Abstract
The functional polymorphism A1082G in the gene (IL10) for interleukin-10 associated with risk of oral squamous cell carcinoma (OSCC). The present case–control study was to evaluate the possible association between IL10 A1082G gene and OSCC in north Indian population. Analysis of IL10 A1082G genotype in 232 OSCC cases and 221 healthy controls of comparable age, gender, smokers, tobacco chewing and alcohol consumption. IL10 A1082G status in cases and controls were evaluated by polymerase chain reaction and restriction fragment length polymorphism (PCR-RFLP). The frequencies of IL10 A1082G polymorphism AA, AG, GG genotypes were 29.74, 68.10 and 2.15% in OSCC cases and 57.46, 42.08 and 0.45% in healthy controls. The average frequency of G mutant allele was 36.20% in OSCC cases compared with 21.50% among the controls and this allele was associated with increased risk for OSCC cases. Heterozygous AG genotype was found statistically significant in OSCC cases than in controls (OR = 1.6, 95% CI = 1.1–2.2, P = 0.003), whereas homozygous mutant GG genotype was not found significant (OR = 4.7, 95% CI = 0.55–41.1, P = 0.2). Moreover, we found that G allele was significant in OSCC cases of tobacco chewing. The frequency of IL10 A1082G polymorphism G allele and AG genotype is associated with OSCC cases as compared with controls; this may be due to smoking and tobacco chewing. Our findings showed that in IL10 A1082G gene polymorphism AG genotype and G allele may participate in the progression of OSCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oral squamous cell carcinoma (OSCC) is the worldwide eighth common human malignancy with more than 4,260,000 patients and ˜128,000 deaths each year (Petersen 2005; Ferlay et al. 2010). The incidence of oral cancer in India is 12.8 per 100,000 in males and 7.5 per 100,000 in females. Among all the oral malignancies, OSCC accounts for more than 90% cases (Neville and Day 2002). Previous studies reveal that OSCC is the most commonly occurring malignancy which is responsible for morbidity and mortality (Williams 2000; Massano et al. 2006). Oral cancer is a multistep process which occurs due to factors like smoking, tobacco chewing, alcohol consumption, nutrition deficiency, genetic and environmental factors (Neville and Day 2002; McDowell 2006).
Interleukin-10 (IL-10) have been associated with increased risk for the development of OSCC, which is an immunosuppressant cytokine that inhibits activation and function of T cells; responsible for angiogenesis, thrombophilia autoimmune disease, inflammation and determines termination of inflammatory responses (de Vries 1995; Fortis et al. 1996). The encoding gene of IL10 is located on chromosome 1 (1q31-1q32). IL10 inhibits synthesis of cytokines and tumour necrosis factor-alpha (TNF- α) in activated macrophage and interferon gamma (IFN γ) by T cells, thus it plays major role in antiinflammatory responses (Vairaktaris et al. 2007a). The synthesis of cytokines is controlled genetically that differs among individuals as functions of polymorphisms within the regulatory region of various genes determine the transcriptional activation (Cantagrel et al. 1999; Vairaktaris et al. 2007b, 2007c; Yapijakis et al. 2009).
The IL10 promoter is composed of three biallelic polymorphisms at position −1082 from the transcription start site which affect the production of IL10 (Rasmussen et al. 2000). IL10 polymorphisms are found to be associated with breast cancer (McCarron et al. 2002), cervical cancer (Trabace et al. 2002), multiple myeloma (Savage et al. 2004), cutaneous malignant melanoma (Palli et al. 2005), oral squamous cell carcinoma (Rhodus et al. 2005), gastric carcinoma (Hatanaka et al. 2001) and hepatocellular carcinoma (Nagata et al. 2002). High expression levels of IL10 have been observed in OSCC cases and were associated with poor prognosis (Karcher et al. 1999; Fujieda et al. 1999). Genetic factors influence or regulate the level of expression of IL10 gene-like polymorphisms at location A1082G in the promoter region of the gene (Lin et al. 2003). Due to relation disequilibrium of polymorphic alleles, high or low expression of IL10 may be examined by studying single polymorphic position (Lin et al. 2003).
