Abstract
The study was designed to explore the efficiency of magnetite nanoparticles (Fe3O4 NPs)/bacterial cell assembly to biodegrade lignin and lignocellulose, decontaminate pulp and paper-contaminated wastewater and optimize lignin adsorption by Fe3O4 NPs. Water samples were collected from three paper and carton manufacturing companies, Alexandria Governorate, Egypt. Pseudomonas otitidis MCC10330, the most active and promising strain among 10 previously screened indigenous and exogenous isolates, was selected and decorated with magnetic Fe3O4 Nanoparticles, that were prepared by the co-precipitation method, characterized and used to decontaminate paper and pulp effluent in a batch mode bioassay for 4 h. Fe3O4 NPs/bacterial cell assembly achieved the highest removals (64.1, 52.0, 54.3 and 66.6%) of TSS, COD, BOD, and Total Tannin and Lignin after 1, 4 and 4 h, reaching residual concentrations (RCs) of 322, 216, 112 and 7 mg/L, which are still slightly higher (5.35, 2.7 and 1.86-fold) than their maximum permissible limits (MPLs), respectively. RCs of pH, DO and TDS in the treated effluent are accepted for safe discharging. Maximum lignin adsorption and removal (82.14%) using Fe3O4 NPs was achieved at the optimized conditions (pH 6, Fe3O4 NPs dosage of 100 mg and 10 min contact time). Results confirmed that the proposed magnetite-coated Pseudomonas otitidis treatment system is highly efficient and recommended to treat the highly contaminated pulp and paper wastewater. Also, as far as we know, this integrated assemblage is the first time to be used as a novel, very promising, eco-friendly, renewable and economical biotechnological approach to minimize/eliminate the involved pollutants with the least running time.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pulp and paper industry consume huge amounts of water during pulping process and manufacturing of paper as well as generating more quantities of polluted and colored wastewater (Ahmed et al. 2022; Kumar et al. 2021a, b), which considered severely polluted and possess dangerous environmental impacts (Ramos et al. 2022). Among others, paper and pulp manufacturing is major energy-consuming industry and constitutes one of the primary sources of industrial wastewater together with some other industries (chemical and petrochemical, food processing, tannery etc.) (Ahmed et al. 2022). Their effluents are contributing directly to the production of greenhouse gases as well as other pollutants including sulfur compounds, nitrogen oxides, chlorinated by-products with high load of lignin fragments, hemi-cellulose, chlorophenolics, resins, fatty acids and inorganic salts, all of which greatly damaging the water quality as reported by Kumar et al. (2020); Kumar et al.(2015) and Tripathy et al. (2022). They are also toxic for aquatic organisms and showed strong mutagenic effects. Pulp and paper effluent characterized by very high organic pollution load originated from the residues of lignin and polysaccharide degradation and reflected by high BOD, COD and TDS(Núñez et al. 2022). Chemical composition and the dark coloration of the black liquor effluents from this industry pose negative environmental impact, especially in the receiving aquatic systems where they adversely affect aquatic fauna and flora (Singh et al. 2022). Such effluents are highly toxic and consisting of more than 250 chemicals of high molecular weight lignin (aromatic rigid polymer presents predominantly in woody plants) (Yao et al. 2021). Lignin is highly recalcitrant compound and very difficult to be degraded either chemically or biologically due to the presence of aryl ether bonds and carbon–carbon linkages (non-hydrolysable) (Le et al. 2022), therefore, degradation of lignin is utmost difficult task to reach the strict levels stated by the environmental regulations (Kumar et al. 2021b).
Abhishek et al. (2017), Hooda et al. (2018) and Khan et al. (2022) stated that efficient treatments are necessary before discharge into water bodies to minimize and avoid the hazardous environmental negative impacts caused by the paper industry by-products. Membrane separation, chemical coagulation, precipitation using metal salts and advanced oxidation processes are available among several traditional physicochemical treatment methods for pulping and paper production wastewater (Esmaeeli et al. 2023; Cyganowski 2021). Other traditional methods used for the treatment of pulp and paper effluents include incineration, photochemical UV/TiO2 oxidation, adsorption on activated carbon and polymer resin as well as chemical coagulation/flocculation by synthetic or natural coagulants (Nordin et al. 2020; Teodor et al. 2021; Moreira et al. 2022; Jagaba et al. 2023), all of which exhibited non-sufficient effectiveness for proper treatment for safe discharging. Selecting highly efficient, eco-friendly and cost-effective decontaminating method is of top priority demands in the field of wastewater treatment (Costa et al. 2017). Biological processes that actively used in bioremediation of pulp and paper industrial wastewater include activated sludge (Kumar et al. 2021a; Kumar et al. 2021b), aerated lagoons (Jansson 2022; Sonkar et al. 2020; Dagar et al. 2022), anaerobic treatment processes (Ribeiro et al. 2023; Goycoechea et al. 2023; Hovikorpi et al. 2020) and sequential anaerobic/aerobic treatment systems (Saski et al. 2020; Coimbra et al. 2021). Microbial fermentation of lignocellulose materials considered an added value approach for remediation of pulp and paper effluent. Previous study reported using wood rotting fungi in valorization and degradation of lignocellulose, since they are effectively producing lignin and polysaccharides-degrading enzymes (Kipping et al. 2024). However, the relatively slow growth of filamentous fungi and loss of polysaccharides during lignin degradation considered as limitations of this method as stated by Sharma and Arora (2015) and Podkościelna et al. (2022). In contrary, cellulose and lignocellulos degrading bacteria are highly active for this industrial effluent compared to other microbes since they produce functional enzymes at high concentration leading to efficient conversion of the secondary degradation metabolites. Many cellulose/lignocellulos degrading bacteria that are used for pulp and paper effluents treatment are either native (belong to the contaminated environments) or exogenous consortia (from outer source) and can be isolated from the contaminated effluent or the pulp mill sludge (Brown et al. 2021). Limitations include high cost, longer times, large spaces for aerobic processes, as well as experiencing difficulty in controlling the microbial populations, pH, temperature, nutrients and toxic or recalcitrant compounds harmful to biological degradation (Esmaeeli et al. 2023). Integration of physical, chemical or biological systems such as coagulation and wet oxidation, ozone and biofilms, chemical oxidation with ozone as well as chlorine with activated sludge sometimes are the optimal solution allowing to benefit from their unique features (Jansson 2022; Jagaba et al. 2023 and Murshid et al. 2023). Also, COD and NH4+–N levels could be reduced to the permitted level using post-treatment electrolysis or ozonation (Saeed et al. 2022).
