Abstract
The eye lens is considered to be among the most radiosensitive human tissues. Brain CT scans may unnecessarily expose it to radiation even if the area of clinical interest is far from the eyes. The aim of this study is to implement a bismuth eye lens shielding system for Head-CT acquisitions in these cases. The study is focused on the assessment of the dosimetric characteristics of the shielding system as well as on its effect on image quality. The shielding system was tested in two set-ups which differ for distance (“contact” and “4 cm” Set up respectively). Scans were performed on a CTDI phantom and an anthropomorphic phantom. A reference set up without shielding system was acquired to establish a baseline. Image quality was assessed by signal (not HU converted), noise and contrast-to-noise ratio (CNR) evaluation. The overall dose reduction was evaluated by measuring the CTDIvol while the eye lens dose reduction was assessed by placing thermoluminescent dosimeters (TLDs) on an anthropomorphic phantom. The image quality analysis exhibits the presence of an artefact that mildly increases the CT number up to 3 cm below the shielding system. Below the artefact, the difference of the Signal and the CNR are negligible between the three different set-ups. Regarding the CTDI, the analysis demonstrates a decrease by almost 12 % (in the “contact” set-up) and 9 % (in the “4 cm” set-up). TLD measurements exhibit an eye lens dose reduction by 28.5 ± 5 and 21.1 ± 5 % respectively at the “contact” and the “4 cm” distance. No relevant artefact was found and image quality was not affected by the shielding system. Significant dose reductions were measured. These features make the shielding set-up useful for clinical implementation in both studied positions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The lens of the eye is one of the most radiosensitive tissue of the human body [1, 2]. Radiation-induced cataract has been highlighted as a possible result of interventional procedures whereas other studies have shown the risk of lens opacities in populations exposed to even low doses of ionizing radiation, including astronauts and patients undergoing computed tomography (CT) [3–6].
Recently, in light of the results of an epidemiological analysis [7], the International Commission on Radiation Protection (ICRP) has lowered the dose threshold for these effects and now recommends, for exposed workers, a dose equivalent limit of 20 mSv to lens in a year, averaged over a 5-year period, not to exceed 50 mSv in any single year. For these reasons, attention on dose reduction techniques is growing and several scientific studies on the topic have been published [8–10]. Gantry tilting, organ-based tube current modulation, bismuth shielding and iterative reconstruction [11, 12] are among the most widely used procedures to reduce the eye lens dose. All authors agree that gantry tilting and tube current modulation (or reduction) techniques [13, 14] have to be preferred to high attenuation-filter (bismuth shielding) ones allowing for dose reduction while maintaining image quality. However, these techniques are not implemented in all available commercial scanners, especially in less recent ones. In these last cases, taking into account their cost of implementation and attenuation characteristics, the bismuth filter may be a valid alternative, for the protection of patient’s eye lenses [15].
The manufacturer of the shield guarantees eye lens dose reduction up to 50 % (40 % mean reduction): these results are on-line with reported data in literature [16]. The default set up for this shielding system is to place the protection dispositive just upon the eyes of the patient. Using this set-up the compliance of uncooperative patient (children, elderly, claustrophobic, etc.) may be compromised in some circumstances. Moreover, cases of artefact due to the contact between the shielding system and the patient have been noted [16]. Avoiding contact between the shielding system and patients’ eyes can bring two important benefits: increase in patient’s compliance and a reduction of biological risk. This study aims at characterizing the clinical impact of a high absorption filter, placed at different eye distances, in head CT-scans.
Materials and methods
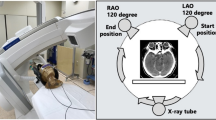
The bismuth shield (3 × 14 cm2, 0.06 mm Pb eq, Attenurad, Vandergrift PA, USA) was placed on an in-house developed height adjustable holder aimed at increasing patients’ compliance (Fig. 1).
After the scout scan the shielding system can be easily implemented in the CT-head holder, thanks to the equipped “Velcro” strips on both the shielding system and the head holder. For stability and resistance purposes a layer of 2 cm of radio-transparent rubber was positioned upon the filter.
CT acquisitions (see Table 1) were performed using a Light Speed L16 (General Electric, Milwaukee USA) CT system.