Thus, the presence of G allele of the IL10 A1082G polymorphism is associated with higher production of IL10 (Lin et al. 2003; Yilmaz et al. 2005). The allele G-transmitting genotypes have been found to be significantly frequent in the cases of lung squamous cell carcinoma (Seifart et al. 2005). IL10 A1082G polymorphism in AA genotype was significantly frequent in prostate cancer and cutaneous malignant melanoma (Howell et al. 2001; McCarron et al. 2002). IL10 A1082G was not found to be associated with oesophageal squamous cell carcinoma and gastroesophageal junction adenocarcinoma (Savage et al. 2004; Guo et al. 2005). In oriental Asians genotypes, high frequency of G allele ranges between 21–84% and 20–52% in Caucasians population (Meenagh et al. 2002; Savage et al. 2004; Guo et al. 2005). The functional polymorphisms IL10 A1082G have influence on the gene expression and associated with risk for OSCC. The present case–control study was to evaluate the possible association between IL10 A1082G and OSCC in north Indian population.
Materials and methods
Samples collection
In this study the population consisted of 232 cases of OSCC and 221 healthy controls. Samples were collected from Department of Oral Pathology and Microbiology, King George’s Medical University, Lucknow, India. This study was carried out between March 2012 and June 2014. Ethical approval was obtained from the institutional ethical committee of King George’s Medical University, Lucknow, India. Informed written consent was obtained from all subjects.
Inclusion and exclusion criteria
All cases were divided into two subgroups according to OSCC status: (i) patients with early cancer stage (stages I and II) and (ii) patients with advanced cancer stage (stages III and IV), according to the American Joint Committee for Cancer staging and end-results reporting (AJCC 1992, Cancer staging manual, http://seer.cancer.gov/manuals/historic/comp_stage1.1.pdf). Clinicopathologic information on each case, including age, gender, alcohol and tobacco usage, tumour location, treatment, clinical and pathologic stages, presence or absence of tumoural recurrence and survival of the patients were obtained from medical records and tumour registries. All cases included presented with primary squamous cell carcinoma of the oral cavity. Cases with a former positive history of other type of cancer or with oral histological lesions other than OSCC were excluded. The control group included healthy blood donors who were recruited by health care professionals and volunteers. Controls were matched to cases with regard to ethnicity, gender, age and a low-risk working environment. Healthy individuals with a positive history of cancer were also excluded from serving as controls as well as with a former positive history of other type of cancer or with oral histological lesions other than OSCC were also excluded.
DNA isolation
Genomic DNA extraction from 232 cases of OSCC and 221 healthy controls was done using a commercially available DNA extraction kit (Medox, Chennai, India) and was stored at −20°C.
IL10 gene A1082G polymorphism
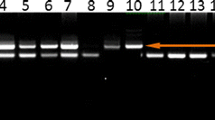
The IL10 A1082G polymorphism was analysed by PCR followed by RFLP. Genomic DNA was amplified (Applied Biosystems, Veriti, Singapore) using the following PCR conditions: 94°C for 4 min, 35 cycles at 94°C for 50 s, 54°C for 1 min, 72°C for 55 s, and finally 72°C for 5 min. The primers used for amplification of the IL10 (A1082G) gene polymorphisms were as follows: forward primer 5\(^{\prime }\)-CTCGCTGCAACCCAACTGGC-\(3^{\prime }\) and reverse primer 5\(^{\prime }\)-TCTTACCTATCCCTACTTCC-3\(^{\prime }\) (Rhodus et al. 2005). Amplification was performed with 20 μL PCR reaction mixture containing 200 ng template DNA, 10 pmol of each primer and 2 × PCR master mixes (Fermentas, Sankt Leon-Rot, Germany). Amplification products were identified in all samples using 2% agarose gel electrophoresis. Subsequently the PCR products were subjected to digestion by MnlI enzyme (NEB, Hertfordshire, UK) to screen for the IL10 A1082G. The enzymatic mixture contained 1 μL restriction enzyme, 1 μL 10 × buffer, 6 μL PCR products and 2 μL distilled water; the mixture was incubated for 5 h at 37°C for digestion. The digested product was electrophoresed on 3% agarose gel electrophoresis at 80 volts for 1 h. In the case of A1082G, an undigested 139 bp band showed wild-type AA genotype, while two bands of 106 and 33 bp confirmed mutant GG genotype and three bands of 139, 106 and 33 bp were detected in the heterozygous AG genotype (Manoochehr et al. 2008) (figure 1).