Nanomaterials-based applications have gained increased attention in water purification and wastewater treatment due to their unique physiochemical properties for elimination of organic and inorganic pollutants as well as toxic metals at minimum energy consumption, low cost and potential reuse of the treated effluents (Kiss 2020; Palani et al. 2021). There are different methods for synthesis of metals NPs to control their size and shape, including co-precipitation, hydrothermal, thermal decomposition, microemulsion, electrochemical deposition, laser pyrolysis, solvothermal, sonochemical, chemical vapor deposition, microwave-assisted method and aerosol pyrolysis. Among these methods, co-precipitation is one of the most used methods in the synthesis of aqueous phases because it is the simplest and efficient. Other methods have many disadvantages including long reaction time, temperature of reaction is high or extremely high, high pressure, poor yield, amounts of solvent required are large, relatively expensive and poor reproducibility (Sulistyaningsih et al. 2015).
Currently, Fe3O4 NPs are widely used in decontamination and purification processes due to their high specific surface area, simple preparation, easy modification and low toxicity (Malik et al. 2022; Ning et al. 2021; Nayl et al. 2022). In addition of being cost-effective, they are converted into hydroxides during treatment, which work as flocculants /solid sorbent, and help removing suspended and colloidal inorganic and organic contaminants (Pandey et al. 2022; Abo-Zeid et al. 2020). Upon the contact with Fe nanoparticles in wastewater treatment systems, reduction reactions help to efficiently eliminate mobile or immobile contaminants (Zhao et al. 2020; Priyadarshini et al. 2022).
Conventional bacterial immobilization possesses many advantages such as efficient, stabilized and continuous catalytic activity in addition to increasing microbial biomass, which allow biodegradation of hazardous pollutants (He et al. 2017; Ranmadugala et al. 2018; Malik et al. 2022). Integration (immobilization) of bacterial cells with Fe3O4 NPs overcome the drawbacks of the conventional bacterial immobilization including limited mass transfer in the inner matrix, matrix fragility, cell leakage from the supporting matrix (solid medium) as well as adverse effects on cell viability and catalytic activity (Yoshimoto et al. 2017). Fe3O4 NPs remarkably upgrade bacterial efficiency, reduces mass transfer problems, facilities separation of the dispersed coated cells and reuse of the biomass, minimizes microbial death and avoids blocking of continuous flow systems as early reported by Khan et al. (2022) and Jabbar et al. (2022). Moreover, microbial cell surfaces are coated with Fe3O4 NPs through strong physical adsorption due to the high specific surface area and high surface energy of NPs, where the smaller size of the NPs increases the surface area of the bacterial cell membrane, which enhances chemical activity and capacity for adsorbing many types of pollutants on their surfaces (Darabdhara et al. 2017). Integration between NPs and bacterial cells upgrades microbial activities and increase their reaction rates through overcoming technological limitations such as reduced solubility of hydrophobic substrate, reduced bioavailability to the microorganisms, kinetic limitations on degrading enzymes, un-healthy microorganisms, in addition to restoring magnetic NPs after complete treatment using external magnetic field for reuse (Ranmadugala et al. 2018; Jabbar et al. 2022). Thus, the current study was designed to develop a new assemblage of magnetite-coated bacteria, as an efficient and magnetically separable system, for bioremediation of pulp and paper industrial wastewater under optimum operation conditions using promising environmental bacterium. This assemblage was expected to have superior efficiency in removing the tested contaminants compared to its individual components since they gather different removal mechanisms (biodegradation, sorption, flocculation) that synergistically promote decontamination process at a remarkable level.
Materials and methods
Wastewater sampling
Wastewater was collected from the end pipes of 3 different pulp and paper manufacturing companies (RAKTA, ALAHLYA and ALMAMOORA), located in Alexandria Governorate, Egypt, during the course of the study. Pulp and paper manufacturing wastewaters are rich in cellulose fibers as well as other organic and inorganic contaminants. They were investigated before and after treatment with the proposed treatment.
Microorganisms
Based on the screening of 6 indigenous bacterial species isolated from pulp and paper-contaminated wastewater and 4 exogenous species (Pseudomonas stutzeri, Bacillus licheniformis, Bacillus sphaericus and Pseudomonas otitidis MCC10330) as well as their efficiency in the reduction of the investigated parameters, P. otitidis MCC10330 (ATCC BAA-1130) considered the most promising (Mohamed 2022). It was selected since it had the broad and highest degradation/accumulation activities for the contaminants in the pulp and paper waste effluent compared to the other tested bacteria. Pseudomonas otitidis MCC10330 was kindly provided, from the microbial collection at the Institute of Graduate Studies & Research, Alexandria University (IGSR). The selected bacterium was maintained on nutrient agar (NA) medium and prior to each experiment, the culture was reactivated overnight. It was selected, decorated with magnetite nanoparticles (NPs) and the efficiency of Fe3O4 NPs / P. otitidis assemblage for decontaminating pulp and paper waste effluent was investigated.
Synthesis and characterization of magnetite NPs
Fe3O4 Nanoparticles (Fe3O4 NPs) were synthesized using reverse co-precipitation method under N2 stream during the whole preparation steps (Sulistyaningsih et al. 2015; Mahmoud et al. 2013). A mixture of 5.2 g of FeCl3·6H2O and 2.674 g of FeSO4·7H2O were dissolved in 50 mL deionized water and stirred for 15 min under inert atmosphere at room temperature. Ammonia solution (25%) was added to the mixture till pH ~ 10 under continuous stirring for another 30 min to achieve nucleation and formation of magnetite nanoparticles. Glycine (1.2 g) was added to the magnetite NPs (Fe3O4) as a surfactant to stabilize their dispersion, adjust particle size and surface functionalization. The black solid product was sonicated using ultrasonic bath for 20 min. Fe3O4 NPs were isolated from the solution using an external magnet, washed three times with deoxygenated water and ethanol then dispersed and stored in ethanol followed by drying at 50 °C until complete dryness. Formation of Fe3O4 NPs followed the equation:
2FeCl3 + FeSO4 + 4H2O + 8NH3 → Fe3O4 + (NH4)2SO4 + 6NH4Cl
Morphological, chemical and thermal stability properties of the Fe3O4 NPs were characterized. Their particles size and morphology were determined using transmission electron microscope (TEM JEOL, JEM 100 CX, Japan). X-ray diffractometer (XRD BRUKER D8 Advance Cu target, Germany) was employed to examine their crystal phase. It was operating with CuKα radiation (λ = 1.54 A°) generated at 40 kV and 40 mA. Stability/degradation processes was estimated by thermal gravimetric analysis (TGA). Fourier Transform Infrared Spectroscopy (FT-IR, Model 8400 S, Shimadzu, Japan) was used to determine the Fe3O4 NPs functional groups.
