Abstract
Background
Exposure of the eye lens to ionizing radiation results in cataract. Several dose optimization techniques to protect the lens are available for computed tomography (CT).
Objective
The radiation dose to the eye lens, volume CT dose index (CTDIvol) and image quality of various methods of dose optimization were evaluated for pediatric head CT: automated tube current modulation (ATCM), automated tube voltage selection (ATVS), organ-based tube current modulation (OBTCM) and bismuth shielding.
Materials and methods
An anthropomorphic phantom of a 5-year-old child was scanned with nine protocols: no dose optimization technique and then adding different dose optimization techniques alone and in combination. Dose to the eye, thyroid and breast were estimated using metal oxide semiconductor field effect transistor (MOSFET) dosimetry. CTDIvol, influence of timing of shield placement, image noise and attenuation values in 13 regions of interest of the head and subjective image quality were compared.
Results
The eye shield significantly reduced the eye lens dose when used alone, to a similar degree as when using all software-based techniques together. When used in combination with software-based techniques, the shield reduced the eye lens dose by up to 45% compared to the no dose optimization technique. Noise was significantly increased by the shield, most pronounced in the anterior portion of the eye.
Conclusion
The combination of ATCM, ATVS, OBTCM and a bismuth shield, with the shield placed after acquiring the localizer image, should be considered to reduce the radiation dose to the eye lens in pediatric head CT.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The lens of the eye is one of the most radiosensitive tissues in the human body [1,2,3]. Exposure to ionizing radiation results in cataract [4]. Until recently, ionizing radiation-induced cataract formation was considered a deterministic effect with a threshold set at 2,000 mGy. Less than 1% of exposed individuals would have been expected to develop cataract at lower doses. In recent years, the threshold has been reduced to 500 mGy [1, 3, 4]. Children are particularly sensitive to ionizing radiation. Computed tomography (CT) of the head is one of the most performed CT examinations in children and usually leads to irradiation of the orbit and lens [4].
CT vendors have implemented several radiation dose optimization techniques. The most used technique is automated tube current modulation (ATCM), which adjusts the tube current according to the patient’s habitus and attenuation while maintaining image quality or noise level, respectively [5]. ATCM is important in pediatric head CT, as head size and density vary widely depending on the patient’s age. Automated tube voltage selection (ATVS), in conjunction with ATCM, automatically selects the combination of tube voltage and tube current, not only tailored to the patient’s anatomy, but also to the type of examination [6,7,8]. A newer technique is organ-based tube current modulation (OBTCM), which minimizes radiation exposure to superficial radiosensitive organs such as the lens [9]. OBTCM reduces the tube current when the X-ray tube rotates over the anterior part of the patient’s body [10].
A conventional method to protect radiosensitive superficial organs is the use of lead-free shields such as those from bismuth, where the shield is placed over the radiosensitive organ and attenuates the X-ray beam before it reaches the patient [11].
The aim of this study was to compare the effect of different dose optimization techniques and bismuth eye shielding individually and in combination on the dose of the lens and on image quality in pediatric head CT.
