Abstract
Biorefineries from paddy residues (rice straw and rice husk) have a high potential to satisfy human society’s need for sustainable fuel and chemical production. Biorefinery systems should be created in a sustainable and eco-friendly manner, accompanying the safe disposal of waste stream produced during processing. This study developed a pilot-scale biorefinery, whose bioethanol production from rice straw was an integrated recovery system for lignin, silica, and nutrient recovery. The recovery yield of silica and lignin from the black liquor of alkaline pretreatment was up to 96%, and the lignin purity reached 79% without the existence of carbohydrate fiber. After the recovery, the final liquid waste mainly contained inorganic matters and has a potential to be reused in the acidification step. The distillation residue was a nitrogen source for simultaneous saccharification and fermentation equivalent to corn steep liquor with the final ethanol concentration of 1.6 wt% in 160 h. The new process can be considered as a zero-waste biorefinery model. The material flow indicated that more valued products produced by the simple method increase the profits of this process. Also, the energy efficiency of the process was 0.53 that demonstrated the process’s economy and sustainability. The proposed system was feasible to foster the sustainable integration of local agricultural development and biomass industry shortly.
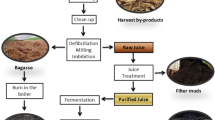
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biorefineries could be the most promising means of creating a sustainable bio-based economy because of their undeniable benefits (de Jong et al. 2012). Biorefinery cleanly produces various fuels, power, or heat that contribute to human energy needs; it also generates various chemical commodities and bioproducts in an environmentally sustainable manner by utilizing local agricultural residues and municipal wastes feedstocks, reducing disposal problems (Aresta et al. 2012). Local agricultural residues used as biorefinery feedstocks are derived from lignocellulose-rich biomass resources, including wood, straw, and grass. Biorefineries have been significantly advanced towards fractionating lignocellulose to its major constituents in the past decade (Saini et al. 2015). These constituents have been studied to process continuously into a range of products and energy using different process configurations with zero-waste generation to create more economically feasible biorefineries (Abraham et al. 2016).
Rice straw is one of the most abundant lignocellulosic residues in many agricultural countries. Rice cultivation generates large amounts of crop residues that do not find alternative use and are left behind in fields or burned. The burning of crop residues obtained from rice cropping systems causes greenhouse gas (GHG) emissions that ultimately lead to climate change. The exploitation of paddy straw for bioethanol generation emits fewer GHG amounts, while it produces lignin and other organic products during the process (Zhang 2020). Thus, paddy field-based biorefineries have gained much attention in recent years (Hatti‐Kaul 2010). Large rice-producing countries have adopted research strategies to develop small-scale biorefineries utilizing crop residues integrated with sustainability local agriculture (Serna‐Loaiza et al. 2019). Several pilot-scale plants have been made available, followed by R&D activities to develop fully-fledged systems. Bruins and Sanders (2012) redesigned the small-scale biorefinery process to minimize capital cost. This study indicated the prospects of residual materials to produce the high-value products. Kolfschoten et al. (2014) also designed small-scale biorefineries for the production of sugar and ethanol in the Netherlands. That process added value to the whole chain, farmers, and suppliers of processing services and covered the additional costs by processing waste materials into biogas. Paddy straw-based biorefinery systems could be based on a biochemical operation mode, including pretreatment or delignification, hydrolysis, and fermentation of cellulosic fractions into ethanol or other value-added products (Abraham et al. 2016). Pretreatment is the most crucial challenge of the biorefinery development to enrich cellulosic components for hydrolysis and fermentation into ethanol. A number of rice straw pretreatment technologies have been developed during the last century via physical, chemical, and biological pathways to achieve a high content of separated lignin without losing cellulosic fractions (Ruiz et al. 2020). Lignin isolated from black liquor of pretreatment obtained after has promising applications in bioplastics, composites, carbon fibers, adsorbents, and dispersants (Kaur and Kuhad 2019). Lignin then is chemically modified to a range of platform chemicals, followed by functionalizing or defunctionalizing into emerging structures and bulk chemicals (Sun et al. 2018). Rice straw is bulky and rigid lignocellulosic biomass containing high silica (SiO2) deposits (7–20%), can be isolated and further purified to amorphous silica that finds use in various industries (Chiew and Cheong 2011). Silica in nanosized materials like resins, catalysts, biological membranes, and batteries plays a significant role in biology, medicine, and electronics (Jeelani et al. 2020). Khaleghian et al. (2017) studied the effects of silica and lignin on hydrolysis of the bioethanol production process from rice straw. They reported using organosolv pretreatment and sodium carbonate pretreatment to remove silica and lignin from rice straw, respectively. The method removed 91% silica in rice straw and eliminated the effect of lignin on enzymatic hydrolysis. However, this study did not address the pretreatment waste (the liquid after organosolv pretreatment and sodium carbonate pretreatment). Also, the organosolv pretreatment required harsh conditions and equipment (180 °C and high-pressure stainless-steel reactor). The protocol caused many difficulties for scaling-up into mass production. Only few research groups (Minu et al. 2012; Kauldhar and Yadav 2018a) published the recovery methods of lignin and silica from the waste stream of rice straw pretreatment to our best knowledge. (Minu et al. 2012) developed the efficient recovery of lignin and silica from black liquor by two-step pretreatment of rice straw by acid and alkaline peroxide. Lignin and silica were isolated from precipitation using dilute H2SO4 for reducing the pH of the black liquor. Chemical characterization of isolated lignin was done by FTIR and compared with commercial lignin to evaluate its potential industrial applications. The filtrate quality of precipitate separation was also monitored in each step by COD and TDS analyses. Kauldhar and Yadav (2018a) reported a simple acid–base hydrolytic method to recover pure silica (SiO2) in a 17-nm size and lignin from straw residues. The physio-chemical characteristics of lignin and nanosilica were determined using FTIR, TGA, XRD, TEM, and SEM studies, along with energy-dispersive X-ray spectroscopy (EDS) analysis. Highly pure nanosilica and lignin were derived by acid precipitation at an overall yield of 9.26% and 2.30%, respectively. The elemental composition of SiO2 and lignin was authenticated by FTIR pattern and EDS analysis. Amorphous structures of both SiO2 and lignin were confirmed by XRD investigation. This process worked perfectly at kilogram scale of paddy waste with a similar yield. Very recently, our group (Do et al. 2020) published a handling method to recover lignin and silica from a waste liquid of similar lab-scale pretreatment. The novel method just included two treatment steps of acid and base (e.g., NaOH) but gave a high recovery yield of 67% and a silica reduction of 94%. This simple and low-cost procedure for lignin and silica recovery is advantageous over existing ones that required harsh conditions or complicated treatment.
In Viet Nam, crop residue-based biorefinery at a small scale was demonstrated by a pilot plant installation. It belongs to a “Biomass Town” project built up to meet biomass-orientated regional material and energy circulations (Mochidzuki et al. 2006). From this perspective, Tran et al. (2013) published a report on bioethanol production from rice straw calculated by engineering data gained from system operation; therein, the process’s energy supply was partly from rice husk carbonization. In this work, 18.4 kg ethanol was obtained from 100 kg of alkali-pretreated rice straw. Steam generated from the rice hush’s carbonization covered more than 90% of the process's total energy. However, this process still has remained some drawbacks related to system design, such as mass and heat loss or unused energy. Also, silica and lignin-rich liquid drained from alkaline pretreatment were not recovered and fully valorized. Some research activities were boosted to improve system design (Le et al. 2020), minimize waste streams (Do et al. 2020), and utilize sustainable feedstocks (Vu et al. 2013). In an approach to improve the process more economically, Tran's group found out that the bottom waste of ethanol distillation might contain a significant nitrogen source. In contrast, corn-steep liquor (CSL), a primary nitrogen source for the yeast of ethanol fermentation, spent highly operational expense and was not commercially available in some countries. Following this work, Khanh et al. (2015) utilized ethanol distillation’s bottom residue to replace CSL’s role for SSF operation. The study confirmed the potential of distillation residue with an equivalent weight of nitrogen to CSL. The reuse of that residue was expected to reduce 98% of the demand for CSL and recycle 26% of water. The research series of Tran et al. (2013), Do et al. (2020), and Khanh et al. (2015) are efforts in biorefinery development and in particular the possibility to produce biofuels and biomaterials from paddy residual biomass. Despite of achieving some positive outcomes, the performance of biorefinery plant in Viet Nam still has not synchronously improved and fully re-evaluated.