Development and characterization of the Fe 3 O 4 NPs-immobilized bacterial assemblage
Pseudomonas otitidis MCC10330 (the most active and promising strain) was cultured for 24 h at 37 °C in nutrient broth (NB) medium till the microbial density reached ∼0.5 g [dry weight cells/L]. Fe3O4-(1.5 g) dissolved in 100 mL solution (50 mL DW + 50 mL absolute ethanol) in a conical flask and incubated for 10 min in a sonicator water bath. Fe3O4 powder breakage was achieved by sonication shock waves that help breaking intermolecular interactions, thus, speed dissolution. Then, a fixed volume of the culture (200 mL = 0.10 g bacterial weight) was mixed with the prepared Fe3O4 NPs solution equivalent to cells: magnetite (g/g) ratio of 1:3 (that resulted in the highest coating efficiency according to a previous study) (El Bestawy et al. 2020) using shaking incubator (NEW BRUNSWICK SCIENTIFIC, NEW BRUNSWICK, N.J., USA) at 180 rpm and 37 °C. After one hour, the Fe3O4 NPs/bacterial assemblage was collected and separated from the supernatant plus the free uncoated cells, which can then be harvested.
Characterization of the Fe3O4 NPs-immobilized bacterial assemblage was previously performed (El Bestawy et al. 2020) using Fourier transform infrared (FT-IR) to determine its functional groups while its morphological characteristics were determined using scanning electron microscope (SEM). Their results described bacteria in the micrograph as rod-shaped cells that immobilized by several layers of Fe3O4 NPs and attributing the strong coverage to the size of the NPs (typically about 2 orders of magnitude smaller than the bacterial cell), which allows the attachment of multiple NPs onto a bacterial cell.
Optimization of lignin removal by Fe 3 O 4 NPs
Removal of lignin by magnetite NPs under different conditions of pH (6–8), amount of nanosorbent (10–150 mg) and contact time (5–15 min) was investigated to determine the optimized conditions for maximum lignin removal from contaminated wastewater effluent.
Treatment bioassay using Fe 3 O 4 NPs/immobilized bacterial assemblage
Schematic diagram of the treatment process of pulp and paper wastewater using the developed Fe3O4 NPs/bacterial biomass assembly is illustrated in Fig. 1. Pseudomonas otitidis MCC10330, the selected bacterium was cultured in 200 mL NB and incubated for 24 h at 37 °C to obtain high cell density. El Bestawy et al. (2020) reported that magnetite–microbial cells assembly prepared at 1:3 ratio bacteria: Fe3O4 w/w provides the highest bacterial growth and immobilization ability, which is supported by Yan et al. (2019). It was left for one hour at 37 °C under 180 rpm shaking speed (the perfect time of coating process). Then, the prepared magnetite–microbial cells assembly culture (200 mL) was seeded into to 800 mL raw industrial wastewater (1L culture) after determination of the start-up bacterial density (CFU/mL). Fe3O4 NPs/immobilized bacterial assemblage in the wastewater sample was incubated for 4 h under shaking (180 rpm) and treated samples were collected at 1 h interval. A control culture (Fe3O4 NPs free–P. otitidis culture) was subjected to the same investigation in parallel and under the same conditions for 4 h to decontaminate pulp and paper wastewater. Tested parameters were determined in the raw and treated wastewater to define the initial pollution load and their residues after treatment as well as the effectiveness of the remediation process.
Characterization of the raw and treated industrial effluent
Total tannin and lignin, the main targeted contaminant in the pulp and paper wastewater, as well as other quality parameters (pH, temperature, dissolved oxygen content, total suspended solids, total dissolved solids, biochemical oxygen demand, chemical oxygen demand and total viable count of bacteria) were determined in the raw and treated wastewater according to the standard techniques of Rice et al. (2017) to determine the removal efficiency at each exposure time.
Statistical analysis
Multifactorial analysis (ANOVA) was performed for the different contaminants in the industrial effluents by Pearson's correlation coefficient (r) at different confidence levels.
Results and discussion
As shown in the previous work by the authors (Mohamed 2022), P. otitidis proved being the most active to remove the involved pollutants. Therefore, it was selected to treat pulp and paper-contaminated wastewater as immobilized cells using magnetite nanoparticles.
Characterization of Fe 3 O 4 NPs
Physical properties of magnetite nanoparticles (Table 1 and Fig. 2A–D) confirmed presence of nanoscale particles and aggregates with substantial interfacial contact areas per unit volume. Sonication was required to disrupt powder agglomerates and effectively break down the weaker Van der Waals forces.
-
1.
TEM analysis illustrated spherical shape of Fe4O3 NPs with size ranging from 8.75 to 13.8 nm and partial aggregation (Fig. 2A).
-
2.
XRD analysis indicated the Fe4O3 NPs crystalline structure with characteristic diffraction peaks at 2θ = 30°, 35°, 43°, 50, 57.2°, 53.7°, 57, 63 and 73.8° as shown in Fig. 2B, which are attributed to the cubic phase structure of Fe4O3 NPs (according to JCPDS card, No. 01-089-0691). Results confirmed that other types of iron oxides are nor present and also signposted that Fe4O3 NPs are well crystallized.
-
3.
Two strong characteristic absorption peaks at 440 cm−1 and 570 cm−1 are shown in the FT-IR analysis of Fe4O3 NPs, which are related to the Fe–O bond (Fig. 2C). Intermolecular hydrogen bonding was illustrated by the absorption peak at ~ 3400 cm−1as shown in all nanomaterials.
-
4.
Fe4O3 NPs showed transition temperatures high thermal stability according to the TGA thermograms (Fig. 2D).
Fe3O4 NPs are partially aggregated as shown in the TEM micrograph, attributed to their superparamagnetic behavior, which is supported by previous studies (Gnanamoorthy et al. 2020; Sallam et al. 2018; El Bestawy et al. 2020). Other characteristics of Fe3O4 NPs are consistent with those reported by other workers concerning FT-IR, X-ray diffraction (Gonçalves et al. 2021) and TGA(Nayl et al. 2022).
