Abstract
Interleukin-18 (IL-18) is one of the immunomodulatory cytokines that plays an important role in cellular functions against tumor development and progression. IL-18 (-607) C/A and (-0137) G/C gene promoter polymorphisms and their haplotypes variants are associated with risk of various cancers. We evaluated a possible association of IL-18 (-607) C/A and (-137) G/C gene promoter polymorphisms in the susceptibility to oral squamous cell carcinoma (OSCC). A total number of 272 patients with OSCC and 185 healthy volunteers were genotyped for the IL-18 (-607) C/A and (-137) G/C polymorphism. Polymorphism variants were examined by using tetra-primer amplification refractory mutation system (T-ARMS). Genotype frequencies were evaluated by chi-square test and odds ratio (OR) relative risk. IL-18 (-137) G/C gene polymorphism was significantly associated with the risk of OSCC as compared to healthy volunteers (genotype GG vs GC: OR 2.238; 95 % CI 1.455-3.441; p = 0.0003 and allele G vs C: OR 1.984; 95 % CI 1.335–2.947; p = 0.0007). The genotype and allele frequencies of the IL-18 promoter -607 C/A polymorphism in OSCC patients were not significantly different than that in healthy controls (p > 0.05). Our results suggest that IL-18 -137 G/C polymorphism is significantly associated with the progression of oral cancer but -607 C/A polymorphism is not associated with this.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oral squamous cell carcinoma (OSCC) is the eighth most common human malignancy worldwide, with more than 4,260,000 cases and ∼128,000 deaths every year [1, 2]. In India, the incidence of oral cancer is 12.8/100,000 in men and 7.5/100,000 in women. Genetic alterations, smoking, and heavy alcohol consumption influence the susceptibility to oral oncogenesis [3]. Interleukin-18 is a pleiotropic proinflammatory cytokine also known as interferon-γ-inducing factor. It is a member of the interleukin-1 (IL-1) super family and is produced by numerous immune and non-immune cells which regulate both innate and adaptive immune responses [4, 5]. Interleukin-18 (IL-18) is a key regulator for the development of oral cancer because of its modulation of cycle progression; it also reduces cell viability and induces apoptosis. The expression and serum level of IL-18 have been reported to increase in various cancers [6–8].
The human IL-18 gene is composed of six exons and five introns located on chromosome 11q22.2–q22.3. Several single nucleotide polymorphisms (SNPs) have been identified in the promoter region of IL-18 gene, but have only two functional gene polymorphisms -607A/C and -137G/C in its promoter region [9]. The IL-18 -607 C to A transversion disrupts a cyclic AMP (cAMP) protein-binding site, while -137 G to C substitution abolishes a histone 4 transcription factor-1 (H4TF-1) nuclear factor-binding site. Individuals with habit of using tobacco (chewing and smoking) and alcohol are more prone to develop oral cancer [10–13]. Several studies have also reported that genetic polymorphisms are responsible for the progression of oral cancer and other malignancy, such as breast cancer [14–21]. However some studies have shown that it is not associated with head and neck squamous cell carcinoma [22, 23]. The purpose of this study is to investigate the genetic variations at positions -607C/A and -137G/C of IL-18 and their interaction in patients with OSCC and healthy controls.
Materials and methods
This case–control study was done on registered patients of Gandhi Memorial and Associated Hospitals, King George’s Medical University, Lucknow. Two hundred seventy two OSCC patients and 185 controls were recruited from the Department of Surgical Oncology. The selection criterion for patients was their OSCC diagnosis that had been histologically/pathologically confirmed. The calculated mean age of cases was 47.67 (SD ± 12.67) years. The control group comprised 185 healthy volunteers who visited with patients or for the general health checkup in our medical university. Inclusion criteria for controls were no evidence of any abnormal growth suggestive of cancer in the individual and family history of any types of cancer or other serious diseases. The control group had a mean age of 43.1 (SD ± 8.31) years. Informed written consent was obtained from all subjects. Ethical clearance was obtained following the guidelines set down by the institute’s ethical committee. Clinical data, including disease stage and history of comorbid conditions (heart disease including myocardial infarct, liver disease, kidney disease, peripheral vascular disease, etc.) was extracted from the patients’ charts.