Statistical analysis
The significance of this study was evaluated by chi-square test. Odds ratio (OR) was calculated as an estimate of relative risk of having disease according to the relative frequency of different genotypes among the cases as well as the controls. ORs are given with 95% confidence interval (CI). P value was considered significant at <0.05. The value was expressed in mean ± (Standard deviation) SD.
Results
In our study, we recruited 232 OSCC cases, including 137 males and 95 females, age ranging from 26 to 60 years. Calculated mean age of cases were 37.11 ± 6.10. The mean age of 221 healthy controls (133 males and 88 females) were 28.28 ± 5.32. All the cases and controls were successfully genotyped by PCR-RFLP. The average IL10 A1082G AA, AG and GG genotype frequencies in total OSCC cases were 29.7, 68.1, 2.15%, and 57.4, 42.0, 0.45% in healthy controls. The frequency of IL10 A1082G and statistical analysis of the cases and controls are shown in tables 1 and 2. The IL10 A1082G high expression mutant G allele frequency observed was 36.20% in OSCC cases and frequency was 21.50% in healthy controls (table 2).
We correlated the IL10 genotypes of OSCC patients under different groups, i.e. age, gender, smoking, tobacco chewing, alcohol consumption, clinical tumour stage (I + II and III + IV), tumour grade (grade 1/2 and grade 3), lymph node (yes and no) and metastasis (M0 and M1) are shown in tables 3 and 4. It is observed that smoking, tobacco chewing and alcohol consumption are risk factors for OSCC, we included these factors in our study. The distribution of patient biological characteristics and selected risk factors are shown in tables 1, 3 and 4.
In this study among 232 cases and 221 controls, we found that IL10 A1082G genotype was present among 158 cases and 93 controls, the GG genotype was present in five OSCC cases and one in healthy controls and the AA genotype was present among 69 OSCC cases and 127 in healthy controls (table 2). The heterozygous AG genotypes were more prevalent in OSCC cases than healthy controls and difference between OSCC cases and controls was statistically significant (OR = 1.6, 95% CI = 1.1–2.2, P = 0.003), whereas homozygous GG genotype was not significant. The frequency of mutant G allele in IL10 A1082G was significantly higher in OSCC cases than in healthy controls (OR = 1.6, 95% CI = 1.2–2.2, P = 0.0004); suggesting that individual alleles were associated with OSCC cases (table 2).
This case–control study signifies the frequency of mutant G allele and heterozygous AG genotype are extremely associated with risk for tobacco chewing in OSCC patients (P=<0.0001 and P=<0.0001) and heterozygous AG genotype statistically significant for smokers in OSCC patients (P =<0.019) but it was not significant in alcohol consumption (table 3). Further, on grouping them under clinical tumour stage (I + II and III + IV), it was evident that heterozygous AG genotype was significant in OSCC patients (P = 0.035). Similarly, in case of metastasis (M0 and M1), we found that homozygous GG genotype was highly significant (P = 0.003) but we did not find any correlation in tumour grade and lymph node with IL10 in OSCC cases (table 4).
