all seeds. Three categories of seeds were distinguished: (a) whole not damaged after gut passage, (b) partially digested (whole but deemed damaged or unviable), and (c) fragments. In this latter case, a relationship was previously established for seed quantification. For this, the number of fragments that constitute one whole seed was determined depending on the species and the mean size of its seeds (Fernández and Saiz 2007). All seeds were recorded, quantified, and characterized. They were identified according to the reference collection-built ad hoc during the sampling period and to the reference collection of seeds at the Ruiz Leal Herbarium (IADIZA-CONICET, Argentina). Seeds were identified to species level when possible. To characterize the seeds of the species found in the field and identified in the feces, a bibliographical search was carried out to build a table containing their ecological and morphological characteristics. All whole seeds were kept in a refrigerator at 8 °C until used for the germination tests.
Germination assay
To evaluate the germination capacity of all whole seeds found in rabbit feces, we performed a laboratory assay in standard conditions common to all species, following the recommendations of the International Seed Testing Association (ISTA 2005) manual. Previous to the assay, we determined if any of the whole seeds of the species extracted from feces required pre-germination treatments to break dormancy, following the recommendations of the ISTA for each species. The seeds were sowed in Petri dishes (maximum of 40 per dish) with humid filter paper for a maximum of 30 days. We also sowed seeds belonging to the same species, but obtained directly from the mature fruits within the study sites, as a control treatment. Seeds from fruits were extracted under a binocular magnifying glass with the help of tweezers and needles, and they were dried with absorbent paper. During the assay, seeds were kept in a germination room (20–30 °C, 12–12 h light–darkness, watered daily) and observed every day to check for root emergence, indicating seed germination.
Viability assay
To evaluate the viability of the seeds that did not germinate, we used a standard bioindicator (2, 3, 5 triphenyltetrazolium chloride (TTC)) that detects seed viability by staining the embryo tissue pink/red (Moore 1985). For it, all seeds were cut open leaving the embryo exposed. Only the seeds with a healthy embryo were incubated in the TTC solution, at room temperature and under darkness for 24 h. We determined seed viability by observing seed coloration under microscope amplification, in comparison to positive control seeds collected from the study sites. We determined the number of unproductive versus viable seeds among those non-germinated, by the TTC assay.
Data analyses
We calculated the number and proportion of feces containing whole, digested, and/or fragmented seeds. Both for seeds in feces and for the control, we calculated the germination percentage (%G) with the equation: %G = (n germinated seeds/total seeds sown) × 100. We analyzed differences in the proportion of germinated seeds obtained from feces and the control treatment using a chi-square test of homogeneity (Zar 2010) for each species by site. The cumulative percent germination was calculated and was drawn in order to demonstrate differences in germination patterns among treatments. The evaluation of seed germination was based on two additional parameters: germination start (GS) and germination rate (GR). The germination start was defined as the time interval (days) between sowing and the emergence of 1/6 of the final germination percentage, and was calculated with the equation: GS = 1/6 × %G (Izhaki and Ne’eman 1997). We calculated the germination rate with the equation: GR = (5/6 × %G) / (T2 − T1), where T1 is the interval (in days) between sowing and emergence of 1/6 x %G of the seedlings, and T2 is the interval (in days) between sowing and emergence of 5/6 x %G of the seedlings (Izhaki and Ne’eman 1997). The parameter GR was calculated in order to describe the rapidity in the % germination per day for each species by site and treatments (Izhaki and Ne'eman 1997).
Results
Feces collection
We collected a total of 1283 pellets. Of these, 71% were from the wetland areas of both study sites. The number of feces per site was 397 pellets at Bardas Blancas (number of transects = 6) and 886 at Llancanelo Reserve (number of transects = 8).
Seeds extraction
We found a total of 855 whole seeds of 6 identified plant species, and 46 whole seeds of one unidentified plant (Table 1). The number of whole seeds per pellet was higher in Bardas Blancas than in Llancanelo Reserve (Table 1). Most of the whole seeds found in feces were of the shrub Frankenia juniperoides (88% in Bardas Blancas and 57% in Llancanelo Reserve). The highest number of fully or partially digested seeds was also observed for the same F. juniperoides (98% and 68%, respectively). The highest number of seed fragments identified was from the shrub Lycium chilensis (55%) and they were detected only in Llancanelo Reserve (Table 1). Of the six identified species, only one was non-native: Sesuvium portulacastrum. Two species were identified at the genus level, with one morphotype for each genus (Arjona sp. and Poa sp.). Three species had incomplete values because data were not available. The whole seeds found in feces had a maximum size of ca. 1.6 × 2.0 mm and corresponded mostly to L. chilensis (Table 1 and Fig. 2).