A CTDI phantom and an anthropomorphic phantom were subjected to Axial scans centered on the shielding system. Because of its intrinsic high homogeneity CTDI phantom scans were utilized for CTDI measurement and image quality assessment while four calibrated TLDs have been positioned on the anthropomorphic phantoms scans (Fig. 2) for dose evaluation.
The CTDI measurements were performed with a 10 cm pencil ionization chamber (10X5-3CT, Radcal Instruments, Monrovia, California, USA) and clinical protocol scanning parameters (see Table 1) were adopted.
For the purposes of this study, three set-ups were defined: at 0 cm distance between shielding system from the top of the phantom (“contact” set up), a second set-up of 4 cm distance (“4 cm” set up, Fig. 3) and a final set-up without the shielding system. Dosimetric characteristics were evaluated through CTDI assessment and TLDs measurements. For each position (right eye, left eye) and each set-up, the measurements were reported 3 times with a different TLDs set every time (2 eyes * 3 distance * 3 times = 18 scans in all).
The thermoluminescent material used was the LiF:Mg,Cu,P (GR200A - Lavoro e Ambiente Srl, Forlì, Italy) chosen for its better performance in terms of sensibility and reproducibility compared to the TLD-100 [17]. Dosimeters are calibrated once a year with X (37–248 keV) and γ (60Co) sources certified by the Italian authority on personal dosimetry (ENEA).
Image quality was assessed by measuring Signal, Noise and Contrast to Noise Ratio (CNR). Three vertical areas and three horizontal areas of interest were defined. The vertical ones are along the midline of the homogenous phantom and on its lateral sides in correspondence to the eyes’ position (see Fig. 4). The three horizontal areas (of the same dimensions) are defined in the upper, the middle and the lower parts of the CTDI phantom (see Fig. 4).
The vertical areas of interest have been divided into regions of interests (ROIs) with dimension of 10x10 pixels. In each ROI the mean Hounsfield units value (HU) was defined as “Signal” and the standard deviation of the HU as “Noise”. In the horizontal area the average Signal (contrast referred to air = −1000 u.a.), standard deviation and contrast to noise ratio CNR (phantom vs. air) of the vertical ROI (10 × 10) belonging to the considered zone were calculated. Signal was not converted in HU because CT-Number of CTDI phantom is proximal to 0 (CT scanner are calibrated with HUwater = 0). Then converting the signal in HU the direct proportionality with density is lost and the relative variation due to shield induced artefact could be incorrectly estimated.
Air measurements (signal and standard deviation) have been performed outside the phantom in the exam without high attenuation filter.
CNR was defined as:
where \(I_{phantom} ,I_{air}\) are respectively the CT numbers of the CTDI phantom and of the air, while σ indicates their standard deviations.
A MATLAB (The MathWorks, Natick, Massachusetts, USA) code was developed for these purposes.
Eye lens doses were evaluated through TLDs dosimeter. Relative attenuation was evaluated as the ratio between the filtered and the full dose scans (without shielding system). The uncertainty of the Relative Attenuation was evaluated as follows:
where Dose filter and Dose W are respectively the measured TLD dose with and without bismuth shielding, while σ indicates their standard deviations.
Results
Dosimetric assessment
The in-house developed holder has proved to be reliable, durable and user friendly. Comparisons of the TLD measurements exhibit a substantial dose reduction at the eyes’ level: by 28.5 % in the setup with the shielding system just upon the phantom, and by 21.1 % for the set up with the shielding system placed 4 cm above the phantom.
Table 2 gives information about the entrance surface dose (ESD) measured with the TLD. The results reported in Table 2 are the averages of the different repeated measurements. Uncertainty was calculated as the standard deviation of the measurements.
Peripheral and central dose measurements in the CTDI phantom lead to the results presented in Table 3.
Image quality
The effect of shielding system, in the “contact” set-up, produced a moderate artefact that caused a gradient in Signal Level increasing the CT-number up to about 4-5 cm from the shielding system (see Fig. 5). 5 cm below the shielding system the increase is limited to a few point (a.u.) of signal.
Signal and contrast-to-noise ratio versus shielding system distance—left, and center. The zero point of the abscissa axis is the beginning of the phantom in the central profile (v.Fig. 2)
Images taken with the shielding system in the 4 cm set up did not show any artefacts.