Bioremediation of pulp and paper industrial effluent using Fe 3 O 4 /P. otitidis assemblage
Immobilization of bacterial cells with nanomaterial such as Fe3O4 NPs is a novel technique attracted much attention due to the remarkable enhancement in their activity and efficiency after acquiring the unique properties of Fe3O4 NPs such as super para-magnetism, biocompatibility, large surface area, high adsorption capacity of different pollutants and high surface energy that remarkably enhance adsorption, biodegradation and elimination of contaminants. Moreover, Fe3O4 NPs are easy to synthesize and can be regenerated and magnetically recyclable, which is very useful, sustainable and saves time in practical applications. The bacteria/ Fe3O4 NPs assemblage efficiency is controlled by physicochemical properties of Fe3O4 NPs, bacterial cells surface properties and environmental/culture conditions (Stylianou et al. 2021). In the present study, most of the Fe3O4 NPs have particle size more than 10 nm in diameter; therefore, they seem to be suitable for assembling with bacteria for remediation processes without the risk of their penetration into bacterial cell membrane, damaging the cells or creation of abnormal cell function at 10 nm or less (Banerjee et al. 2021).
Fe3O4 NPs/P. otitidis assemblage (3:1w/w) and NPs-free P. otitidis culture (control) were examined at 0, 1, 2, 3 and 4 h to decontaminate pulp and paper wastewater as shown in Table 2 and Figs. 3,4, and 5. Bioremediation assay concluded the following points:
-
1.
Raw wastewater recorded 7.0, 7.61, 686, 898, 450, 245 and 21 mg/L for pH, DO, TDS, TSS, COD, BOD and total tannin and lignin, respectively.
-
2.
Magnetite/bacterial cells assembly recorded the highest removals (64.1, 52.0, 54.3 and 66.6%) of TSS, COD, BOD and total tannin and lignin after 1, 4, 4 and 3 h reaching RCs of 322, 216, 112 and 7 mg/L, respectively.
-
3.
The highest removals of TSS, COD, BOD and total tannin and lignin achieved by the control (Fe3O4 NPs-free P. otitidis culture) recorded 82.8, 52.7, 50.0 and 57.1% after 2, 4, 4 and 2 h, respectively, which is attributed mainly to microbial degradation process. Compared with Fe3O4/P. otitidis culture, it is clear that Fe3O4 NPs enhanced the removal of total tannin and lignin reaching 66.6% instead to 57.1% achieved by the control culture.
-
4.
The RCs of pH, DO and TDS in the effluent treated with the proposed system are accepted for safe discharging, while TSS, COD and BOD levels are slightly higher (5.35, 2.7 and 1.86-fold) than their MPLs (60, 80 and 60 mg/L, respectively). Yet, there is no MPL stated for tannin and lignin in the Egyptian regulations.
-
5.
Although a very short time (4 h) was applied for this treatment bioassay, considerably very high reductions were achieved. Some of the tested pollutants are still higher than their MPLs for the safe discharge, which may be attributed to the short treatment time, small inoculum size or dose of the nanoparticles.
In nature, many species of fungi and bacteria can degrade lignin by secreting enzymes (Janusz et al. 2017). Metabolic pathway (mechanism) of lignin by the degrading microorganisms takes place through 2 steps (Zhao et al. 2022). De-polymerization of lignin is the first and most sophisticated step during lignin bioconversion into valuable products. Lignin de-polymerization involves two sequential processes; firstly, lignin is decomposed into oligomers or monomers (Xu et al. 2018), and secondly these products are completely degraded into carbon dioxide, water and minerals. Various specialized microbial enzymes that can depolymerize or mineralize lignin components have been isolated from specialized fungi and bacteria. They include class II heme-containing peroxidases, laccases and other auxiliary enzymes (Odwa et al. 2020). Minerals and low molecular weight phenolic compounds in the lignin as well as nutrient balance (C/N ratio) in the surrounding medium are stimulatory factors, which directly influence the production of ligninolytic enzymes (Raychaudhuri and Behera 2022). The second step of lignin biotransformation is microbial catabolism of aromatic derivatives (low molecular weight) of lignin, which is used by many microorganisms as carbon and energy sources for cell growth (Levy-Booth et al. 2022).
Bacteria/Fe3O4 NPs assemblage considered highly efficient system for wastewater treatment, where it characterized by cost-effective preparation, desirable electrical conductivity, paramagnetic and absorptive activity as well as being eco-friendly to the environment (Fang et al. 2022). In the present study, the proposed Fe3O4 NPs/P. otitidis assemblage could achieve considerably very high reductions of TSS, COD, BOD and total tannin and lignin within very short time (4 h), confirming tremendous enhancement in the bacterial activity due to the presence of the nanoparticles, as reported also by El Bestawy et al. (2020) and Liu et al. (2020). Compared to other conventional treatment methods, the proposed technology can be considered as an efficient, simple, economical and flexible alternative to be used in wastewater bioremediation (Table 3).
RCs of pH, DO and TDS in the effluent treated with the proposed system are accepted for safe discharging, while TSS, COD and BOD levels (5.35, 2.7 and 1.86-fold) were slightly higher than their MPLs, respectively. The main causes of having RCs higher than their MPLs (short treatment time, small inoculum size or dose of the nanoparticles) can be optimized to produce high quality and safe effluent. This is supported by other workers. Results indicated also high recyclability of Fe3O4 NPs and recommend its use as a good adsorbent to remove lignin from aqueous solutions (Li et al. 2024).
Stimulatory effect of the pulp and paper industrial effluent on the growth of the tested bacterium
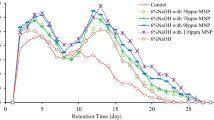
Effects of the tested wastewater on the stimulation or inhibition of the tested bacterium growth estimated as total viable count (TVC) were investigated at the starting and ending points of the treatment process (Fig. 6). P. otitidis decorated with Fe3O4 NPs at 3:1 ratio (magnetite NPs: bacteria) recorded the highest growth rate (11.0 × 106 CFU/mL) after 4 h, which is equivalent to 7.4-fold higher density than the initial inoculum at the starting point, indicating high metabolic activity. Moreover, Fe3O4 NPs coated bacteria showed remarkable increase in the growth rate compared to the control (Fe3O4 NPs-free culture) that reached the highest growth rate of only 1.8 × 106 CFU/mL after 2 h followed by slow decline till the end of the bioassay. This clearly confirmed the stimulation and protective effect of the Fe3O4 NPs on the growth of the associated bacteria as well as their low toxicity on living bacterial cells as stated by Konate et al. (2018) and El Bestawy et al. (2020).