Materials and methods
Scans and dosimetry
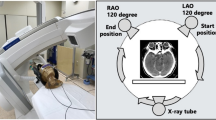
A commercially available anthropomorphic pediatric phantom (ATOM dosimetry verification phantom Model 705; CIRS, Norfolk, VI) simulating an average 5-year-old child was used for the measurements. Five metal oxide semiconductor field effect transistor (MOSFET) dosimeters (Model TR-RD-70-W; Best Medical, Ottawa, Canada & Model TN-1002RD; Thomson Electronic, Ottawa, Canada) with active detector areas of 200 × 200 µm were placed on the following organs: right eye, left eye, right thyroid lobe, left thyroid lobe and right breast. Due to the construction, the detectors have an inherent buildup of about 1-mm epoxy resin. The MOSFET detectors were calibrated individually as described in Best Medical operators’ manual procedure on the surface of a soft-tissue phantom (Virtual Water, Standard Imaging, MOSFET slab) in an orthovoltage beam (WOmed T-200, 100 kV, 6 mm Al, St. Gangloff, Germany). The dose was verified against a calibrated ionization chamber (soft X-ray chamber 23342 incl. Phantom, PTW-Freiburg, Germany) for that beam energy at METAS (Bern, Switzerland) [12]. Since the eye lens, thyroid gland and mammary glands are very superficially located organs, the signal registered by the MOSFET electrodes was used to estimate organ absorbed doses. The phantom was placed in a supine position and scanned with a third-generation dual-source CT scanner (SOMATOM Force; Siemens Healthineers, Forchheim, Germany). The scanner is equipped with ATCM (Care Dose 4D), ATVS (Care kV), and OBTCM (X-Care). Under optimal conditions, OBTCM reduces the mA to 20% for the anterior 90° projection. For the remaining degrees of projection, it increases the mA to preserve image quality. All scans were performed in single-source mode with a detector collimation of 192 × 0.6 mm. Gantry rotation time was 1.0 s at a pitch of 0.55. Initially, a reference scan was obtained without any dose-optimization techniques by using the vendor’s standard setting (100 kV, 220 mAs). For all acquisitions, the scan length was constant from vertex to the first cervical vertebra and measured 11.8 cm.
There were nine different protocols (A-I):
-
A: No dose optimization technique (reference scan)
-
B: ATCM + ATVS
-
C: ATCM + ATVS + OBTCM
-
D: Bismuth shield placed after localizer (lateral) + no other dose optimization technique
-
E: Bismuth shield placed after localizer (lateral) + ATCM + ATVS
-
F: Bismuth shield placed after localizer (lateral) + ATCM + ATVS + OBTCM
-
G: Bismuth shield placed before localizer (lateral) + ATCM + ATVS + OBTCM
-
H: Bismuth shield placed before localizer (anteroposterior) + ATCM + ATVS
-
I: Bismuth shield placed before localizer (anteroposterior) + ATCM + ATVS + OBTCM
For each protocol, the phantom was scanned five times (with the same settings) to account for interscan variations. The commercially available bismuth eye shield, consisting of one layer of bismuth-impregnated latex, was 16-cm long, 4-cm wide and equivalent to a 0.07-mm Pb layer (CTEYEPROTEC 2 mm; XProtect, Reinach, Switzerland). A 0.5-cm thick layer of foam is attached by the manufacturer behind the bismuth layer. The shield was placed over the eyes in protocols D-I (Fig. 1), in protocols D-F after acquiring the localizer image and in protocols G-I before acquiring the localizer image to account for the influence of dense material in the localizer scan regarding ATCM. To describe the change in mAs due to ATCM, we recorded the mAs on every 50th image in protocol B and compared it to protocol A. The organ absorbed doses (eyes, thyroid, breast) were obtained from the MOSFET dosimeters in real time and the volume CT dose index (CTDIvol) was noted from the scanner. Institutional review board approval was not required as the study did not involve human subjects.
Image quality assessment
Computed tomography images were reconstructed in the axial plane with advanced modeled iterative reconstruction (ADMIRE) at a strength level of 2 out of 5, a soft-tissue kernel (Hr40), a slice thickness of 1 mm and an increment of 0.7. Images were stored in the hospital picture archiving and communication system (ImpaxEE, Version R20.XIX; Agfa Healthcare, Mortsel, Belgium).
For quantitative image quality assessment, 13 circular 100-mm2 regions of interest (ROI) were placed, 4 in the eye sockets and 9 in the brain (Fig. 2). These ROIs were always placed by the same radiologist (S.M.) with 7 years of experience and pasted to the corresponding images in every protocol. Attenuation values (in Hounsfield units [HU]) and noise (standard deviation [SD] of the mean HU) measurements were recorded for every ROI. Each parameter was measured on five consecutive images at the level of the eyes, starting one slice below the dosimeter electrodes.