The plot in Fig. 5 outlines the trend of Signal and CNR versus the distance from the filter. Quantitative analysis of the data is reported in Table 4.
Mean CNRs in the ROI included in phantom were measured along the three vertical profiles (see Table 5). In the plots above a comparison between CNRs with filters and without filters is provided (Fig. 5).
Data analysis shows a degradation of image quality. In particular a moderate decrease of the average CNR value, ranging between 8 % (in the “4 cm”) to 20 % (in the “contact” set up) was found. CNR is influenced by the presence of the artefact that increases the signal in the first ROI. Thus, to quantify the variation of the image quality, we included the analysis of the Standard Deviations of the Signal below the shielding system. According to the distance from the top of the phantom, three ranges were identified, namely “top” range (up to 5 cm from top), “medium” range (from 5 up to 10 cm) and “bottom” ranges (from 10 cm up to the end) of the phantom(see Fig. 4). Noise distribution was considered along these three vertical areas.
In Table 5 it can be seen a non-negligible noise increase in the set up with the shielding system placed in contact with the patient in the “Top” area. In this area the Standard Deviation is three times its value for the central profile. In the “Medium” and “Bottom” range the Noise increase is moderate and it never exceeds 1 a.u., becoming negligible in the “Bottom” range were the filter was placed at 4 cm from the patient. In these last two areas a double paired two tails T Test showed a not significant (p < 0.05) difference as visible in Fig. 6.
Noise increase in the left profile is moderately higher than the increase in the right one. This is probably due to the presence of the plastic support of the shielding system on that side of the head support.
Discussion
The American Association of Medical Physics (AAPM) recently (2012) recommended the use of alternative systems (tube current modulation, tube current reduction, iterative reconstruction, etc.) for dose reduction in CT-scans. The report focused on the fact that these systems are not compromised by the disadvantage (artefact) of the high attenuation filter [18]. However these systems (all above iterative reconstruction algorithm) are not available in every CT-scannner and may be not suitable for all anatomical regions (e.g. tube current modulation is not widely used in head CT-scan). In these cases the high attenuation filter should represent a cheap, effective and easy to implement alternative in head CT-scans where the area of clinical interest is far from eyes.
The shielding system, developed in this study, has proved to be reliable, durable and user-friendly. Moreover, the set-ups presented here do not involve direct contact of the shielding system with the patient, allowing for multiple use of the shield.
The dosimetric analysis shows a moderate global dose reduction induced by the presence of the filter. In fact, the CTDIvol decreases by nearly 10 % in both studied set-ups. A greater dose reduction was observed at eyes’ level in the TLDs measurements (−21.1, −28.5 %). These data are consistent with what declared from manufacturer (40 % dose reduction for 0.06 mm Pb eq) with the bismuth shield placed in contact with the eyes of the patient. The decrease of variation of ESD can be attributed to the greater distance between the patient and the shield.
The CTDI phantom images elaborations showed a degradation of image quality up to about 5 cm below the shielding system. In fact in the set-up where the bismuth shield was placed in contact of the phantom it is possible to see the presence of an artefact involving growth of the CT number by nearly 250 % compared to the non-artefacted signal. In the “medium” and “bottom” range CNRs of the 3 different set-ups are comparable; it is thus possible to say that in these areas image quality is not significantly affected by the presence of the shield. The choice of evaluating image quality only in a homogenous phantom and not in the anthropomorphic one was made with the intent to objectify the effect of the shielding system. The presence of inhomogeneity (like cranial fossa, orbits or air cavity), acting on the quality of X-ray spectrum (spatial difference in beam hardening), could influence the variation of CT signal.
No artefact, at the phantom level, was evident in the set-up with the shielding system placed at 4 cm from the top of the phantom.
By observing that usually the distance between eye lens and the brain is about 4 cm, it is possible to conclude that no image degradation will affect brain region in Head CT examination when using both shielding system “set ups”.
Conclusions
The presence of the shielding system can lead to a reduction of the entrance surface dose by 21 % (in the “4 cm” set up) and by 29 % (in the “contact” set up). Our results, when considering the distance from the eye lens to the filter in the two studied set-ups, are comparable with the manufacturer data.