Stimulation of bacterial growth rate with increase in magnetite concentration, especially at magnetite to bacterial cells ratio 3:1 (w/w), was supported by other workers who reported growth enhancement up to 500 ppm magnetite NPs (Konate et al. 2018). It was also proved that the 3:1 ratio resulted in the highest values of biomass (7.4-fold higher density than the initial inoculum) after only 4 h. Fe3O4-bacterial culture biomass showed continuous increase till the last exposure compared to the start-up density. Such results confirmed the regular ascending stimulation effects of the nanoparticles on the growth and multiplication of the tested bacteria till the end of the experiment. This stimulation resulted from the super paramagnetic behavior of Fe3O4 NPs accompanied by their low toxicity on the living cells. Moreover, glycine modification of Fe3O4 NPs with different surfactants has negligible toxicity on eukaryote cells compared to free NPs (Kafayati et al. 2013; El Bestawy et al. 2020).
Optimization of lignin adsorption using synthesized magnetite NPs
In order to reach the maximum lignin removal, optimization of lignin (100 mg/L solution) adsorption on magnetite NPs under different pH values, nanoadsorbent dosage and contact times was investigated. Results revealed the following points:
-
1.
Results showed decrease in lignin adsorption with the increase in pH value from 6.0 to 8.0 (Fig. 7A). Therefore, 6 considered the optimal pH.
-
2.
There was a regular increase in the RE of lignin (28 mg/L) with increase in Fe3O4 NPs dosage (10, 25, 50, 100, 150 mg), reaching the highest removal of 82.14% at 100 mg adsorbent and still constant at 150 mg (Fig. 7B). Therefore, Fe3O4 NPs dosage of 100 mg was considered the optimal dose.
-
3.
Similarly, results indicated increase in the RE of lignin (initial concentration of 35 mg/L) with increase in contact time (5, 10 and 15 min) reaching the highest removal (71.4%) after 10 min, which still constant up to the last contact time of 15 min (Fig. 7C). Therefore, 10 min was considered the optimal contact time.
-
4.
It was then concluded that the optimized conditions for the maximum lignin adsorption and removal using Fe3O4 NPs were found to be achieved at pH 6, Fe3O4 NPs dosage of 100 mg and 10 min contact time.
Optimization of lignin adsorption on Fe3O4 NPs under different conditions; pH (A), nanoadsorbent dosage (mg) (B) and contact time. Such conditions are the key factors that are greatly controlling the efficiency of the remediation process as well as adjusting the shortest time for the best treatment, which is the main focus of the present study
The effect of solution pH on lignin absorption may be attributed to the influence on the surface properties of the adsorbent and the ionization/dissociation of lignin. Decrease of absorption was detected with pH increase. Similar findings reported that adsorption decreased with increase in pH in the range 2.0–9.0. Moreover, the maximum lignin adsorption was achieved at 10 min contact time and increased with increasing Fe3O4 NPs dosage, as also supported by other workers (Li et al. 2024).
It is worth to clarify that only 0.3 g is required to prepare the Fe3O4 NPs/P. otitidis assemblage (as shown in the material and methods) that was used to remediate pulp and paper manufacturing wastewater with 21 mg/L total lignin. In case of using Fe3O4 NPs only, dosage of 100 mg Fe3O4 NPs is required for lignin (28 mg/L) removal. Considering the fact that Fe3O4 NPs are easy to synthesize and can be regenerated and magnetically recyclable, as shown by Stylianou et al. (2021) and Sulistyaningsih et al. (2015), it is confirmed that the proposed technology is very useful, sustainable, fast and cost-effective in practical applications for treatment of real pulp and paper manufacturing wastewater on industrial scale. Moreover, no sludge was produced during the treatment with the proposed system; pollutants were digested (biologically decomposed), which is another valuable advantage. This is clearly shown in Fig. 8, which compares between pulp and paper effluent before and after treatment with the proposed technology. Moreover, comparison with similar previous works confirmed that the proposed technology present superior solution for the removal of lignin and other included contaminants from heavily polluted real pulp and paper manufacturing raw effluents. Not only the high removal efficiency, but economically, it is much cheaper than other technologies, very fast (within 4 h) and performed at the prevailed conditions with no agitation, temperature or pH adjustment are required. Therefore, the proposed treatment considered highly effective, environmentally friendly, renewable and strongly recommended for use for decontamination of pulp and paper industrial wastewater.
Statistical analysis
Multifactorial analysis (ANOVA) for the different contaminants in the industrial effluents was performed during the batch bioassay of Fe3O4/P. otitidis assemblage by Pearson's correlation coefficient (r) at different confidence levels (95 and 99%) and presented in Table 4. Data in the table concluded the following points:
-
1.
BOD showed highly significant positive correlations (at 0.01) with only COD, while it was insignificantly correlated with other tested parameters.
-
2.
BOD showed significant positive correlations (at 0.05) with total tannin and lignin
-
3.
COD showed significant positive correlations (at 0.05) with total tannin and lignin
-
4.
No other significant correlations were detected.
-
5.
Statistical analysis confirmed that high and significant correlations were recorded among most of the tested parameters during the bioremediation process and are all affecting positively or negatively the remediation efficiency.
Conclusions
Results of the present study achieved the following conclusions.
-
1.
Pulp and paper effluent contained extremely high levels of all the tested pollutants, especially COD, BOD and TSS, which creates many difficulties in its treatment and dangerously affecting the receiving environments.
-
2.
P. otitidis MCC10330 (ATCC BAA-1130) proved being the most active when previously screened with other 9 bacterial species. Therefore, it was selected, immobilized using magnetite nanoparticles and used to treat pulp and paper-contaminated wastewater.
-
3.
Using the proposed magnetite-coated bacterial assembly system is remarkably efficient since it enhanced the growth of P otitidis and achieved the highest removals of TSS, COD, BOD, total tannin and lignin, prevented sludge production and reduced treatment time during the treatment process. This is also confirmed by comparison with previous works (Table 3).
-
4.
Although the very short time (4 h) applied for this bioassay, considerably very high reductions were achieved. However, some of the tested pollutants (TSS, COD, and BOD) are still slightly higher than their MPLs for the safe discharge, which may be attributed to the short treatment time, small inoculum size or dose of the nanoparticles. To overcome pollutants RCs that are not compiling with the environmental laws, it is highly recommended to scale up a suitable unit for remediation, increase Fe3O4 NPs dose and bacterial inoculum size as well as performing physical pre-treatment step (sedimentation) before treatment using the proposed Fe3O4/bacteria assembly system.
-
5.
Finally, the proposed assemblage can be directly augmented, at the optimized dose, into the existing treatment system (i.e., activated sludge) without the need to any infrastructures, or used as a very compacted tertiary biofilter unit after the secondary (biological) treatment in a continuous system, all of which confirmed the ease of application.
Data availability
Data obtained and analyzed in this study are presented in the main work. The manuscript was not submitted to a preprint server before submitting it to “Applied Water Science” and data are not shared with any other party.