Subjective image quality was assessed independently by two blinded pediatric radiologists (S.L.W. and S.M. with 11 and 7 years of experience, respectively) using a 4-point Likert scale (excellent, good, fair, nondiagnostic). Images of every scan for all protocols were assessed from upper to lower orbital rims and images were divided into four regions (anterior orbit, posterior orbit, anterior portion of the brain, posterior portion of the brain).
Statistical analysis
Statistical analysis was performed with the software R (version 4.0.2. R Core Team, Vienna, Austria). Radiation doses measured from the electrodes attached to both eyes, as well as those attached to the left and right thyroid lobes, were combined by calculating their mean for each of the 45 scans. SDs of the HU readings from the five consecutive images per scan and per ROI were combined by calculating pooled standard deviations. Percentage changes in radiation doses between two protocols were calculated as the difference between means divided by the mean of the less protected protocol. For these differences, 95% confidence intervals (CI) were obtained by fitting linear models to the log-transformed dose with protocol as predictor. Models were fitted to all data, using protocol A as reference, and to selected pairs of protocols for specific questions. The CIs for model coefficients were back-transformed to obtain CIs for percentage change. The significance of differences between protocol A and other protocols was tested using the same linear model and Dunnett-type multiple comparisons.
Standard deviations of HU readings in each of the 13 ROIs were compared between specific pairs of treatments using Wilcoxon rank sum tests. Pooled SDs per protocol and per ROI were calculated as the square root of the mean squared SDs for each scan. Percentage changes between two protocols were calculated from these pooled SDs. Using the adjusted bootstrap percentile (BCa) method, 95% CIs for the % change were obtained.
To analyse the subjective ratings of image quality, means of the ten (two readers x five scans) ratings per location and per protocol were calculated. The four categories of the Likert scale were treated as numeric values from 1 = excellent to 4 = nondiagnostic. The ten replicated ratings per location and protocol were mostly identical and differed by, at most, one point. Therefore, no further statistics were calculated.
Results
Radiation dose
Radiation dose received by the eyes increased by 20% with the use of the ATCM + ATVS (protocol B) but reduced by 22−45% with all other combinations of protective measures (Fig. 3, Table 1). The use of a bismuth eye shield during the CT scan reduced radiation to the eyes by 18−35% when used alone or when used in combination with dose optimization techniques (Fig. 3, Table 2). Conversely, mean dose was slightly higher (+ 10%, 95% CI from -2% to + 25%) with the eye shield alone (protocol D) than with the use of all software-based techniques together (protocol C). The combination of a bismuth eye shield with all dose optimization techniques (protocol F) resulted in the highest reduction of the mean dose (45% [95% CI from − 37% to − 51%]) compared to the reference scan (protocol A). Placing the bismuth eye shield before the localizer acquisition had little effect on the radiation dose received by the eyes. The difference in mean dose ranged from − 3% to + 11%, and all 95% CIs included zero (details not shown).
Distribution of radiation dose received by the eyes with nine protocols (n = 5 scans per protocol). Box shading corresponds to different dose-modulation techniques, whereas the three main groups correspond to the use of a bismuth eye shield. In protocols H and I, the localizer was carried out in an anteroposterior projection and in lateral projection in the other protocols
Radiation dose received by the thyroid gland was slightly increased by all protective measures used either separately or in combination. However, all changes were small, (+ 3% to + 24% compared to protocol A), with CIs including zero (Table 3). Placing the bismuth eye shield before the localizer acquisition did not affect the radiation dose received by the thyroid gland.
Radiation dose received by the breast was not consistently affected by protective measures used either separately or in combination. Differences in mean dose between protocols corresponded to those expected by chance due to the variability of individual measurements (see width of 95% confidence intervals in Table 3).
Volume computed tomography dose index
The distribution of CTDIvol (mGy) values in each protocol is shown in Fig. 4.