The work presented here was to upgrade the lab-scale lignin and silica recovery protocol of Do and co-worker in integration with the Tran's pilot-scale process and Khanh’s research results. The primary goal of this study is to assess the scalability of Do’s protocol. The recovery process was conducted on both bench and pilot scale to verify method compatibility on a larger scale. Simultaneously, obtained products were characterized by FTIR, TGA, and SEM to ensure the stable quality of products on a larger scale. The material and energy balance of the upgraded process were re-assessed to clear its efficiency. Our perspective about this process's prospect was also discussed briefly to orient a strategy the next study stage.
Materials and methods
Raw materials and reagents
Rice straw (Oryza sativa L.) and rice husk, used for pilot-scale biorefinery, were harvested in Vietnam. After harvesting, rice straw was air-dried to attain less than 15% moisture content. The dry matter content of rice straw was measured by Sartorius moisture analyzer MA37.
All reagents were purchased from commercial suppliers with the pure grade, including sodium hydroxide (NaOH) and hydrochloride acid (HCl).
Microorganisms and inoculum preparation for SSF process.
Acremonium cellulase (Meiji Seika Co.), which has 360 FPU (filter paper unit) /g-enzyme, was used for SSF process. Dry yeast (S.Cerevisiae, Ethanol RedTM) was pre-cultivated. The pre-cultivation media (sucrose and CSL; corn steep liquor) prepared in deionized water were autoclaved at 121 °C for 15 min before the dry yeast loading. The yeast was pre-cultivated in the shaking incubator (110 rpm) at 35 °C for 16–24 h. Optical density (O.D.) of pre-cultivation broth was measured at 600 nm with UV–Vis instrument DR 5000 (Hach). The volume of yeast culture needed for SSF was calculated according to SSF experimental protocols NREL/TP-510-42630 (Dowe and McMillan 2008).
Process description of pilot-scale biorefinery to obtain black liquor and distillation residue
The biorefinery process was modified from the operational protocol previously reported by (Tran et al. 2015). The dried straw was sliced into 2–4 cm pieces and then puffed by a puffing machine combining wetting straw with 15–17% water. The puffed rice straw was alkaline pretreated by soaking with 1 wt% NaOH aqueous solution at 50 °C in 24 h. After pretreatment, the mixture was packed in filter cloth bags, squeezed by a filter press at pressure up to 11–13 kgf/cm2. The resulting filtrate, known as black liquor, was obtained for the next experiments. The solid matter was subsequently neutralized with HCl to get a mixture of pH 5–6 and re-squeezed before being fed SSF (simultaneous saccharification and fermentation). Pretreated rice straw, pre-cultivated yeast, and enzyme were loaded in turn into an 800-L SSF reactor. A multi-step fed-batch SSF was applied to maximize obtained ethanol concentration. SSF broth was distilled to obtain an ethanol solution with 48–50 vol% and was removed water by another distillation column to obtain 97 vol% ethanol as the final product. The distillation residue remained after distillation was obtained for further uses. The simplified flowsheet for the whole process was shown in Supplementary Figure S1 (Fig. S1).
Lignin and silica recovery at lab-scale
The method for recovering lignin and silica used in this study was developed by our team (Do et al. 2020) with a slight modification to ensure that it is simple, mild, safe, and more applicable to a larger scale. Various steps for lignin and silica recovery are shown visually in Fig. 1a. Firstly, 1L of black liquor was adjusted to pH 9 by HCl 20 w/v% and settled in 24 h to remove silica from the liquid. After that, the desilication liquid was acidified again by HCl 20 w/v% to pH 3 and settling in 12 h to recover lignin. The changing color of black liquor from dark brown to light brown was used to determine lignin and silica sedimentation. The obtained silica and lignin were rinsed three times with distilled water and dried at 105 °C in 24 h.