References
Abhishek A, Dwivedi A, Tandan N, Kumar U (2017) Comparative bacterial degradation and detoxification of model and kraft lignin from pulp paper wastewater and its metabolites. Appl Water Sci 7:757–767. https://doi.org/10.1007/s13201-015-0288-9
Abo-zeid Y, Ismail NSM, McLean GR, Hamdy NM (2020) A molecular docking study repurposes FDA approved iron oxide nanoparticles to treat and control COVID-19 infection. Eur J Pharm Sci 153:105465. https://doi.org/10.1016/j.ejps.2020.105465
Ahmed M, Mavukkandy MO, Giwa A et al (2022) Recent developments in hazardous pollutants removal from wastewater and water reuse within a circular economy. NPJ Clean Water 5:12. https://doi.org/10.1038/s41545-022-00154-5
Banerjee A, Choudhury M, Chakravorty A, et al (2021) Microbiological degradation of organic pollutants from industrial wastewater. In: Nanobiotechnology for green environment. CRC Press, pp 83–114
Brown DM, Pawlak J, Grunden AM (2021) Bacterial valorization of pulp and paper industry process streams and waste. Appl Microbiol Biotechnol 105:1345–1363. https://doi.org/10.1007/s00253-021-11107-2
Coimbra ECL, Mounteer AH, do Carmo ALV et al (2021) Electrocoagulation of kraft pulp bleaching filtrates to improve biotreatability. Process Saf Environ Prot 147:346–355. https://doi.org/10.1016/j.psep.2020.09.039
Costa S, Dedola DG, Pellizzari S et al (2017) Lignin biodegradation in pulp-and-paper mill wastewater by selected white rot fungi. Water 9:935. https://doi.org/10.3390/W9120935
Cyganowski P (2021) Fully recyclable gold-based nanocomposite catalysts with enhanced reusability for catalytic hydrogenation of p-nitrophenol. Colloids Surf A Physicochem Eng Asp 612:125995. https://doi.org/10.1016/j.colsurfa.2020.125995
Dagar S, Singh SK, Gupta MK (2022) Economics of advanced technologies for wastewater treatment: evidence from pulp and paper industry. Front Environ Sci. https://doi.org/10.3389/fenvs.2022.960639
Darabdhara G, Boruah PK, Hussain N et al (2017) Magnetic nanoparticles towards efficient adsorption of gram positive and gram negative bacteria: an investigation of adsorption parameters and interaction mechanism. Colloids Surf A Physicochem Eng Asp 516:161–170. https://doi.org/10.1016/j.colsurfa.2016.12.003
El BE, El-Shatby BF, Eltaweil AS (2020) Integration between bacterial consortium and magnetite (Fe3O4) nanoparticles for the treatment of oily industrial wastewater. World J Microbiol Biotechnol 36:141. https://doi.org/10.1007/s11274-020-02915-1
Esmaeeli A, Sarrafzadeh M-H, Zeighami S et al (2023) A comprehensive review on pulp and paper industries wastewater treatment advances. Ind Eng Chem Res 62:8119–8145. https://doi.org/10.1021/acs.iecr.2c04393
Fang Y, Hu J, Wang H et al (2022) Development of stable agar/carrageenan-Fe3O4-Klebsiella pneumoniae composite beads for efficient phenol degradation. Environ Res 205:112454. https://doi.org/10.1016/j.envres.2021.112454
Gnanamoorthy G, Muthukumaran M, Varun Prasath P et al (2020) Enhanced photocatalytic performance of Sn 6 SIO8 nanoparticles and their reduced graphene oxide (rGO) nanocomposite. J Nanosci Nanotechnol 20:5426–5432. https://doi.org/10.1166/JNN.2020.17814
Gonçalves J, Nunes C, Ferreira L et al (2021) Coating of magnetite nanoparticles with fucoidan to enhance magnetic hyperthermia efficiency. Nanomaterials 11:2939. https://doi.org/10.3390/NANO11112939/S1
Goycoechea N, Borges I, Castello E, Borzacconi L (2023) Improvements in the anaerobic digestion of biological sludge from pulp and paper mills using thermal pretreatment. Waste Manage Res 41:1331–1341. https://doi.org/10.1177/0734242X231154198
He S, Zhong L, Duan J et al (2017) Bioremediation of wastewater by iron Oxide-Biochar nanocomposites loaded with photosynthetic bacteria. Front Microbiol 8:247898. https://doi.org/10.3389/FMICB.2017.00823/BIBTEX
Hooda R, Bhardwaj NK, Singh P (2018) Brevibacillus parabrevis MTCC 12105: a potential bacterium for pulp and paper effluent degradation. World J Microbiol Biotechnol 34:1–10. https://doi.org/10.1007/S11274-018-2414-Y/METRICS
Hovikorpi KS, Vakkilainen EK, Simao G et al (2020) NCG system and safety in modern large eucalyptus Kraft pulp mills. Appita Magazine 1:67–76
Jabbar KQ, Barzinjy AA, Hamad SM (2022) Iron oxide nanoparticles: Preparation methods, functions, adsorption and coagulation/flocculation in wastewater treatment. Environ Nanotechnol Monit Manag 17:100661. https://doi.org/10.1016/j.enmm.2022.100661
Jagaba AH, Birniwa AH, Usman AK et al (2023) Trend and current practices of coagulation-based hybrid systems for pulp and paper mill effluent treatment: mechanisms, optimization techniques and performance evaluation. J Clean Prod 429:139543. https://doi.org/10.1016/j.jclepro.2023.139543
Jansson K (2022) Development of advanced oxidation processes for the Finnish pulp and paper industry water treatment. Åbo Akademi University Press, 2022. ISBN 978–952–389–032–9
Janusz G, Pawlik A, Sulej J, Swiderska-Burek U, Jarosz-Wilkolazka A, Paszczynski A (2017) Lignin degradation: microorganisms, enzymes involved, genomes analysis and evolution. FEMS Microbiol Rev 41(6):941–962. https://doi.org/10.1093/femsre/fux049
Kafayati ME, Raheb J, Torabi Angazi M, Alizadeh S, Bardania H (2013) The effect of magnetic Fe3O4 nanoparticles on the growth of genetically manipulated bacterium, Pseudomonas aeruginosa (PTSOX4). Iran J Biotechnol 11(1):41–46
Khan A, Malik S, Ali N, et al (2022a) Chapter 16 - Treatment of pulp and paper industry waste effluents and contaminants. In: Bhat R, Kumar A, Nguyen TA, Sharma S (eds) Nanotechnology in Paper and Wood Engineering. Elsevier, 349–370
Khan J, Ali MI, Jamal A et al (2022b) Response of mixed bacterial culture towards dibenzothiophene desulfurization under the influence of surfactants and microscopically (SEM and TEM) characterized magnetic Fe3O4 nanoparticles. Microsc Res Tech 85:3838–3849. https://doi.org/10.1002/jemt.24230