Distribution of volume computed tomography dose index (CTDIvol) with nine protocols (n = 5 scans per protocol). Boxes correspond to different dose-modulation techniques, whereas the three main groups correspond to the use of a bismuth eye shield. Thick lines indicate that several values were equal to the median
There was a consistent, large increase in CTDIvol due to OBTCM application, and a smaller increase in CTDIvol due to ATCM + ATVS. There was no effect of the bismuth shield, neither when using it during the scan (comparing protocols D − F to corresponding protocols A − C) nor when already using it during localizer acquisition (comparing protocols G and I to the corresponding protocols C and F), except for the case of protocol H, which had much higher CTDIvol than protocols B and E.
mAs
Compared to protocol A (no dose optimization techniques), the average mAs for protocol B (ATCM + ATVS) was 12% higher in the skull base region but 4% lower in the rest of the skull.
Image quality
Noise was unchanged or slightly reduced using software-based dose optimization (protocols B and C), meaning that scatter noise was reduced. Conversely, noise was significantly increased by a bismuth eye shield. The increase in variability was largest in the anterior eye (+ 54% and + 62%), much smaller for the posterior eye (+ 14% and + 9%), and consistently around 20% for the brain (Table 4). In summary, image quality was impaired by a bismuth eye shield.
Placing the bismuth shield before the localizer acquisition further increased scatter noise by approximately 5% (protocol G). However, compared to protocol F, the difference was not statistically significant. Placing the bismuth eye shield before the localizer acquisition without using OBTCM (protocol H) mitigated the increase in scatter noise caused by the eye shield. With this combination of protective measures, noise in the brain was not significantly increased compared to protocols without any protection. It should be noted that this combination had the highest CTDIvol values. Figure 5 shows a sample image of every protocol (A-I).
Mean attenuation values were increased by the bismuth shield regardless of dose optimization technique. Comparing protocols F and C, the increase in mean attenuation due to the bismuth shield was approximately + 300% for the anterior eye and + 100% for the posterior eye, but only + 5% to + 15% for all brain regions (Table 5).
Subjective image quality was unaffected by the dose optimization techniques without the bismuth shield, but partly reduced by the bismuth eye shield. Without the eye shield, image quality was mostly rated as excellent. With the eye shield, image quality was always rated as excellent in the posterior brain and as good in the anterior brain, while declining to fair in the posterior eye and to poor (nondiagnostic) in the anterior eye.
Discussion
Children are more sensitive to ionizing radiation than adults and are more likely to undergo multiple CT exams during their lifetime [9]. Irradiating the lens of the eye can cause cataract formation. Based on new epidemiological evidence, the International Commission on Radiological Protection (ICRP) concluded in 2012 that the lens is more radiosensitive than previously thought and among the most radiosensitive tissues of the human body [1, 3]. For a long time, a deterministic effect was assumed for cataract induction with a threshold of 2,000 mGy, which was later reduced to 500 mGy, so that < 1% of the exposed population were likely to develop radiation-induced cataracts with exposure doses below the threshold [1, 3, 4, 9, 13, 14]. Certain authors have even put a stochastic effect up for discussion, meaning a zero-dose threshold [1, 3, 4].
The risk of cataract formation is likely to be small for the vast majority of children undergoing head CT, primarily due to the small cumulative number of examinations [4]. However, special attention should be paid to the lens dose in children who undergo multiple brain CT scans. Several methods are available to optimize radiation dose in CT exams. These include ATCM, ATVS, OBTCM and lead-free protection devices such as bismuth shields [11].
ATCM adjusts the tube current along the longitudinal axis based on the localizer and/or angularly based on axial images during the scan [15,16,17,18,19]. In the scanner used for this study, ATCM modulated longitudinally and angularly. ATCM is usually activated in pediatric head CT since the size of the skull can vary greatly depending on age.
OBTCM reduces the tube current when the X-ray tube rotates over the anterior part of the patient’s body, reducing radiation dose to superficial radiosensitive organs [9, 10, 20, 21]. In the scanner used for this study, the tube current was reduced to 20% for the anterior 90° projection (Fig. 6). For the remaining projections, it increases the tube current to preserve overall image quality. The total exposure during one tube rotation should be equal to the exposure without OBTCM. Not all CT vendors increase the tube current for the remaining projection [9, 20, 22,23,24]. There is very little literature on OBTCM in children, and none on the combination with bismuth shielding [9, 10, 25].