Discussion
Previous studies revealed that increased levels of IL10 have been reported in patients with OSCC, suggesting that this pleiotropic cytokine might play imperative role in malignancy (Fortis et al. 1996; Fujieda et al. 1999; Karcher et al. 1999). The gene expression of inflammation causing factors is affected by functional DNA polymorphisms which may confer susceptibility, progression and severity of disease (Savage et al. 2004; Tsai et al. 2005; Xu et al. 2005; Vairaktaris et al. 2005, 2007d). The combined effects in a series of gene polymorphisms of factors participating in the same biological pathway may provide study which may illustrate the relative significance of each polymorphic site in the final clinical result. Previously few studies have reported increased serum/ saliva levels of IL10 in OSCC cases (Jablonska et al. 1997; St John et al. 2004; Palli et al. 2005). It has been reported that there are highly significant contributions of cytokines, specifically IL6, IL10 and TNF α, which have an imperative role in the occurrence of OSCC cases (Fortis et al. 1996; Petersen 2005). IL10 A1082G has been found to promote tumour proliferation by suppressing the immune and inflammatory responses (Fortis et al. 1996; Hatanaka et al. 2001; Nagata et al. 2002).
The expression of IL10 A1082G is affected by SNP in its promoter region. In the present study, IL10 A1082G in heterozygous AG genotype was significantly frequent in OSCC cases, regardless of their family history of cancer, thrombophilia and cancer stages. The high expression of G allele behaves as a dominant trait. Based on such results, this may be concluded that high levels of IL10 A1082G gene expression may have an important role in increasing susceptibility for development of oral cancer (Vairaktaris et al. 2008). This is in accordance with the previously observed increase of IL10 A1082G gene expression in OSCC cases (Fujieda et al. 1999; Karcher et al. 1999).
In India, studies have been done on IL10 and found it to play an important role in instigating different types of diseases like atopic asthama, asthama, type 2 diabetes mellitus, chronic hepatitis and gastric cancer (Chatterjee et al. 2005; Chand-Bhayal et al. 2012; Saxena et al. 2013; Srivastava et al. 2014; Raeiszadeh et al. 2015). Previous studies reported that IL10 genotypes were associated with increased risk of gastric cancer, type 2 diabetes mellitus, asthama and chronic hepatitis B (Chand-Bhayal et al. 2012; Saxena et al. 2013; Srivastava et al. 2014; Raeiszadeh et al. 2015).
In our study IL10 gene polymorphism is found to be associated with OSCC in north Indian population. The present findings are in accordance with previously reported observed increase of IL10 genotype in OSCC in Greek and German populations (Vairaktaris et al. 2008). Previous studies infer that the mutant G allele association was studied on the OSCC in early stages rather than advanced stages (Vairaktaris et al. 2008) and showed that IL10 may increase susceptibility to oral cancer in a synergistic manner with other cytokines. IL10 inhibits some cytokines, it may act as a cooperative growth factor. Such a role of IL10 in OSCC might be extremely potent (Vairaktaris et al. 2008).
In the studied individuals, a significant difference was observed in the total group and subgroups of OSCC patients. IL10 A1082G genotype AG heterozygous was significantly more frequent in patients with OSCC, in spite of their family history of either cancer and regardless of their cancer stage. Similar pattern was also observed in OSCC patients with smoking, tobacco chewing and clinical tumour stage. We found that effect of the high expression G allele is more pronounced in tobacco chewing and metastasis.
According to the above finding, this study showed that the significant contributions of cytokines IL10 A1082G the occurrence of OSCC and for the first time, we report IL10 in north Indian population. In conclusion, the most important finding of our study implies that IL10 A1082G polymorphisms AG genotype and mutant G allele may participate in the progression of OSCC in north Indian population.
References
Cantagrel A., Navaux F., Loubet-Lescoulie P., Nourhashemi F., Enault G., Abbal M. et al. 1999 Interleukin-1 beta, interleukin-1 receptor antagonist, interleukin-4, and interleukin-10 gene polymorphisms: relationship to occurrence and severity of rheumatoid arthritis. Arthritis Rheum. 42, 1093–1100.