Blood collection and molecular analyses
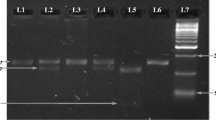
Blood samples (4 ml) were collected in EDTA tubes from both cases and healthy controls and stored at −80 °C until DNA extraction. Genomic DNA extraction from blood samples were carried out using the salting out method [24]. Genotyping of IL-18 -607 (C/A) polymorphism was performed by using tetra-primer amplification refractory mutation system (T-ARMS) [25]. IL-18 (-607) C/A and (-137) G/C promoter region SNP were analyzed by using genomic DNA isolated from blood. The PCR was performed in a total volume of 20 μl consisting 40 ng genomic DNA, 1 picomole/primers at concentration of 1×, 1× PCR master mixture. The amplification was carried out by an initial denaturation at 94 °C for 5 min, followed by 35 cycles of 94 °C for 30 s, 56 °C for 30 s, and 72 °C for 45 s, with a final extension of 72 °C for 5 min. In each polymorphic position, a common reverse primer and two sequence-specific forward primers were used. For the IL-18 (-607) C/A promoter region-specific PCR, a common reverse primer 5′-TAA CCT CAT TCA GGA CTT CC-3′ and two sequence-specific forward primers 5′-GTT GCA GAA AGT GTA AAA ATT ATT AC-3′ and 5′-GTT GCA GAA AGT GTA AAA ATT ATT AA-3′ were used. An amplification product of 196 bp was obtained. A control forward primer 5′-CTT TGC TAT CAT TCC AGG AA-3′ was used for amplification of a 301 bp fragment covering the polymorphic site (Table 1, Fig. 1). This product was provided as an internal positive amplification control. For the IL-18 (-137) G/C promoter region specific PCR, a common reverse primer 5′-AGG AGG GCA AAA TGC ACT GG-3′ and two sequence-specific forward primers 5′-CCC CAA CTT TTA CGG AAG AAA AG-3′ and 5′-CCC CAA CTT TTA CGG AAG AAA AC-3′ were used to amplify a 261-bp product. A control forward primer 5′-CCA ATA GGA CTG ATT ATT CCG CA-3′ was used for amplification of 446-bp product (Table 1, Fig. 2). This fragment served as an internal positive amplification control covering the polymorphic site. Amplification products were identified in all cases using 2 % agarose gel electrophoresis. The specific products generated by PCR from homozygous individuals consisted of only one DNA fragment and those from heterozygous individuals consisted of two specific DNA fragments. There were three genotypes named as AA, AC, and CC at position -607, and three named GG, GC, and CC at position -137 [18].
Statistical analysis
Demographic and clinical data between groups were compared by chi-square test. IL-18 genotype and allele frequencies of the OSCC patients were compared to the respective frequencies of the control groups using the chi-square test. Odds ratios (ORs) were calculated to estimate relative risk conferred by a particular allele and genotype. ORs are given with 95 % confidence interval (CI). P value was considered significant at <0.05.
Results
Characteristics of the study population
The demographic profile of OSCC patients and healthy controls are shown in (Table 2). The demographic profile included gender, age, TNM staging of OSCC, and various habitual risks which are known to be contributing factors in the progression of OSCC. Our study covered 272 OSCC cases, including 219 males and 53 females, their ages ranging from 20 to 70 years and 185 healthy controls, including 142 males and 43 females, their ages ranging from 20 to 70 years. In this study, we observed that smoking and tobacco chewing are significantly associated, while alcohol consumption is not significantly associated with OSCC, as shown in Table 2. The frequency of various tumor stage, tumor T status, lymph node status, metastasis, and grades among the patients is shown in Table 2. Stages I and II were present in 64.34 % patients, whereas 35.66 % patients belonged to stages III and IV. The other statuses of tumor were tumor T status (≤T2 71 %, >T2 29 %), lymph node status (N0 76.47 % and N1 + N2 23.53 %), metastasis (M0 98.53 % and M1 1.47 %), and cell differentiated grade (grade 1: 10.66 % and >grade 1: 89.34 %), as shown in Table 2.