ATVS selects the tube voltage based on each patient’s individual attenuation on the localizer image and on the diagnostic task of the CT examination [6, 8, 26].
Bismuth shields are placed on the patient’s body to protect superficial radiosensitive organs such as the lens of the eye, thyroid, breast and gonads. The radiation-absorbing material causes beam-hardening artifacts and may increase the attenuation value recorded under the shield [11]. Special attention must therefore be paid to image quality and radiation dose [11, 27, 28].
The combination of ATCM, ATVS and OBTCM (protocol C) significantly reduced the radiation dose received by the eyes. Papadakis and Damilakis [9] demonstrated a reduction of the eye lens dose in children of 13% in ATCM combined with OBTCM compared with ATCM alone using a CT system of a different vendor.
In our study, we used the vendor’s setting (protocol A) as the basis. In 2018, Sadigh et al. [29] published an article about doses in pediatric non-contrast head CTs in multiple centers in the United States. CTDIvol values ranged from 22 to 47 mGy. The vendor’s setting used in our study (protocol A) had a CTDIvol of 19,26 mGy. Radiation dose received by the eyes was significantly increased when using the combination of ATCM and ATVS without OBTCM (protocol B). This effect was reduced using bismuth shields. ATCM and ATVS do not necessarily lower radiation dose, as these optimizations attempt to maintain image quality for diagnostic purposes by adapting the dose according to the patient’s attenuation [5]. This reduces radiation dose in regions of the body with a low density (i.e. lung tissue) and increases it in regions with a high density such as the skull base and other bony structures. The increase of radiation dose by using ATCM and ATVS (protocol B) is therefore most likely explained by the dense bony structures of the skull, especially the skull base. ATCM typically reduces mAs outside the skull base. While radiation dose received by the eyes increases in scans with ATCM + ATVS without OBTCM and without bismuth protection, it may decrease in regions with less dense structures (e.g., cerebral hemispheres, bone marrow), which is beneficial in terms of brain tumor and leukemia development. However, this was not investigated in this work and should not discourage the use of ATCM + ATVS.
Likewise, OBTCM is known to compensate for the decreased output in the anterior projections with increased output everywhere else. While the dose to the eye lens is decreased, the overall impact on patient dose, including brain and bone marrow dose, has not been evaluated in this study.
In our study, the use of a bismuth eye shield significantly reduced radiation to the eyes when used alone (protocol D, to a similar degree as with the use of tube modulation alone) and when used in combination with tube modulation (protocols E-I). It further reduced the radiation dose of the eye lens when used in combination with all radiation dose optimization techniques (protocol F), reducing the dose by 45% compared to a no dose optimization technique (protocol A). The total reduction is similar to that reported by Wang et al. [23], who examined the combination of OBTCM and bismuth shield in adults, using an older scanner from the same vendor. They were able to show a reduction in lens dose of 47%. Mehnati et al. [11] stated in a meta-analysis that the protective effect of bismuth shielding of the eye may be further enhanced in scanners with an increased number of detector rows.
In contrast to studies performed with older scanners, placing the bismuth eye shield before the localizer image acquisition did not significantly increase the radiation dose received by the eyes [11, 30].
Also, the bismuth shield of the eye did not significantly increase the CTDIvol, except when used during the localizer acquisition in the anteroposterior projection (Fig. 1) combined with ATCM and ATVS (protocol H). In that case, the CTDIvol was significantly higher compared to the corresponding protocol without a bismuth shield on the localizer acquisition. This can be attributed to the scanner detecting a relatively large and dense foreign body on the anteroposterior (but not on the lateral) localizer and responding by increasing radiation dose to maintain image quality.
In scans with ATCM and OBTCM, image noise increased slightly in the orbit compared to scans without OBTCM. This is in line with the recent publication about OBTCM in pediatric CT by Papadakis and Damilakis [9]. In the remainder of the head, image noise decreases when using OBTCM. This contrasts with Papadakis and Damilakis [9] who used a scanner from a different vendor.