Chand-Bhayal A., Krishnaveni D., Pandu Ranga Rao K., Prabhakar B., Vidyasagar A., Murali Krishna B. et al. 2012 Association of interleukin-10 promoter polymorphism (–1082 G/A) and gastric cancer in Andhra Pradesh population of south India. Iran J. Cancer Prev. 3, 117–123.
Chatterjee R., Batra J., Kumar A., Mabalirajan U., Nahid S., Niphadkar P. V. and Ghosh B. 2005 Interleukin-10 promoter polymorphisms and atopic asthma in north Indians. Clin. Exp. Allergy 35, 914–919.
de Vries J. E. 1995 Immunosuppresive and anti-inflammatory properties of interleukin-10. Ann. Med. 27, 537–541.
Ferlay J., Shin H. R., Bray F., Forman D., Mathers C. and Parkin D. M. 2010 Cancer incidence and mortality worldwide. International Agency for Research on Cancer GLOBOCAN 2008 (http://globocan.iarc.fr/factsheets/populations/factsheet.asp?uno=900).
Fortis C., Foppoli M., Gianotti L., Galli L., Citterio G., Consogno G., Gentilini O. and Braga M. 1996 Increased interleukin-10 serum levels in patients with solid tumors. Cancer Lett. 104, 1–5.
Fujieda S., Sunaga H., Tsuzuki H., Fank G. K. and Saito H. 1999 IL 10 expression is associated with the expression of platelet-derived endothelial cell growth factor and prognosis in oral and oropharyngeal carcinoma. Cancer Lett. 136, 1–9.
Guo W., Wang N., Wang Y. M., Li Y., Wen D. G., Chen Z. F. et al. 2005 Interleukin-10 –1082 promoter polymorphism is not associated with susceptibility to esophageal squamous cell carcinoma and gastric cardiac adenocarcinoma in a population of high-incidence region of north China. World J. Gastroenterol. 11, 858–862.
Hatanaka H., Abe Y., Naruke M., Tokunaga T., Oshika Y., Kawakami T. et al. 2001 Significant correlation between interleukin 10 expression and vascularization through angiopoietin/TIE2 networks in nonsmall cell lung cancer. Clin. Cancer Res. 7, 1287–1292.
Howell W. M., Turner S. J., Bateman A. C. and Theaker J. M. 2001 IL 10 promoter polymorphisms influence tumor development in cutaneous Malignant melanoma. Genes Immun. 2, 25–31.
Jablonska E., Piotrowski L. and Grabowska Z. 1997 Serum levels of IL-1b, IL-6, TNF α, sTNF-RI and CRP in patients with oral cavity cancer. Pathol. Oncol. Res. 3, 126–129.
Karcher J., Reisser C., Daniel V. and Herold-Mende C. 1999 Cytokine expression of transforming growth factor-beta 2 and interleukin-10 in squamous cell carcinomas of the head and neck: comparison of tissue expression and serum levels. HNO 47, 879–884.
Lin M. T., Storer B., Martin P. J., Tseng L. H., Gooley T., Chen P. J. and Hansen J. A. 2003 Relation of an interleukin-10 promoter polymorphism to graft-versus-host disease and survival after hematopoietic-cell transplanation. N. Engl. J. Med. 349, 2201–2210.
Manoochehr R., Simin K. and Maryam B. 2008 Interleukin-10 gene polymorphisms and susceptibility to brucellosis in Iranian patients. Iran. J. Immunol. 5, 131–135.
Massano J., Regateiro F. S., Januario G. and Ferreira A. 2006 Oral squamous cell carcinoma: review of prognostic and predictive factors. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 102, 67–76.