Results (no artefacts in the brain area, good dose reduction) show that the set up with the contact shielding system seems to be a very attractive option but, in case of uncooperative patient, also the other set up (4 cm distance from the patient) appears to provide good results in terms of eye lens dose reduction.
References
Protection ICR (1990) Recommendations of the international commission on radiological protection. ICRP Publication 60: Pergamon, Oxford
Council NR (1990) Health effects exposure to low levels of ionizing radiation. In: BEIRV. National Academy, Washington
Hein E, Rogalla P, Klingebiel R, Hamm B (2002) Low-dose CT of the paranasal sinuses with eye lens protection: effect on image quality and radiation dose. Eur Radiol 12:1693–1696
Wagner LK, Eifel PJ, Geise RA (1994) Potential biological effects following high X-ray dose interventional procedures. J Vasc Interv Radiol 5:71–84
Nishizawa K, Maruyama T, Takayama M, Okada M, Hachiya J, Furuya Y (1991) Determinations of organ doses and effective dose equivalents from computed tomographic examination. Br J Radiol 64:20–28
Ciraj-Bjelac O, Rehani MM, Sim KH, Liew HB, Vano E, Kleiman NJ (2010) Risk for radiation-induced cataract for staff in interventional cardiology: is there reason for concern? Catheter Cardiovasc Interv 76:826–834
Protection ICoR (2011) Statement on tissue reactions. Protection ICoR, Elsevier
Wang J, Duan X, Christner JA, Leng S, Grant KL, McCollough CH (2012) Bismuth shielding, organ-based tube current modulation, and global reduction of tube current for dose reduction to the eye at head CT. Radiology 262:191–198
Hopper KD, Neuman JD, King SH, Kunselman AR (2001) Radioprotection to the eye during CT scanning. Am J Neuroradiol 22:1194–1198
Raissaki M, Perisinakis K, Damilakis J, Gourtsoyiannis N (2010) Eye-lens bismuth shielding in paediatric head CT: artefact evaluation and reduction. Pediatr Radiol 40:1748–1754
McLaughlin DJ, Mooney RB (2004) Dose reduction to radiosensitive tissues in CT. Do commercially available shields meet the users’ needs? Clin Radiol 59:446–450
Heaney DE, Norvill CA (2006) A comparison of reduction in CT dose through the use of gantry angulations or bismuth shields. Aust Phys Eng Sci Med (supported by the Australasian College of Physical Scientists in Medicine and the Australasian Association of Physical Sciences in Medicine) 29:172–178
Nikupaavo U, Kaasalainen T, Reijonen V, Ahonen SM, Kortesniemi M (2015) Lens dose in routine head CT: comparison of different optimization methods with anthropomorphic phantoms. Am J Roentgenol 204:117–123
Reimann AJ, Davison C, Bjarnason T, Thakur Y, Kryzmyk K, Mayo J et al (2012) Organ-based computed tomographic (CT) radiation dose reduction to the lenses: impact on image quality for CT of the head. J Comput Assist Tomogr 36:334–338
Huggett J, Mukonoweshuro W, Loader R (2013) A phantom-based evaluation of three commercially available patient organ shields for computed tomography X-ray examinations in diagnostic radiology. Radiat Prot Dosim 155:161–168
Hopper KD, King SH, Lobell ME, TenHave TR, Weaver JS (1997) The breast: in-plane X-ray protection during diagnostic thoracic CT—shielding with bismuth radioprotective garments. Radiology 205:853–858
Zha Z, Wang S, Shen W, Zhu J, Cai G (1993) Preparation and Characteristics of LiF:mg, Cu, P thermoluminescent material. Radiat Prot Dosimetry 47:111–118
Medicine AAoPi (2012) Use of bismuth shielding for the purpose of dose reduction in CT scanning. American Association of Physicists in Medicine, College Park
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ciarmatori, A., Nocetti, L., Mistretta, G. et al. Reducing absorbed dose to eye lenses in head CT examinations: the effect of bismuth shielding. Australas Phys Eng Sci Med 39, 583–589 (2016). https://doi.org/10.1007/s13246-016-0445-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13246-016-0445-y