As in adults [23], image noise was significantly increased by a bismuth eye shield in our study. This increase was greatest for the anterior portion of the orbit, much smaller for the posterior portion of the orbit and around 20% for the brain. Mean attenuation values were increased by the eye shield by approximately + 300% for the anterior part of the orbita, but only + 5% to + 15% for all brain regions. Therefore, evaluating the orbital and periorbital soft tissues of the skull could be more difficult and the use of a bismuth eye shield could be inappropriate, for example following direct trauma of the orbit or the facial bones. On the other hand, using a bismuth shield in children for most indications of CT of the brain has no significant negative impact. Placing the bismuth shield before the localizer acquisition creates a slightly further reduction in image quality. Therefore, placement of the bismuth shield after the localizer acquisition is preferable.
This study has some limitations. First, the full potential of ATVS cannot be shown as we have only examined one phantom size. Second, the impact of patient positioning on radiation dose and image noise was not examined. Third, we did not investigate whether the acquisition of the localizer in anteroposterior or posteroanterior direction influences the radiation dose when using the bismuth shield. Fourth, this study was limited to a single OBTCM technique and may not apply to other CT scanners with different OBTCM techniques. Fifth, there are other dose reduction options, but we have not investigated them. The lens can be spared by tilting the gantry, but the gantry cannot be tilted with the scanner we used. With inclination of the head, the lens can also be spared, but this was not possible with the phantom used. By performing the examination in non-helical mode, the lens could also be spared.
Conclusion
The combination of ATCM, ATVS, OBTCM and bismuth eye shield significantly reduces the radiation dose of the lens while producing some reduction in image quality of the anterior aspect of the orbit. Therefore, a bismuth eye shield should be considered for head CT in children when the anterior aspect of the orbit is not of diagnostic importance.
References
Ainsbury EA, Barnard S, Bright S et al (2016) Ionizing radiation induced cataracts: Recent biological and mechanistic developments and perspectives for future research. Mutat Res Rev Mutat Res 770(Pt B):238–261
Poppe E (1957) Experimental investigations on cataract formation following whole-body roentgen irradiation. Acta Radiol 47:138–148
Stewart FA, Akleyev AV, Hauer-Jensen M et al (2012) ICRP publication 118: ICRP statement on tissue reactions and early and late effects of radiation in normal tissues and organs–threshold doses for tissue reactions in a radiation protection context. Ann ICRP 41:1–322
Harbron RW, Ainsbury EA, Barnard SGR et al (2019) Radiation dose to the lens from CT of the head in young people. Clin Radiol 74:816 e819-816 e817
Yabuuchi H, Kamitani T, Sagiyama K et al (2018) Clinical application of radiation dose reduction for head and neck CT. Eur J Radiol 107:209–215
Spearman JV, Schoepf UJ, Rottenkolber M et al (2016) Effect of automated attenuation-based tube voltage selection on radiation dose at CT: an observational study on a global scale. Radiology 279:167–174
Papadakis AE, Damilakis J (2019) Automatic tube current modulation and tube voltage selection in pediatric computed tomography: a phantom study on radiation dose and image quality. Invest Radiol 54:265–272
Santos J, Foley S, Paulo G et al (2015) The impact of pediatric-specific dose modulation curves on radiation dose and image quality in head computed tomography. Pediatr Radiol 45:1814–1822
Papadakis AE, Damilakis J (2020) Evaluation of an organ-based tube current modulation tool in pediatric CT examinations. Eur Radiol 30:5728–5737
Boos J, Kropil P, Klee D et al (2014) Evaluation of the impact of organ-specific dose reduction on image quality in pediatric chest computed tomography. Pediatr Radiol 44:1065–1069