Germination assay
Three of the seven plant species with whole seeds obtained from feces germinated after 30 days: F. juniperoides (at both sites), L. chilensis, and S. portulacastrum (only at the Llancanelo Reserve) (Fig. 2). Of the seed species obtained from the fruits (control), only F. juniperoides germinated both in Bardas Blancas as in Llancanelo Reserve. Fourteen percent of the 901 whole seeds from feces germinated after 30 days and 0.9% died due to fungal attack. Forty one percent of the total control seeds germinated after 30 days and 1.8% were attacked by fungi. The germination percentage was significantly higher in the control treatment than in the digested seeds for F. juniperoides both in Llancanelo Reserve (51% and 31%, respectively; χ2 = 26.86, p < 0.0001, df = 1) and in Bardas Blancas (58% and 7%, respectively; χ2 = 177.10, p < 0.0001, df = 1) (Fig. 3). Three percent of L. chilensis seeds extracted from feces showed radicle protrusion during the assay, while none of the seeds from the fruits germinated (χ2 = 4.07, p < 0.05, df = 1) (Fig. 3). The seeds of S. portulacastrum obtained from fruits did not germinate after 30 days, while those obtained from feces did (5%; χ2 = 4.1, p < 0.05, df = 1) (Fig. 3). The F. juniperoides control seeds germinated first and had the highest germination rate than those seeds from feces at both Bardas Blancas and Llancanelo Reserve (Table 2 and Fig. 4).
Germination percentage of F. juniperoides (ntreatment = 326, ncontrol = 326), L. chilensis (ntreatment = 120, ncontrol = 120), and S. portulacastrum (ntreatment = 75, ncontrol = 75) in Llancanelo Reserve; and of F. juniperoides in Bardas Blancas (ntreatment = 293, ncontrol = 293). ntreatment: whole seeds found in European rabbit feces and exposed to the assay; ncontrol: whole seeds extracted from the plants and exposed to the assay. *p < 0.05; ***p < 0.0001
Viability assay
Of the 721 seeds from the feces that did not germinate during the assay, 12% were unproductive seeds (Table 3). All non-germinated seeds from the feces that presented a healthy embryo were deemed non-viable by the TTC assay (Table 3). Of the 468 non-germinated control seeds, 19% had an empty seed or a poorly developed embryo. Eleven percent of the control seeds corresponding to F. juniperoides and L. chilensis were positive by the TTC assay (Table 3). All seeds belonging to A. patagonica, F. denudata, and Poa sp. were judged non-viable (Table 3).
Discussion
Our results show that the European rabbit is a legitimate disperser of at least three plant species in its expansion range in the arid ecosystems of Argentina. In these study areas, the non-native plant species represent less than 10% of the total species (54 in Bardas Blancas and 52 in Llancanelo Reserve). According to our results, the rabbit disperses 2 to 4% of the native plant species and 2% of the non-native plant species recorded in the field. In its native range, the rabbit disperses 8% of the plant species in a coastal dune (Dellafiore et al. 2009) and 50% of the plants in a Mediterranean dehesa (Malo and Suárez 1995). These differences in the percent of dispersed plants by the same vector in different environments may be related to both the number of species likely to be dispersed (survival capacity of seeds when passing through the rabbit’s digestive tract) and their abundance in the originating community (supply of fruits and/or seeds) (Traveset 1998). For invasion ranges, the eco-evolutionary experience of both the introduced species and the recipient community (Saul et al. 2013) and the possibility of establishment of novel interactions (ecological fitting, Janzen 1985), could also be factors that explain these differences. In this sense, it is expected that the incidence and extent of the novel interactions increase with residence time. The analysis of these factors is beyond the scope of the present work and reinforces the need for further studies considering the abundance of rabbits and of plant resources, through different phenological stages in the arid landscapes of Argentina.
The load of whole seeds per rabbit pellet recorded in Argentina is below the values reported in sand dunes in Spain, 0.94–0.97 (Calviño-Cancela 2002; Larrinaga 2010), but higher than the lowest values reported in an acid grassland in England, 0.1–0.5 seeds/pellet (Pakeman et al. 1999). Regarding the relationship between numbers of plant species dispersed versus those eaten, rabbits dispersed from 6 to 17% of the species recorded in their diet at the same two study sites in Argentina (Bobadilla et al. 2022). In dune environments as well as in woods, scrub, and pastures, some of the species dispersed by rabbits also have been cited as part of their diet (Dellafiore et al. 2007, 2009). Indeed, few studies have determined the number of species consumed by rabbits versus the number of species dispersed by endozoochory at any given site (Godó et al. 2022).