The genotype and allele frequencies of IL-18 promoter
The genotype and allele frequencies of the IL-18 (-607) C/A and (-137) G/C polymorphisms among the cases and controls are shown in Table 3. The frequencies of the CC, CA, and AA genotypes of IL-18 (-607) C/A were 29.04, 56.62, and 14.34 % in the study cases and were 35.14, 51.89, and 12.97 % in controls, respectively, and the frequencies of the GG, GC, and CC genotypes of IL-18 (-137) G/C were 82.25, 18.75, and 0.00 % in the study cases and 65.65, 34.05, and 00 % in controls, respectively (Table 3). There were significant differences in the genotype and allele frequencies of the IL-18 (-137) G/C polymorphism between OSCC patients and control groups. The difference between OSCC cases and controls was statistically significant in heterozygous (GC) and homozygous (GG) genotype (OR 2.238, 95 % CI 1.455–3.441, p = 0.0003). C allele was significantly increased as compared with the G allele (OR 1.984, 95 % CI 1.335–2.947, p = 0.0007) as shown in Table 3. However, genotype and allele frequencies of the IL-18 (-607) C/A polymorphism in OSCC patients were not significantly different from healthy controls (p > 0.05) as shown in Table 3.
Association between OSSC and genotypes with environmental factors
This study also explored the possible association of environmental risk factors with IL-18 (-607) A/C and (-137) G/C gene polymorphisms on OSCC susceptibility. The genotype and allele frequencies of the IL-18 (-137) G/C were statistically significant (p < 0.05) among subjects not exposed to susceptible environmental factors (nonsmoking, nontobacco, nonalcoholic) and those exposed to these environmental factors (smokers and tobacco chewing). However, IL-18 (-607) A/C showed no significant (p > 0.05) association of these environmental factors with OSCC patients (Tables 4 and 5).
Association of genotypes with tumor stage/grade
We stratified the patients into two categories viz low-risk OSCC and high-risk OSCC groups. Low OSCC group comprised of stage I + II, ≤T2, N0, M0, and grade 1 and high-risk group comprised of patients with stage III + IV, >T2, N1 + N2, M1, and >grade 1. Low-risk OSCC was taken as a reference. There was no significant difference in between the genotype distribution of the IL-18 (-607) A/C and (-137) G/C gene polymorphism and tumor status (stage III + IV, >T2, N1 + N2, M1, and >grade 1) as shown in Table 6.
Discussion
Accumulating evidence points out that IL-18, a pleiotropic cytokine, enhances Th1 or Th2 immune response, depending on its cytokine milieu and genetic background [26]. In the presence of IL-12, IL-18 induces interferon-γ (IFN-γ) secretion from natural killer (NK) cells and T cells, strongly activates the Th1 immune responses, which is considered to be an essential cytokine in the host defense against tumor cells [27]. A recent study shows that the IL-18 is expressed in a biologically active form and regulates the immune response in gastrointestinal tract, but lymph node and liver metastasis are associated with neoplastic transformation during IL-18 decrease [26]. In contrast, IL-18 through vascular endothelial growth factor, enhances the immune response stimulation which increases the metastasis and tumor growth [27, 28]. In both condition, it may influence the clinical outcome of cancer patients [20, 29].
Cytokines play a complex role in malignant transformation [30]. The proinflammatory cytokine IL-18 showed protective effects against cancer [31–33]. Despite the conventional view of IL-18 as an anticancer agent, recent data suggest a procancerous activity for this multifunctional cytokine under some conditions [31]. IL-18 is an interferon-γ inducing cytokine produced during normal immune responses and cancers [21, 34–36]. Several studies on the other cancer types found that the IL-18 inhibited the angiogenesis and tumor suppression [21, 37–40]. Considering the above, the purpose of this study was to investigate whether the IL-18 promoter (-607) C/A and (-137) G/C genes polymorphism have any association with risk for OSCC.
Several studies have shown that polymorphisms of cytokine genes influence cytokine production, which may or may not be associated with susceptibility to certain diseases [11–22, 25]. IL-18 promoter polymorphisms (-607 or -137) were significantly associated with esophageal squamous cell carcinoma and prostate cancer in Chinese populations, colorectal cancer in a Greek population, ovarian cancer in native Hawaiians, and breast cancer in Iranians [17–20, 23]. In contrast, there was no significant association between IL-18 polymorphism and squamous cell carcinoma of the head and neck in Iran and squamous oral carcinoma in Greece [21, 22]. These discrepant results may be explained by the dual effects of IL-18 on tumor immune response or different tumor sites [22, 31]. In the present study, we evaluated two SNPs at positions -607 (C/A) and -137 (G/C) of IL-18 promoter in OSCC patients and healthy controls. Finally, we observed a significant increase in the GC genotypes and C allele at position -137 of the IL-18 gene in OSCC patients. There was no significant association of -607 genotypes and alleles with these.