Mehnati P, Malekzadeh R, Sooteh MY (2019) Use of bismuth shield for protection of superficial radiosensitive organs in patients undergoing computed tomography: a literature review and meta-analysis. Radiol Phys Technol 12:6–25
Gargett MA, Briggs AR, Booth JT (2020) Water equivalence of a solid phantom material for radiation dosimetry applications. Phys Imaging Radiat Oncol 14:43–47
Chodick G, Bekiroglu N, Hauptmann M et al (2008) Risk of cataract after exposure to low doses of ionizing radiation: a 20-year prospective cohort study among US radiologic technologists. Am J Epidemiol 168:620–631
Worgul BV, Kundiyev YI, Sergiyenko NM et al (2007) Cataracts among Chernobyl clean-up workers: implications regarding permissible eye exposures. Radiat Res 167:233–243
Tack D, De Maertelaer V, Gevenois PA (2003) Dose reduction in multidetector CT using attenuation-based online tube current modulation. AJR Am J Roentgenol 181:331–334
Kalra MK, Rizzo S, Maher MM et al (2005) Chest CT performed with z-axis modulation: scanning protocol and radiation dose. Radiology 237:303–308
McCollough CH, Bruesewitz MR, Kofler JM Jr (2006) CT dose reduction and dose management tools: overview of available options. Radiographics 26:503–512
Lee CH, Goo JM, Ye HJ et al (2008) Radiation dose modulation techniques in the multidetector CT era: from basics to practice. Radiographics 28:1451–1459
Hoang JK, Yoshizumi TT, Choudhury KR et al (2012) Organ-based dose current modulation and thyroid shields: techniques of radiation dose reduction for neck CT. AJR Am J Roentgenol 198:1132–1138
Franck C, Smeets P, Lapeire L et al (2018) Estimating the patient-specific dose to the thyroid and breasts and overall risk in chest CT when using organ-based tube current modulation. Radiology 288:164–169
Euler A, Szucs-Farkas Z, Falkowski AL et al (2016) Organ-based tube current modulation in a clinical context: Dose reduction may be largely overestimated in breast tissue. Eur Radiol 26:2656–2662
Duan X, Wang J, Christner JA et al (2011) Dose reduction to anterior surfaces with organ-based tube-current modulation: evaluation of performance in a phantom study. AJR Am J Roentgenol 197:689–695
Wang J, Duan X, Christner JA et al (2012) Bismuth shielding, organ-based tube current modulation, and global reduction of tube current for dose reduction to the eye at head CT. Radiology 262:191–198
Lungren MP, Yoshizumi TT, Brady SM et al (2012) Radiation dose estimations to the thorax using organ-based dose modulation. AJR Am J Roentgenol 199:W65-73
Yamauchi-Kawaura C, Yamauchi M, Imai K et al (2013) Image quality and age-specific dose estimation in head and chest CT examinations with organ-based tube-current modulation. Radiat Prot Dosimetry 157:193–205
Lee KH, Lee JM, Moon SK et al (2012) Attenuation-based automatic tube voltage selection and tube current modulation for dose reduction at contrast-enhanced liver CT. Radiology 265:437–447
Hopper KD, King SH, Lobell ME et al (1997) The breast: in-plane x-ray protection during diagnostic thoracic CT–shielding with bismuth radioprotective garments. Radiology 205:853–858
Hopper KD (2002) Orbital, thyroid, and breast superficial radiation shielding for patients undergoing diagnostic CT. Semin Ultrasound CT MR 23:423–427
Sadigh G, Kadom N, Karthik P et al (2018) Noncontrast head CT in children: national variation in radiation dose indices in the United States. AJNR Am J Neuroradiol 39:1400–1405
Colletti PM, Micheli OA, Lee KH (2013) To shield or not to shield: application of bismuth breast shields. AJR Am J Roentgenol 200:503–507
Acknowledgments
We thank Damian Koller and Evelyn Wirth for their excellent technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Markart, S., Fischer, T.S., Wildermuth, S. et al. Organ-based tube current modulation and bismuth eye shielding in pediatric head computed tomography. Pediatr Radiol 52, 2584–2594 (2022). https://doi.org/10.1007/s00247-022-05410-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-022-05410-x