It is generally assumed that seeds are accidentally ingested by herbivores when consuming vegetative parts of plants (the “foliage is fruit” hypothesis of Janzen 1984). This could be the case of L. chilensis, because its foliage represents an important food item in the rabbit’s diet, but not for F. juniperoides or S. portulacastrum, whose seeds but not their vegetative parts are present in the diet (Bobadilla et al. 2022). Similarly, Pakeman et al. (1999) observed in England that rabbits play a more frugivorous role than an indirect removal of seeds by herbivory, actually selecting the fruits of some of the species that they disperse. Such evidence is consistent with our results and with other studies showing that the European rabbit actively selects small seeds (Larrinaga 2010) or mainly consumes small fruits (Guerrero-Campos et al. 2023) both in native and non-native areas.
The proportion of fragmented seeds in rabbit feces ranged 6–22% between the two study sites. In addition, we found > 40% digested whole seeds. Thus, our results highlight the complex relationship between endozoochory and predation (involving mastication and digestion) (Malo and Yanes 1999). According to Dellafiore et al. (2007), in a sand dune in Spain the plants dispersed by endozoochory were also preyed on by rabbits. Indeed, the passing of seeds through an herbivore’s digestive tract can increase their mortality if seed coats are weak, and increase their germination by scarification during digestion (Janzen et al. 1985). Our germination tests allowed to determine that the rabbit’s gut passage of seeds favored the germination of L. chilensis and S. portulacastrum, while for F. juniperoides the seeds collected directly from the plant germinated earlier and in greater quantity. Therefore, as observed by Dellafiore et al. (2007, 2009) in Spain, we found that for some plant species the passage of seeds through the digestive tract of rabbits is only a dispersion means, while for others it also favors subsequent germination, which indicates that rabbits act as legitimate dispersers. Contrariwise, in Argentina, rabbits act as illegitimate dispersers for those plant species whose whole but non-viable seeds were found in the feces (Arjona sp., F. denudata, Poa sp.).
Viability tests indicate that all seeds ingested by rabbits that did not germinate were not viable. Nevertheless, viable seeds from the controls were found for F. juniperoides and L. chilensis. Our results indicate that the control seeds of L. chilensis, were viable but did not germinate during the time that the test lasted, while those in the feces did, showing that their passage through the rabbit’s gut accelerated their germination rate. It is important to point out that during water deficit periods, seeds are naturally bound to germinate quickly to ensure species survival in arid ecosystems (Contreras Quiroz et al. 2015). Therefore, the improved germination time of L. chilensis seeds dispersed by rabbits could be relevant in such environments.
Herbivory effects involve digestion, intestinal retention period, seed size, and seed coat hardness (Campos et al. 2008). Considering that rabbits carry out caecotrophy (ingestion of their own soft feces), seeds may pass more than once through their digestive tract, affecting their germination pattern according to the number of intestinal passes (Castro et al. 2008; Mancilla-Leyton et al. 2013). Previous studies showed significant increases in the germination percentage of seeds that passed twice through rabbit gut (Castro et al. 2008; Mancilla-Leytón et al. 2013). Also, negative associations have been observed between seed size and the percentage of whole seeds recovered in rabbit feces: seeds < 4 mm were more likely to pass whole through their digestive tract (Malo et al. 2000; Pakeman et al. 2002). Consistent with these reports, in our study, whole seeds in rabbit feces did not exceed 2 × 1.5 mm. This suggests a maximum seed size for their intact passage through the rabbit gut and reinforces the need for further studies that assess morphometric variables of whole and damaged seeds in rabbit feces.
Of the three species of seeds dispersed by rabbits in our study, two were native (L. chilensis and F. juniperoides) and one was non-native (S. portulacastrum). Our results show a significant increase in the germination percentage of seeds of L. chilensis. This native shrub is a good forage species, especially important in arid and semi-arid ecosystems where most plants have low nutritional value due to their high fiber content (Noy-Meir 1973; Cuevas et al. 2013). Indeed, L. chilensis is the fifth most frequent food item in the rabbit diet during the wet season at Llancanelo Reserve (Bobadilla et al. 2022), and its fleshy and juicy fruits constitute a nutritious, accessible, and abundant food during summer (Giorgetti et al. 2000). Therefore, the rabbit–L. chilensis interaction may depict a scenario of positive ecological effects between a non-native disperser and a native plant in this study site. Regarding the other native plant, F. juniperoides, our results show a significant reduction in the percentage and rate of seed germination after the passage through the rabbit’s gut. This is thus, a legitimate but inefficacious or inefficient dispersal service. However, the rabbit–F juniperoides interaction provides another dispersal mechanism for this native plant that could provide an advantage if its seeds are deposited in suitable sites, far from the parental plant (Schupp et al. 2010).