McCarron S. L., Edwards S., Evans P. R., Gibbs R., Dearnaley D. P., Dowe A. et al. 2002 Influence of cytokine gene polymorphisms on the development of prostate cancer. Cancer Res. 62, 3369–3372.
McDowell J. D. 2006 An overview of epidemiology and common risk factors for oral squamous cell carcinoma. Otolaryngol. Clin. North. Am. 39, 277–294.
Meenagh A., Williams F., Ross O. A., Patterson C., Gorodezky C., Hammond M. et al. 2002 Frequency of cytokine polymorphisms in populations from western Europe, Africa, Asia, the middle East and south America. Hum. Immunol. 63, 1055– 1061.
Nagata J., Kijima H., Hatanaka H., Tokunaga T., Takagi A., Mine T. et al. 2002 Correlation between interleukin 10 and vascular endothelial growth factor expression in human esophageal cancer. Int. J. Mol. Med. 10, 169–172.
Neville B. W. and Day T. A. 2002 Oral cancer and precancerous lesions. CA Cancer J. Clin. 52, 195–215.
Palli D., Saieva C., Luzzi I., Masala G., Topa S., Sera F. et al. 2005 Interleukin-1 gene polymorphisms and gastric cancer risk in a high-risk Italian population. Am. J. Gastroenterol. 100, 1941–1948.
Petersen P. E. 2005 Strengthening the prevention of oral cancer: the WHO perspective. Commun. Dent. Oral. Epidemiol. 33, 397–399.
Raeiszadeh J. S., Mahesh P. A., Jayaraj B. S., Holla A. D., Vishweswaraiah S. and Ramachandra N. B. 2015 IL 10 and IL17F promoter single nucleotide polymorphism and asthma: a case–control study in south India. Lung Jun. 25 (In press).
Rasmussen S. K., Urhammer S. A., Jensen J. N., Hansen T., Borch-Johnsen K. and Pedersen O. 2000 The –238 and –308 G →A polymorphisms of the tumor necrosis factor alpha gene promoter are not associated with features of the insulin resistance syndrome or altered birth weight in Danish Caucasians. J. Clin. Endocrinol. Metab. 85, 1731–1734.
Rhodus N. L., Ho V., Miller C. S., Myers S. and Ondrey F. 2005 NF-kappaB dependent cytokine levels in saliva of patients with oral preneoplastic lesions and oral squamous cell carcinoma. Cancer Detect. Prev. 29, 42–45.
Savage S. A., Abnet C. C., Haque K., Mark S. D., Qiao Y. L., Dong Z. W. et al. 2004 Polymorphisms in interleukin -2, -6, and -10 are not associated with gastric cardia or esophageal cancer in a high risk chinese population. Cancer Epidemiol. Biomarkers Prev. 13, 1547–1549.
Saxena M., Srivastava N. and Banerjee M. 2013 Association of IL-6, TNF- α and IL 10 gene polymorphisms with type 2 diabetes mellitus. Mol. Biol. Rep. 40, 6271–6279.
Seifart C., Plagens A., Dempfle A., Clostermann U., Vogelmeier C., von Wichert P. and Seifart U. 2005 TNF-alpha, TNF-beta, IL -6, and IL 10 polymorphisms in patients with lung cancer. Dis. Markers 21, 157–165.
Srivastava M., Ranjan A., Choudhary J. K., Tripathi M. K., Verma S., Dixit V. K. et al. 2014 Role of proinflammatory cytokines (interferon gamma) and anti-inflammatory cytokine (interleukin-10) gene polymorphisms in chronic hepatitis B infection: an Indian scenario. J. Interferon Cytokine Res. 34, 547–551.
St John M. A., Li Y., Zhou X., Denny P., Ho C. M., Montemagno C. et al. 2004 Interleukin 6 and interleukin 8 as potential biomarkers for oral cavity and oropharyngeal squamous cell carcinoma. Arch. Otolaryngol. Head Neck Surg. 130, 929–935.