In our study, heterozygous genotype (GC) of IL-18 (-137) G/C have shown 2.2 fold risk for OSCC which, further, suggested a role of genetic variation in IL-18 (-137) G/C promoter in the etiology of OSCC. Variant C allele of IL-18 (-137) was also associated with two fold risk for OSCC. This result is supported by different studies in various cancers like esophageal squamous cell carcinoma, prostate cancer, lung cancer, cervical cancer, and bladder cancer [17, 18, 41–43]. However, some studies found no such association in case of head and neck carcinoma, renal cell carcinoma, nasopharyngeal carcinoma, and recurrent spontaneous abortion [22, 44–46]. We observed that the IL-18 (-607) C/A genotype and allele were not associated with risk of OSCC. This observation is supported by different other studies on various cancers like oral cancer, head and neck carcinoma, esophageal squamous cell carcinoma, breast cancer, renal cell carcinoma, and recurrent spontaneous abortion [17, 21–23, 41, 44, 46]. Our result was inconsistent with other studies, which have found significant association in the colorectal cancer and lung cancer [41, 47].
The results of our study show that the IL-18 (-607) C/A polymorphism does not independently confer risk to OSCC, whereas (-137) G/C polymorphism significantly increased risk to oral cancer. This result is supported by various studies in different cancers like head and neck cancer and oral cancer [4, 6, 7, 13]. In our study, IL-18 (-607) C/A and (-137) G/C polymorphisms had no significant effect on tumor, lymph node, metastasis, and grade stage in OSCC patients. Our result was conflicting with other studies, which have found significant association in the oral cancer and bladder cancer [13, 43].
In conclusion, we have evaluated the association and clinical significance of IL-18 in OSCC. Our results showed significant association between IL-18 (-137) G/C gene polymorphism and OSCC. This polymorphism may be a factor that increases the susceptibility to risk of OSCC as well as a protective factor against progression of OSCC. Our results did not indicate any significant association between IL-18 (-607) C/A polymorphisms and the occurrence of OSCC. These results suggest that the IL-18 gene may be contribute to an inherited predisposition to OSCC, although additional studies with larger sample sizes will be necessary to confirm our findings.
References
World Health Organization. Strengthening the prevention of oral cancer: the WHO perspective. http://www.who.int/oral_health/publications/CDOE05_vol33_397_9/en/ (accessed on 26 Oct 2013)
International Agency for Research on Cancer. GLOBOCAN 2008. http://globocan.iarc.fr/factsheets/populations/factsheet.asp?uno=900 (accessed on 26 Oct 2013)
Williams HK. Molecular pathogenesis of oral carcinoma. J Clin Pathol. 2000;53:165–72.
Martone T, Bellone G, Pagano M, Beatrice F, Palonta F, et al. Constitutive expression of interleukin-18 in head and neck squamous carcinoma cells. Head Neck. 2004;26:494–503.
Srivastava S, Salim N, Robertson MJ. Interleukin-18: biology and role in the immunotherapy of cancer. Curr Med Chem. 2010;17:3353–7.
Nilkaeo A, Bhuvanath S. Role of interleukin-18 in modulation of oral carcinoma cell proliferation. Mediat Inflamm. 2006;3:67120.
Liu W, Han B, Sun B, Gao Y, Huang Y, et al. Overexpression of interleukin-18 induces growth inhibition, apoptosis and gene expression changes in a human tongue squamous cell carcinoma cell line. J Int Med Res. 2012;40:537–44.
Park S, Cheon S, Cho D. The dual effects of interleukin-18 in tumor progression. Cell Mol Immunol. 2007;4:329–35.
Giedraitis V, He B, Huang WX, Hillert J. Cloning and mutation analysis of the human IL-18 promoter: a possible role of polymorphisms in expression regulation. J Neuroimmunol. 2001;112:146–52.
Loyha K, Vatanasapt P, Promthet S, Parkin DM. Risk factors for oral cancer in northeast Thailand. Asian Pac J Cancer Prev. 2012;13:5087–90.
Ray JG, Ganguly M, Rao BS, Mukherjee S, Mahato B, et al. Clinico-epidemiological profile of oral potentially malignant and malignant conditions among areca nut, tobacco and alcohol users in Eastern India: a hospital based study. J Oral Maxillofac Pathol. 2013;17:45–50.