For the non-native S. portulacastrum, our results show a significant increase in the germination percentage of its seeds. This is a perennial herb with a wide geographic distribution on all continents and is considered non-native in our study sites (Méndez 2005; Jocou and Gandullo 2020). It presents typical anatomical features of wetland plants, combined with xeromorphic characters (Apóstolo 2005) that could act as adaptive strategies for acclimatizing to these wetlands in arid environments. Its success as a colonizing species in other regions has been associated to its ability to be propagated by vegetative fragments, while presenting a continuous flowering and fruiting cycle (Lonard and Judd 1997). This reproductive strategy enables fruits and seeds to be available throughout the year, which is especially important in arid landscapes with periods where food is less abundant for herbivores (dry season). There are no records of endozoochorial seed dispersers for this species (Lonard and Judd 1997; Bohley et al. 2017), so the European rabbit would be its first described animal vector. Therefore, the incipient new interaction of rabbit–S. portulacastrum could have a reciprocal effect on one another, potentially aiding their mutual invasive process (and subsequent impacts) in the wetlands of Bardas Blancas and Llancanelo Reserve. These habitat types are especially important in arid biomes (Blendiger and Alvarez 2002). In particular, the wetland in Llancanelo Reserve has a high level of endemism, made up of different plant communities distributed in two vegetation units: hygrophilic (humid) and halophilic (saline) (Roig et al. 2000).
Our findings add to other studies documenting the European rabbit as a relevant disperser of several plant species both native and non-native in indigenous and invaded ranges in different ecosystems (Malo et al. 2000; Castro et al. 2008; Dellafiore et al. 2009; Salas-Pascual et al. 2009; Bobadilla et al. 2020). From a conservation viewpoint, these interactions may be deemed negative if they facilitate the invasion of non-native plant species, or positive if the dispersal of native species occurs (McConkey et al. 2012). Nevertheless, it is known that exotic lagomorphs may be less efficient in seed dispersal than are native frugivores which may be specialized dispersers of certain plants, considerably changing seed removal rate, seed availability, as well as seed and seedling survival (Godó et al. 2022). In our study, rabbits legitimately dispersed two native and one non-native plant species by consuming and disseminating whole seeds, supporting our hypothesis. On the order hand, due to Llancanelo Reserve being at the edge of the expansion range of the European rabbit in arid ecotones of Argentina, the ecological interaction of rabbits with different plant species takes special importance for two reasons. First, because of the seed dispersal service that rabbits perform for recruiting native plants into the wetlands inside the protected area. Second is for their spreading of non-native plants, aiding their invasive process. Our study highlights the complex ecological role played by the European rabbit in simultaneously facilitating recruitment of native plants but also that of non-native species, thus conducive to invasional meltdown (Simberloff and Von Holle 1999).
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abalos RM (2016) Plantas del monte argentino: guía de campo. Ecoval, Córdoba, Argentina.
Apóstolo NM (2005) Caracteres anatómicos de la vegetación costera del Río Salado (Noroeste de la provincia de Buenos Aires, Argentina). Boletín De La Sociedad Argentina De Botánica 40(3–4):215–227
Barbar F, Ignazi GO, Hiraldo F, Lambertucci SA (2018) Exotic lagomorph may influence eagle abundances and breeding spatial aggregations: a field study and meta-analysis on the nearest neighbor distance. PeerJ 6:e4746. https://doi.org/10.7717/peerj.4746