Trabace S., Brioli G., Lulli P., Morellini M., Giacovazzo M., Cicciarelli G. and Martelletti P. 2002 Tumor necrosis factor gene polymorphism in migraine. Headache 42, 341–345.
Tsai M. H., Chen W. C., Tsai C. H., Hang L. W. and Tsai F. J. 2005 Interleukin-4 gene, but not the interleukin-1 beta gene polymorphism, is associated with oral cancer. J. Clin. Lab. Anal. 19, 93–98.
Vairaktaris E., Yapijakis C., Wiltfang J., Ries J., Vylliotis A., Derka S., Vasiliou S. and Neukam F. W. 2005 Are factor V and prothrombin mutations associated with increased risk of oral cancer? Anticancer Res. 25, 2561–2565.
Vairaktaris E., Yapijakis C., Serefoglou Z., Derka S., Vassiliou S., Nkenke E. et al. 2007a The interleukin-8 (–251A/T) polymorphism is associated with increased risk for oral squamous cell carcinoma. Eur. J. Surg. Oncol. 33, 504–507.
Vairaktaris E., Yannopoulos A., Vassiliou S., Serefoglou Z., Vylliotis A., Nkenke E. et al. 2007b Strong association of interleukin-4 (–590 C/T) polymorphism with increased risk for oral squamous cell carcinoma in Europeans. Oral. Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 104, 796– 802.
Vairaktaris E., Serefoglou Z., Yapijakis C., Stathopoulos P., Vassiliou, S., Derka S. et al. 2007c The interleukin-1 beta gene polymorphism + 3953 C/T is not associated with risk for oral . Anticancer Res. 27, 3981–3986.
Vairaktaris E., Yapijakis C., Tsigris C., Vassiliou S., Derka S., Nkenke E. et al. 2007d Association of angiotensin converting enzyme gene insertion/deletion polymorphism with increased risk for oral cancer. Acta Oncol. 46, 1097–1102.
Vairaktaris E., Yapijakis C., Serefoglou Z., Derka S., Vassiliou S., Nkenke E. et al. 2008 The interleukin-10 (−1082A/G) polymorphism is strongly associated with increased risk for oral squamous cell carcinoma. Anticancer Res. 28, 309– 314.
Williams H. K. 2000 Molecular pathogenesis of oral squamous carcinoma. Mol. Pathol. 53, 165–172.
Xu J., Lowey J., Wiklund F., Sun J., Londmark F., Hsu F. C. et al. 2005 The interaction of four genes in the inflammation pathway significantly predicts prostate cancer risk. Cancer Epidemiol. Biomarkers Prev. 14, 2563–2568.
Yapijakis C., Serefoglou Z., Vylliotis A., Nkenke E., Derka S., Vassiliou S. et al. 2009 Association of polymorphisms in tumor necrosis factor alpha and beta genes with increased risk for oral cancer. Anticancer Res. 29, 2379–2386.
Yilmaz V., Yentur S. P. and Saruhan-Direskeneli G. 2005 IL -12 and IL 10 polymorphisms and their effects on cytokine production. Cytokine 30, 188–194.
Acknowledgements
This study was supported by Department of Biochemistry, King George’s Medical University, Lucknow, Uttar Pradesh, India.
Author information
Authors and Affiliations
Corresponding author
Additional information
[Hussain S. R., Ahmad M. K., Mahdi A. A., Naqvi H., Ahmad M. W., Srivastava S., Nigam K. and Gupta S. 2016 Association of interleukin-10 (A1082G) gene polymorphism with oral squamous cell carcinoma in north Indian population. J. Genet. 95, xx–xx]
Rights and permissions
About this article
Cite this article
HUSSAIN, S.R., AHMAD, M.K., MAHDI, A.A. et al. Association of interleukin-10 (A1082G) gene polymorphism with oral squamous cell carcinoma in north Indian population. J Genet 95, 249–255 (2016). https://doi.org/10.1007/s12041-016-0626-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12041-016-0626-1