Kipping L, Jehmlich N, Moll J et al (2024) Enzymatic machinery of wood-inhabiting fungi that degrade temperate tree species. ISME J. https://doi.org/10.1093/ismejo/wrae050
Kiss É (2020) Nanotechnology in Food Systems: a Review. Acta Alimentaria AAlim 49:460–474. https://doi.org/10.1556/066.2020.49.4.12
Konate A, Wang Y, He X et al (2018) Comparative effects of nano and bulk-Fe3O4 on the growth of cucumber (Cucumis sativus). Ecotoxicol Environ Saf 165:547–554. https://doi.org/10.1016/j.ecoenv.2018.09.053
Kumar A, Anushree KJ, Bhaskar T (2020) Utilization of lignin: a sustainable and eco-friendly approach. J Energy Inst 93:235–271. https://doi.org/10.1016/j.joei.2019.03.005
Kumar A, Saxena G, Kumar V, Chandra R (2021a) Chapter 15-Environmental contamination, toxicity profile and bioremediation approaches for treatment and detoxification of pulp paper industry effluent. In: Saxena G, Kumar V, Shah MP (eds) Bioremediation for Environmental Sustainability. Elsevier, pp 375–402
Kumar A, Srivastava NK, Gera P (2021b) Removal of color from pulp and paper mill wastewater- methods and techniques- A review. J Environ Manage 298:113527. https://doi.org/10.1016/j.jenvman.2021.113527
Kumar M, Revathi K, Khanna S (2015) Biodegradation of cellulosic and lignocellulosic waste by Pseudoxanthomonas sp R-28. Carbohydr Polym 134:761–766. https://doi.org/10.1016/j.carbpol.2015.08.072
Le TM, Tran UPN, Duong YHP et al (2022) Sustainable bioethanol and value-added chemicals production from paddy residues at pilot scale. Clean Technol Environ Policy 24:185–197. https://doi.org/10.1007/s10098-021-02097-w
Levy-Booth DJ, Navas LE, Fetherolf MM et al (2022) Discovery of lignin-transforming bacteria and enzymes in thermophilic environments using stable isotope probing. ISME J 16:1944–1956. https://doi.org/10.1038/s41396-022-01241-8
Li S, Li X, Li S et al (2024) In-situ preparation of lignin/Fe3O4 magnetic spheres as bifunctional material for the efficient removal of metal ions and methylene blue. Int J Biol Macromol 259:128971. https://doi.org/10.1016/j.ijbiomac.2023.128971
Liu Y, Feng H, Fu R et al (2020) Induced root-secreted d-galactose functions as a chemoattractant and enhances the biofilm formation of Bacillus velezensis SQR9 in an McpA-dependent manner. Appl Microbiol Biotechnol 104:785–797. https://doi.org/10.1007/s00253-019-10265-8
Mahmoud ME, Abdelwahab MS, Fathallah EM (2013) Design of novel nano-sorbents based on nano-magnetic iron oxide–bound-nano-silicon oxide–immobilized-triethylenetetramine for implementation in water treatment of heavy metals. Chem Eng J 223:318–327. https://doi.org/10.1016/j.cej.2013.02.097
Malik S, Kishore S, Shah MP, Kumar SA (2022) A comprehensive review on nanobiotechnology for bioremediation of heavy metals from wastewater. J Basic Microbiol 62:361–375. https://doi.org/10.1002/jobm.202100555
Mohamed AA (2022) Magnetite nano particles modified biodegradation of lignin and lignocellulose constituents in the pulp and paper industry black liquor. MSc, Institute of Graduate Studies and Research, Alexandria University.
Moreira WM, Viotti PV, de Moura AA et al (2022) Synthesis of a biobased resin and its screening as an alternative adsorbent for organic and inorganic micropollutant removal. Environ Sci Pollut Res 29:79935–79953. https://doi.org/10.1007/s11356-021-18250-3
Murshid S, Antonysamy A, Dhakshinamoorthy G et al (2023) A review on biofilm-based reactors for wastewater treatment: Recent advancements in biofilm carriers, kinetics, reactors, economics, and future perspectives. Sci Total Environ 892:164796. https://doi.org/10.1016/j.scitotenv.2023.164796
Nayl AA, Abd-Elhamid AI, Ahmed IM, Bräse S (2022) Preparation and characterization of magnetite talc (Fe3O4@Talc) nanocomposite as an effective adsorbent for Cr(VI) and Alizarin red S dye. Materials 15:3401. https://doi.org/10.3390/MA15093401/S1
Ning J, Chen D, Liu Y et al (2021) Efficient adsorption removal and adsorption mechanism of basic fuchsin by recyclable Fe3O4@CD magnetic microspheres. J Cent South Univ 28:3666–3680. https://doi.org/10.1007/s11771-021-4845-0
Nordin A, Strandberg A, Elbashir S et al (2020) Co-combustion of municipal sewage sludge and biomass in a grate fired boiler for phosphorus recovery in bottom ash. Energies (basel) 13:1708
Núñez D, Oulego P, Collado S et al (2022) Recovery of organic acids from pre-treated kraft black liquor using ultrafiltration and liquid-liquid extraction. Sep Purif Technol 284:120274. https://doi.org/10.1016/j.seppur.2021.120274
Odwa DVB, Viljoen-Bloom M, van Zyl WH (2020) Microbial lignin peroxidases: applications, production challenges and future perspectives. Enzyme Microb Technol 141:109669. https://doi.org/10.1016/j.enzmictec.2020.109669
Palani G, Arputhalatha A, Kannan K et al (2021) Current trends in the application of nanomaterials for the removal of pollutants from industrial wastewater treatment—a review. Molecules 26:2799. https://doi.org/10.3390/MOLECULES26092799
Pandey K, Sharma S, Saha S (2022) Advances in design and synthesis of stabilized zero-valent iron nanoparticles for groundwater remediation. J Environ Chem Eng 10:107993. https://doi.org/10.1016/j.jece.2022.107993
Podkościelna B, Matuszewska A, Stefaniuk D et al (2022) Interactions between biofiller-modified polymeric composites and wood-rotting fungi in terms of their biotechnological applications. Ind Crops Prod 186:115125. https://doi.org/10.1016/j.indcrop.2022.115125
Priyadarshini A, Kumar Sahoo P, Ghosal A, Kumar Sahoo N (2022) Stabilization of zero-valent iron for wastewater treatment: challenges and future prospective. Mater Today Proc 67:1073–1079. https://doi.org/10.1016/j.matpr.2022.07.029
Ramos MDN, Rangel AS, Azevedo KS et al (2022) Characteristics and treatment of Brazilian pulp and paper mill effluents: a review. Environ Monit Assess 194:651. https://doi.org/10.1007/s10661-022-10331-1