Bernardello LM (1983) Estudios en Lycium (Solanaceae). III. Estructura y desarrollo de fruto y semilla en Lycium y Grabowskia. Boletín De La Sociedad Argentina De Botánica 22:1–4
Blackburn TM, Essl E, Evans T, Hulme PE, Jeschke JM et al (2014) A unified classification of alien species based on the magnitude of their environmental impacts. PLoS Biol. https://doi.org/10.1371/journal.pbio.1001850
Blendinger PG, Alvarez ME (2002) Ensambles de aves de los bañados de Carilauquen (Laguna Llancanelo, Mendoza, Argentina): consideraciones para su conservación. Hornero 17:71–83
Bobadilla SY, Marchetta A, Dacar MA, Ojeda RA, Cuevas MF (2020) Food habits of European rabbit and its role as seed dispersal of two Mosqueta roses: facilitation among non-native species in a semiarid protected area of Argentina? Biol Invasions. https://doi.org/10.1007/s10530-020-02205-9
Bobadilla SY, Dacar MA, Jaksic FM, Ojeda RA, Cuevas MF (2022) Spatial and trophic niche of an assemblage of native and non-native herbivores of arid Argentina. J Mammal. https://doi.org/10.1093/jmammal/gyab171
Bobadilla SY, Ojeda RA, Cuevas MF (2021) Invasive European wild rabbits (Oryctolagus cuniculus) in Argentina: state of the art and prospect for research. In: Jaksic FM and Castro SA (eds) Biological invasions in the South American Anthropocene: Global Causes and Local Impacts. Springer, Cham, Switzerland, pp 187–201
Bohley K, Winter PJD, Kadereit G (2017) A revision of Sesuvium (Aizoaceae, Sesuvioideae). Syst Bot 42(1):124–147. https://doi.org/10.1600/036364417X694575
Bonino NA, Amaya J (1985) Distribución geográfica y control del conejo silvestre europeo Oryctolagus cuniculus en la Argentina. IDIA 429:25–50
Bonino NA, Borrelli L (2006) Variación estacional de la dieta del conejo silvestre europeo (Oryctolagus cuniculus) en la región andina de Neuquén, Argentina. Ecol Austral 16:7–13
Bonino N, Soriguer R (2009) The invasion of Argentina by the European wild rabbit Oryctolagus cuniculus. Mammal Rev 39:159–166
Braga RR, Gómez-Aparicio L, Heger T, Vitule JRS, Jeschke JM (2018) Structuring evidence for invasional meltdown: broad support but with biases and gaps. Biol Invasions. https://doi.org/10.1007/s10530-017-1582-2
Cabrera AL (1994) Regiones fitogeográficas Argentinas. In: Kugler WF (ed) Enciclopedia Argentina de Agricultura y Jardinería, Tomo II, Fascículo 1. ACME S.A.C.I., Buenos Aires, 1–85
Calviño-Cancela M (2002) Spatial patterns of seed dispersal and seedling recruitment in Corema album (Empetraceae): the importance of unspecialized dispersers for regeneration. J Ecol 90:775–784
Calviño-Cancela M (2011) Seed dispersal of alien and native plants by vertebrate herbivores. Biol Invasions. https://doi.org/10.1007/s10530-010-9877-6
Campos CM, Peco Vázquez B, Campos VE, Malo Arrazola JE, Giannoni SM, Suárez Cardona F (2008) Endozoochory by native and exotic herbivores in dry areas: consequences for germination and survival of Prosopis seeds. Seed Sci Res. https://doi.org/10.1017/S0960258508940344
Castro S, Bozinovic F, Jaksic FM (2008) Ecological efficiency and legitimacy in seed dispersal of an endemic shrub (Lithrea caustica) by the European rabbit (Oryctolagus cuniculus) in central Chile. J Arid Environ. https://doi.org/10.1016/j.jaridenv.2007.12.012
Contreras Quiroz MDR, Moreno MP, Jurado E (2015) Seed germination of plant species from semiarid zones after hydration–dehydration treatments. Revista Chapingo Serie Zonas Áridas 14:41–50
Correa-Cuadros JP, Flores G, Muñoz-Rodríguez MA, Briceño C, Díaz M, Strive T, Vásquez F, Jaksic FM (2022) History, control, epidemiology, ecology, and economy of the invasion of European rabbits in Chile: a comparison with Australia. Biol Invasions. https://doi.org/10.1007/s10530-022-02915-2
Courchamp F, Chapuis JL, Pascal M (2003) Mammal invaders on islands: impact, control and control impact. Biol Reviews. https://doi.org/10.1017/S1464793102006061
Cuevas MF, Ojeda RA, Dacar MA, Jaksic FM (2013) Seasonal variation in feeding habits and diet selection by wild boars in a semi-arid environment of Argentina. Acta Theriol. https://doi.org/10.1007/s13364-012-0105-x