Gupta B, Ariyawardana A, Johnson NW. Oral cancer in India continues in epidemic proportions: evidence base and policy initiatives. Int Dent J. 2013;63:12–25.
Tsai H-T, Hsin C-H, Hsieh Y-H, Tang C-H, Yang S-F, et al. Impact of interleukin-18 polymorphisms -607A/C and -137G/C on oral cancer occurrence and clinical progression. PLoS One. 2013;8(12):e83572. doi:10.1371/journal.pone.0083572.
Teng YH, Liu TH, Tseng HC, Chung TT, Yeh CM, et al. Contribution of genetic polymorphisms of stromal cell-derived factor-1 and its receptor, CXCR4, to the susceptibility and clinicopathologic development of oral cancer. Head Neck. 2009;31:1282–8.
Chen MK, Chiou HL, Su SC, Chung TT, Tseng HC, et al. The association between hypoxia inducible factor-1alpha gene polymorphisms and increased susceptibility to oral cancer. Oral Oncol. 2009;45:e222–6.
Chen MK, Tsai HT, Chung TT, Su SC, Kao TY, et al. Glutathione S-transferase P1 and alpha gene variants; role in susceptibility and tumor size development of oral cancer. Head Neck. 2010;32:1079–87.
Wei YS, Lan Y, Liu YG, Tang H, Tang RG, Wang JC. Interleukin-18 gene promoter polymorphisms and the risk of esophageal squamous cell carcinoma. Acta Oncol. 2007;46:10906.
Liu Y, Lin N, Huang L, Xu Q, Pang G. Genetic polymorphisms of the interleukin-18 gene and risk of prostate cancer. DNA Cell Biol. 2007;26:6138.
Nikiteas N, Yannopoulos A, Chatzitheofylaktou A, Tsigris C. Heterozygosity for interleukin-18 -607 A/C polymorphism is associated with risk for colorectal cancer. Anticancer Res. 2007;27:384953.
Bushley AW, Ferrell R, McDuffie K, Terada KY, Carney ME, Thompson PJ, et al. Polymorphisms of interleukin [IL]- 1alpha, IL-1beta, IL-6, IL-10, and IL-18 and the risk of ovarian cancer. Gynecol Oncol. 2004;95:6729.
Vairaktaris E, Serefoglou ZC, Yapijakis C, Agapi C, Vassiliou S, Nkenke E, et al. The interleukin-18 -607A/C polymorphism is not associated with risk for oral cancer. Anticancer Res. 2007;27:4011–4.
Asefi V, Mojtahedi Z, Khademi B, Naeimi S, Ghaderi A. Head and neck squamous cell carcinoma (HNSCC) is not associated with interleukin-18 promoter gene polymorphisms, a case control study. J Laryngol Otol. 2009;123:4448.
Khalili-Azad T, Razmkhah M, Ghiam AF, Doroudchi M, Talei AR, Mojtahedi Z, et al. Association of interleukin-18 gene promoter polymorphisms with breast cancer. Neoplasma. 2009;56:225.
Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215.
Taheri M, Hashemi-Shahri SM, Hamzehnejadi M, Naderi M, Moazeni-Roodi A, Bahari G, et al. Lack of association between interleukin-18 -607 C/A gene polymorphism and pulmonary tuberculosis in Zahedan, southeast Iran. Prauge Med Rep. 2012;113:16–22.
Haghshenas MR, Hosseini SV, Mahmoudi M, Saberi-Firozi M, Farjadian S, Ghaderi A. IL-18 serum level and IL-18 promoter gene polymorphism in Iranian patients with gastrointestinal cancers. J Gastroenterol Hepatol. 2009;24:1119–22.
Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 is a unique cytokine that stimulates both Th1 and Th2 responses depending on its cytokine milieu. Cytokine Growth Factor Rev. 2001;12:53–72.
Kim KE, Song H, Kim TS, et al. Interleukin-18 is a critical factor for vascular endothelial growth factor-enhanced migration in human gastric cancer cell lines. Oncogene. 2007;26:1468–76.
Mojtahedi Z. Interleukin (IL)-18 may enhance Th1 response in early cancer but aggravate malignant disease in its later stages. Med Hypotheses. 2005;65:995–6.