Ranmadugala D, Ebrahiminezhad A, Manley-Harris M et al (2018) Magnetic immobilization of bacteria using iron oxide nanoparticles. Biotechnol Lett 40:237–248. https://doi.org/10.1007/s10529-017-2477-0
Ribeiro JP, Cruz NC, Neves MC et al (2023) Granulated biomass fly ash coupled with fenton process for pulp and paper wastewater treatment. Environ Pollut 317:120777. https://doi.org/10.1016/j.envpol.2022.120777
Rice EW, Baird RB, Eaton AD (2017) Standard methods for the examination of water and wastewater; American Public Health Association (APHA), American Water Works Association (AWWA) and Water Environment Federation (WEF). In: Federation, American Public Health Association. ISBN: 9780875532875. https://www.standardmethods.org/
Raychaudhuri A, Behera M (2022) Microbial degradation of lignin: conversion, application, and challenges (Chapter 9). In Development in Wastewater Treatment Research and Processes, Editor(s): Shah MP, Rodriguez-Couto S, Riti Thapar Kapoor RT, Elsevier, pp. 195–219
Saeed MA, Niedzwiecki L, Arshad MY et al (2022) Combustion and explosion characteristics of pulverised wood, valorized with mild pyrolysis in pilot scale installation, using the modified ISO 1m3 dust explosion vessel. Appl Sci 12:12928
Sallam SA, El-Subruiti GM, Eltaweil AS (2018) Facile synthesis of Ag–γ-Fe2O3 superior nanocomposite for catalytic reduction of nitroaromatic compounds and catalytic degradation of methyl orange. Catal Letters 148:3701–3714. https://doi.org/10.1007/s10562-018-2569-z
Saski EK, Jokela JK, Salkinoja-Salonen MS (2020) Biodegradability of different size classes of bleached kraft pulp mill effluent organic halogens during wastewater treatment and in lake environments. In: Environmental Fate and Effects of Pulp and Paper. CRC Press, pp 179–193
Sharma RK, Arora DS (2015) Fungal degradation of lignocellulosic residues: an aspect of improved nutritive quality. Crit Rev Microbiol 41:52–60. https://doi.org/10.3109/1040841X.2013.791247
Singh AK, Kumar A, Chandra R (2022) Environmental pollutants of paper industry wastewater and their toxic effects on human health and ecosystem. Bioresour Technol Rep 20:101250. https://doi.org/10.1016/j.biteb.2022.101250
Sonkar M, Kumar V, Dutt D (2020) Use of paper mill sludge and sewage sludge powder as nitrogen and phosphorus sources with bacterial consortium for the treatment of paper industry wastewater. Biocatal Agric Biotechnol 30:101843. https://doi.org/10.1016/j.bcab.2020.101843
Stylianou M, Vyrides I, Agapiou A (2021) Oil biodesulfurization: a review of applied analytical techniques. J Chromatogr B 1171:122602. https://doi.org/10.1016/j.jchromb.2021.122602
Sulistyaningsih T, Siswanta D, Rusdiarso B (2015) Synthesis and characterization of magnetite obtained from mechanically and sonochemically assisted co-precipitation and reverse co-precipitation methods. Int J Mater Mech Manf 5(1):16–19
Teodor N, Asachi G, Dan S, Dragoi EN (2021) Differential evolution-based optimization of corn stalks black liquor decolorization with active carbon adsorption and TiO2 promoted photochemical degradation. https://doi.org/10.21203/RS.3.RS-658361/V1
Tripathy AP, Dixit PK, Panigrahi AK (2022) Impact of effluent of pulp & paper industry on the flora of river basin at Jaykaypur, Odisha, India and its ecological implications. Environ Res 204:111769. https://doi.org/10.1016/j.envres.2021.111769
Xu R, Zhang K, Liu P, Han H, Zhao S, Kakade A, Khan A, Du D, Li X (2018) Lignin depolymerization and utilization by bacteria. Biores Technol 269:557–566. https://doi.org/10.1016/j.biortech.2018.08.118
Yan Y, Fu D, Shi J (2019) Screening and immobilizing the denitrifying microbes in sediment for bioremediation. Water 11:614. https://doi.org/10.3390/W11030614
Yao T, Feng K, Xie M et al (2021) Phylogenetic Occurrence of the Phenylpropanoid Pathway and Lignin Biosynthesis in Plants. Front Plant Sci. https://doi.org/10.3389/fpls.2021.704697
Yoshimoto S, Ohara Y, Nakatani H, Hori K (2017) Reversible bacterial immobilization based on the salt-dependent adhesion of the bacterionanofiber protein AtaA. Microb Cell Fact 16:123. https://doi.org/10.1186/s12934-017-0740-7
Zhao X, Liu W, Cai Z et al (2020) Reductive immobilization of uranium by stabilized zero-valent iron nanoparticles: Effects of stabilizers, water chemistry and long-term stability. Colloid Surf A Physicochem Eng Asp 604:125315. https://doi.org/10.1016/j.colsurfa.2020.125315
Zhao L, Zhang J, Zhao D, Jia L, Qin B, Cao X, Zang L, Lu F, Liu F (2022) Biological degradation of lignin: a critical review on progress and perspectives. Ind Crops Prod 188 Part B:115715
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors declare that no funds, grants or other support were received from any organization during the preparation of the submitted work. The authors have no relevant financial or nonfinancial interests to disclose.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest or have also no competing interests to declare that are relevant to the content of this article.
Ethical approval
The authors confirmed that all elements of the submission are in compliance with the journal publishing ethics policy and that we have not submitted the manuscript to any preprint server before submitting it to Applied Water Sciences.
Informed consent
The authors declare that no human participants and/or animal studies were involved in the submitted research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
EL-Bestawy, E., Hassan, S.W.M. & Mohamed, A.A. Enhanced biodegradation of lignin and lignocellulose constituents in the pulp and paper industry black liquor using integrated magnetite nanoparticles/bacterial assemblage. Appl Water Sci 14, 211 (2024). https://doi.org/10.1007/s13201-024-02255-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-024-02255-7