Cuevas MF, Chillo V, Marchetta A, Ojeda RA (2011) Mammalia, Lagomorpha, Leporidae, Oryctolagus cuniculus Linnaeus, 1758: new record and its potential dispersal corridors for northern Mendoza, Argentina. Check List 7(4):565–566. https://doi.org/10.15560/7.4.565
Dacar MA, Dalmasso AD, Bobadilla SY, Cuevas MF (2019) Rol del ganado doméstico en el establecimiento de la especie invasora rosa mosqueta (Rosa rubiginosa L.) en los Andes áridos, Argentina. Mastozool Neotrop 26(2):331–339. https://doi.org/10.31687/saremMN.19.26.2.0.17
Davey C, Sinclair ARE, Pech RP, Arthur AD, Krebs CJ, Newsome AE, Hik D, Molsher R, Allcock K (2006) Do exotic vertebrates structure the biota of Australia? An experimental test in New South Wales. Ecosyst 9:992–1008. https://doi.org/10.1007/s10021-004-0173-0
Dellafiore CM, Gallego Fernández JB, Muñoz Vallés S (2007) The contribution of endozoochory to the colonization and vegetation composition of recently formed sand coastal dunes. Res Lett Ecol 1:1–3
Dellafiore CM, Fernández JG, Vallés SM (2009) The rabbit (Oryctolagus cuniculus) as a seed disperser in a coastal dune system. Plant Ecol. https://doi.org/10.1007/s11258-009-9639-7
Dénes FV, Tella JL, Zulian V, Prestes NP, Martínez J, Hiraldo F (2018) Combined impacts of multiple non-native mammals on two life stages of a critically endangered Neotropical tree. Biol Invasions. https://doi.org/10.1007/s10530-018-1758-4
De Fina AL, Giannetto F, Richard AE, Sabella LS (1964) Difusión geográfica de los cultivos índices de la provincia de Mendoza y sus causas. Boletín de Estudios Geográficos 44:151–154. https://bdigital.uncu.edu.ar/12226
Fernández A, Saiz F (2007) The European rabbit (Oryctolagus cuniculus) as a seed disperser of the invasive opium poppy (Papaver somniferum) in Robinson Crusoe Island. Chile Mastozool Neotrop 14:19–27
Foster CN, Barton PS, Lindenmayer DB (2014) Effects of large native herbivores on other animals. J Appl Ecol. https://doi.org/10.1111/1365-2664.12268
Giorgetti HD, Manuel Z, Montenegro OA, Rodríguez GD, Busso CA (2000) Phenology of some herbaceous and wood species in central, semiarid Argentina. Phyton-Revista Internacional De Botánica Experimental 69:91–108
Godó L, Valkó O, Borza S, Deák B (2022) A global review on the role of small rodents and lagomorphs (clade Glires) in seed dispersal and plant establishment. Global Ecol Conserv 33:e01982 https://doi.org/10.1016/j.gecco.2021.e01982
Gubelin P, Correa-Cuadros JP, Ávila-Thieme MI, Flores-Benner G, Duclos M, Lima M, Jaksic FM (2023) European rabbit invasion in a semi-arid ecosystem of Chile: how relevant is its role in food webs? Life 13(4):1–20. https://doi.org/10.3390/life13040916
Guerrero-Campos M, Mendes SB, Marrero P et al (2023) Introduced rabbits as seed-dispersing frugivores: a study case on a environmentally diverse oceanic island (Tenerife, Canaries). Biol Invasions 25:2117–2129. https://doi.org/10.1007/s10530-023-03026-2
Henríquez EB, Isenrath GBD, Cona ML, Campos CM (2014) Dispersión endozoocórica por Lepus europaeus (Lagomorpha, Leporidae) en el ecotono Monte-Patagonia, Argentina. Mastozool Neotrop 21:211–217
Herrera CM (1995) Plant–vertebrate seed dispersal systems in the Mediterranean: ecological, evolutionary, and historical determinants. Annu Rev Ecol Evol Syst. https://doi.org/10.1146/annurev.es.26.110195.003421
Herrera CM, Pellmyr O (2002) Plant animal interactions: an evolutionary approach. John Wiley and Sons, Oxford, UK
Holmgren M (2002) Exotic herbivores as drivers of plant invasion and switch to ecosystem alternative states. Biol Invasions. https://doi.org/10.1023/A:1020535628776
Howe HF (1986) Seed dispersal by fruit-eating birds and mammals. In: Murray DR (ed) Seed dispersal. Academic Press, New York, pp 123–189
Howe FH, Smallwood J (1982) Ecology of seed dispersal. Annu Rev Ecol Evol Syst. https://doi.org/10.1146/annurev.es.13.110182.001221
ISTA (2005) International Rules for Seed Testing. Edition 2005. ISTA, Bassersdorf, Switzerland.