Aggarwal BB, Shishodia S, Sandur SK, Pandey MK, Sethi G. Inflammation and cancer: how hot is the link? Biochem Pharmacol. 2006;72:1605–21.
Vidal-Vanaclocha F, Mendoza L, Telleria N, Salado C, Valcárcel M, Gallot N, et al. Clinical and experimental approaches to the pathophysiology of interleukin-18 in cancer progression. Cancer Metastasis Rev. 2006;25:417–34.
Lian H, Jin N, Li X, Mi Z, Zhang J, Sun L, et al. Induction of an effective anti-tumor immune response and tumor regression by combined administration of IL-18 and apoptin. Cancer Immunol Immunother. 2007;56:181–92.
Herzyk DJ, Bugelski PJ, Hart TK, Wier PJ. Preclinical safety of recombinant human interleukin-18. Toxicol Pathol. 2003;31:554–61.
Lebel-Binay S, Thiounn N, De Pinieux G, Viellefond A, Debré B, Bonnefoy JY, et al. IL-18 is produced by prostate cancer cells and secreted in response to interferons. Int J Cancer. 2003;106:827–35.
Riedel F, Adam S, Feick P, Haas S, Götte K, Hörmann K. Expression of IL-18 in patients with head and neck squamous cell carcinoma. Int J Mol Med. 2004;13:267–72.
Lissoni P, Brivio F, Rovelli F, Fumagalli G, Malugani F, Vaghi M, et al. Serum concentrations of interleukin-18 in early and advanced cancer patients: enhanced secretion in metastatic disease. J Boil Regul Homoest Agents. 2000;14:275–7.
Cao R, Farnebo J, Kurimoto M, Cao Y. Interleukin-18 acts as an angiogenesis and tumor suppressor. FASEB. 1999;13:2195–202.
Golab J. Interleukin-18-interferon Á inducing factor- · novel player in tumor immunotherapy? Cytokine. 2000;12:332–8.
Wang ZY, Gaggero A, Rubartelli A, Rosso O, Miotti S, Mizzanzanica D, et al. Expression of interleukin-18 in human ovarian carcinoma and normal ovarian epithelium: evidence for defective processing in tumor cells. In J Cancer. 2002;98:873–8.
Nilkaeo A, Bhuvanath S. Role of interleukin-18 in modulation of oral carcinoma cell proliferation. Mediat Inflamm. 2006;3:1–6.
Farjadfar A, Mojtahedi Z, Ghayumi MA. Interleukin-18 promoter polymorphism is associated with lung cancer: a case–control study. Acta Oncol. 2009;48:971–6.
Sobti RC, Shekari M, Tamandani DM, Malekzadeh K, Suri V. Association of interleukin-18 gene promoter polymorphismon the risk of cervix carcinogenesis in North Indian population. Oncol Res. 2008;17:159–66.
Jaiswal PK, Singh V, Srivastava P, Mittal RD. Association of IL-12, IL-18 variants and serum IL-18 with bladder cancer susceptibility in North Indian population. Gene. 2013;519:128–34.
Sáenz-López P et al. Impact of interleukin-18 polymorphisms -607 and -137 on clinical characteristics of renal cell carcinoma patients. Hum Immunol. 2010;71:309–13.
Farhat K, Hassen E, Bouzgarrou N, Gabbouj S, Bouaouina N, Chouchane L. Functional IL-18 promoter gene polymorphisms in Tunisian nasopharyngeal carcinoma patients. Cytokine. 2008;43:132–7.
Naeimi S, Ghiam AF, Mojtahedi Z, Dehaghani AS, Amani D, Ghaderi A. Interleukin-18 gene promoter polymorphisms and recurrent spontaneous abortion. Eur J Obstet Gynecol Reprod Biol. 2006;128:5–9.
Nikiteas N, Yannopoulos A, Chatzitheofylaktou A, Tsigris C. Heterozygosity for interleukin-18 -607A/C polymorphism is associated with risk for colorectal cancer. Anticancer Res. 2007;27:3849–53.
Acknowledgments
This work was financially supported by a grant from Indian Council of Medical Research (ICMR).
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singh, P.K., Ahmad, M.K., Kumar, V. et al. Effects of interleukin-18 promoter (C607A and G137C) gene polymorphisms and their association with oral squamous cell carcinoma (OSCC) in northern India. Tumor Biol. 35, 12275–12284 (2014). https://doi.org/10.1007/s13277-014-2538-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-014-2538-0