Izhaki I, Ne'eman G (1997) Hares (Lepus spp.) as seed dispersers of Retama raetam (Fabaceae) in a sandy landscape. J Arid Environ 37:343-354. https://doi.org/10.1006/jare.1997.0273
Jaksic M, Iriarte JA, Jiménez JE, Martínez DR (2002) Invaders without frontiers: cross-border invasions of exotic mammals. Biol Invasions. https://doi.org/10.1023/A:1020576709964
Jaksic FM, Castro SA (2021) Biological invasions in the South American Anthropocene: global causes and local impacts. Springer Nature, Cham, Switzerland
Janzen DH (1970) Herbivores and the number of tree species in tropical forests. Am Nat 104:501–528
Janzen DH (1984) Dispersal of small seeds by big herbivores: foliage is fruit. Am Nat 123:338–353
Janzen DH, Demment MW, Robertson JB (1985) How fast and why do germinating guanacaste seeds (Enterolobium cyclocarpum) die inside cows and horses? Biotropica 17:322–325
Jocou AI, Gandullo R (2020) Diversidad de plantas vasculares de los humedales de la Norpatagonia (Argentina). Revista del Museo Argentino de Ciencias Naturales 22(2):134–154.https://doi.org/10.22179/revmacn.22.688
Jordano P (1987) Patterns of mutualistic interactions in pollination and seed dispersal: connectance, dependence asymmetries, and coevolution. Am Nat. https://doi.org/10.1086/284665
Jordano P, Forget PM, Lambert JE, Böhning-Gaese K, Traveset A, Wright SJ (2011) Frugivores and seed dispersal: mechanisms and consequences for biodiversity of a key ecological interaction. Biol Lett. https://doi.org/10.1098/rsbl.2010.0986
Larrinaga AR (2010) Rabbits (Oryctolagus cuniculus) select small seeds when feeding on the fruits of Corema album. Ecol Res. https://doi.org/10.1007/s11284-009-0635-0
Lonard RI, Judd FW (1997) The biological flora of coastal dunes and wetlands. Sesuvium portulacastrum (L.) L. J Coast Res 13(1):96–104 https://www.jstor.org/stable/4298595
Long JL (2003) Introduced Mammals of the World: Their History, Distribution and Influence. CSIRO Publishing, Collingwood
Lowe S, Browne M, Boudjelas S, De Poorter M (2000) 100 of the world’s worst invasive alien species: a selection from the global invasive species database. Published by The Invasive Species Specialist Group (ISSG) a specialist group of the Species Survival Commission (SSC) of the World Conservation Union (IUCN), Auckland, New Zealand. https://portals.iucn.org/library/sites/library/files/documents/2000-126.pdf. Accessed 27 Mar 2023
Lüdtke R, Souza-Chies TTD, Miotto STS (2009) O gênero Monnina (Polygalaceae) na Região Sul do Brasil. Acta Botanica Brasilica 23:175–195
Malo JE, Jiménez B, Suárez F (2000) Herbivore dunging and endozoochorous seed deposition in a Mediterranean dehesa. J Range Manag 53:322–328
Malo JE, Suárez F (1995) Establishment of pasture species on cattle dung: the role of endozoochorous seeds. J Veg Sci 6:169–174. https://doi.org/10.2307/3236211
Malo JE, Yanes M (1999) ¿Endozoocoria o depredación? La ingestión de legumbres de Retama sphaerocarpa por el conejo (Oryctolagus cuniculus). Dissertation, Almería
Mancilla-Leytón JM, González-Redondo P, Vicente AM (2013) Effects of rabbit gut passage on seed retrieval and germination of three shrub species. Basic Appl Ecol. https://doi.org/10.1016/j.baae.2013.08.005
McConkey KR, Prasad S, Corlett RT, Campos-Arceiz A, Brodie JF, Rogers H, Santamaria L (2012) Seed dispersal in changing landscapes. Biol Conserv 146:1–13
Méndez E (2005) La vegetación de la Reserva Provincial Laguna de Llancanelo (Mendoza, Argentina). Candollea 60:123–148
Méndez E (2014) La Vegetación de los Altos Andes Centrales: Bardas Blancas-Paso Pehuenche (Malargüe, Mendoza, Argentina). Boletín De La Sociedad Argentina De Botánica 49:257–281
Moore RP (1985) Handbook on tetrazolium testing. International seed testing association, Zurich
Muñoz-Gallego R, Fedriani JM, Traveset A (2019) Non-native mammals are the main seed dispersers of the ancient Mediterranean palm Chamaerops humilis L. in the Balearic Islands: rescuers of a lost seed dispersal service? Front Ecol Evol 7:161. https://doi.org/10.3389/fevo.2019.00161
Norte F (2000) Mapa climático de Mendoza. In: Abraham EM and Rodríguez Martínez F (eds) Argentina. Recursos y Problemas Ambientales de la Zona Árida. Primera Parte. Provincia Mendoza, San Juan y La Rioja. PAN, Mendoza, pp 25–27
Noy-Meir I (1973) Desert ecosystems: environment and producers. Annu Rev Ecol Evol Syst 4:25–51
Ojeda RA, Mares MA (1982) Conservation of South American mammals: Argentina as a paradigm. In: Mares MA and Genoways HM (eds) Mammalian biology in South America. University of Pittsburgh, Pittsburgh, pp 505–521
Ojeda RA, Tabeni S (2009) The mammals of the Monte Desert revisited. J Arid Environ 73:173–181. https://doi.org/10.1016/j.jaridenv.2007.09.008
Pakeman RJ, Engelen J, Attwood JP (1999) Rabbit endozoochory and seed bank build-up in an acidic grassland. Plant Ecol. https://doi.org/10.1023/A:1009864030814
Pakeman RJ, Digneffe G, Small JL (2002) Ecological correlates of endozoochory by herbivores. Funct Ecol 16:296–304
Palma Leotta M, Torres J, Cisneros H, Caliri M, Ordoñez M, Gorla NBM (2019) Aportes de la teledetección para la caracterización de amenazas para la conservación del sitio Ramsar humedal Llancanelo, Malargüe, Argentina. Boletín De Estudios Geográficos 112:83–113
Reid N (1989) Dispersal of mistletoes by honeyeaters and flower peckers: components of seed dispersal quality. Ecology. https://doi.org/10.2307/1938420
Roig FA, Martínez Carretero EE, Méndez E (2000) Mapa de vegetación de la provincia de Mendoza. In: Abraham EM and Rodríguez Martínez F (eds) Argentina. Recursos y Problemas Ambientales de la Zona Árida. Primera Parte. Provincia Mendoza, San Juan y La Rioja. PAN, Mendoza, pp 63–64
Salas-Pascual S, Lugo SF, Cigala AN (2009) Interaction between two exotic invading species: endozoochory of Acacia farnesiana seeds by the European rabbit (Oryctolagus cuniculus). Open for Sci J 2:86–90
Salgado I (2016) Conejo Oryctolagus cuniculus (Linnaeus, 1758). In: Calzada J, Clavero M, Fernández A (eds) Guía virtual de los indicios de los mamíferos de la Península Ibérica, Islas Baleares y Canarias. Sociedad Española para la Conservación y Estudio de los Mamíferos (SECEM). http://www.sarem.es/guiadeindiciosmamiferos. Accessed 21 Nov 2021
Saul WC, Jeschke JM, Heger T (2013) The role of eco-evolutionary experience in invasion success. NeoBiota 17:57–74. https://doi.org/10.3897/neobiota.17.5208
Schupp EW, Jordano P, Gomez JM (2010) Seed dispersal effectiveness revisited: a conceptual review. New Phytol. https://doi.org/10.1111/j.1469-8137.2010.03402.x
Schupp EW, Heaton HJ, Gomez JM (1997) Lagomorphs and the dispersal of seeds into communities dominated by exotic annual weeds. Great Basin Naturalist 57(3):253–258
Schupp EW (1993) Quantity, quality and the effectiveness of seed dispersal by animals. In: Fleming TH and Estrada A (eds) Frugivory and seed dispersal: ecological and evolutionary aspects. Springer, Netherlands, pp 15–29
Simberloff D, Von Holle B (1999) Positive interactions of nonindigenous species: invasional meltdown? Biol Invasions. https://doi.org/10.1023/A:1010086329619
Traveset A, Richardson DM (2006) Biological invasions as disruptors of plant reproductive mutualisms. Trends Ecol Evolut. https://doi.org/10.1016/j.tree.2006.01.006
Traveset A (1998) Effect of seed passage through vertebrate frugivores’ guts on germination: a review. Perspect. Plant Ecol Evol Syst 1(2):151–190. https://doi.org/10.1078/1433-8319-00057
Westcott DA, Fletcher CS (2011) Biological invasions and the study of vertebrate dispersal of plants: opportunities and integration. Acta Oecol 37:650–656. https://doi.org/10.1016/j.actao.2011.04.007
Zar JH (2010) Biostatistical analysis, 5th edn. Prentice Hall, Hoboken, New Jersey
Acknowledgements
We are thankful to all the volunteers, students, and colleagues for helping us with the field work. Lucrecia Pearson assisted with the English version of this typescript. We are grateful to the staff of the Natural Reserve of Laguna de Llancanelo and that of the Natural Reserve Caverna de las Brujas-Bardas Blancas village. Diego Zeverini and Paula Cornejo provided advice and assistance with laboratory work and Lorena Bonjour assisted with botanical identification. We thank two anonymous reviewers for their valuable comments on this paper. We acknowledge the continuous support of our respective institutions: IADIZA-CONICET and CCT Mendoza of Argentina and the Center of Applied Ecology and Sustainability (CAPES) of Chile.
Funding
This study was supported by the Rufford Foundation (21499–1), Sociedad Argentina para el Estudio de los Mamíferos (Osvaldo Reig Postgraduate Award 2018), Agencia Nacional de Promoción Científica y Tecnológica (PICT 4504/2017), and ANID PIA/BASAL FB0002.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by S.Y. Bobadilla, E. T. Olivares, F. M. Jaksic, R. A. Ojeda, and M. F. Cuevas. The first draft of the manuscript was written by S.Y. Bobadilla and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Communicated by: Thales Renato Ochotorena de Freitas
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bobadilla, S.Y., Olivares, E.T., Jaksic, F.M. et al. European rabbit (Oryctolagus cuniculus) as seed disperser in arid ecotones of Argentina: non-native herbivore facilitation of native and non-native plants. Mamm Res 68, 625–635 (2023). https://doi.org/10.1007/s13364-023-00712-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13364-023-00712-3