Lignin and silica recovery at pilot scale
Based on the lignin and silica recovery technique in the lab-scale, the small pilot-scale experiment was performed to follow the setup shown in Fig. 1b. Similar to the lab-scale experiment, 50 L of black liquor was added into the acidification tank with HCl 20% to lower the pH of black liquor to 9. The pH 9 liquid was settled in 24 h in the clarifier basin and filtered by a press filter to obtain the precipitation. Afterward, the filtrate continuously went through the next vessels to be adjusted their pH value to 3. The liquid was then stable for 12 h for lignin sedimentation before separating by press filter to recovery lignin.
Reuse of distillation residue as a nitrogen source for SSF
After collection, the distillation residue is dried at ambient conditions for 2–3 days to remove water, then ground to obtain a source of nutrients (Fig. S2). The nitrogen content and nutrient of distillation residue after ground comparison to CSL are shown in Table S1. The total nitrogen content is measured by elemental analysis (CHNS) of materials, performed by EuroEA3000 for measuring. Amino-acid nitrogen was measured by the Sorensen formol titration method.
Analytical methods
The composition of paddy residues and lignin products was determined by the method published at Technical Report NREL/TP-510-42618 of Nation Renewable Energy Laboratory (NREL) (Sluiter et al. 2008). The ash content of the obtained lignin was measured by the calcined method at 900 ± 25 °C for 6 h in Nabertherm muffle furnace model LT3/11. The chemical structure of products was determined by Fourier-transform infrared spectroscopy (FT-IR). This measure was carried out on KBr pellets ranging from 400 to 4000 cm−1 with a 4 cm−1 resolution on the PerkinElmer Frontier IR instrument. The polymer's thermal degradation was monitored by thermal gravity analysis (TGA) using Linseis TGA PT 1600. The sample was heated from room temperature to 800 °C with a heating rate of 20 °C/min in argon. Scanning electron microscope (SEM) micrographs of lignin and silica were recorded using Hitachi FESEM S-4800.
Results and discussion
Raw material
Black liquor is a waste flux of bioethanol production after converting lignocellulosic biomass (rice straw) to bioethanol by the following processes: cutting, puffing (steam explosion pretreatment), alkaline pretreatment, enzymatic reaction, and distillation, as visually shown in Fig. S1. According to Kargbo et al. (2010), silica content was confirmed as approximately 75 wt% of rice straw ash. Moreover, the bonding interaction between lignin and silica was verified elaborately. Both of them were solubilized in black liquor in the pretreatment step. The obtained lignin or silica was natural coprecipitation of silica and lignin or even four components in black liquor. The composition of raw rice straw material used for bioethanol production in pilot runs and black liquor obtained from this process are provided in Table 1. The results illustrate that lignin and silica are the dominant constituents of black liquor; therein, they account for more than 50 wt% of black liquor. Moreover, there is only 15% hemicellulose and cellulose in black liquor. This demonstrates the potential of black liquor obtained from bioethanol production in the recovery of lignin and silica.
Lignin and silica isolation from black liquor in different scales
The methods used to extract lignin and silica from black liquor are developed and shown in Fig. 1a. The lignin and silica recovery results in the bench- and pilot-scale are shown in Table 2. The physical changing of each precipitation (e.g., color, state) was recorded. Like Do et al. (2020), the liquor's color changed from dark brown to olive-brown with decreasing pH and contrasted with the precipitate color. The precipitate state also changes from dense gel at basic pH 9 and a slurry at acidic pH 3. This phenomenon demonstrated the decolorization of black liquor again due to lignin precipitation, as reported by García et al. (2009). Simultaneously, the recovery efficiency of lignin and silica at lab and pilot scales was above 90%, of which the immaculateness of lignin was over 70%. While the total volume of HCl, used in 2 different volumetric scales, is directly proportional to black liquor volume. This result shows that the laboratory-scale process is suitable for growing and expanding industrial scale. Moreover, with simplicity, safety, and green chemicals, this process can be considered a potential process for the comprehensive development of lignocellulosic biomass to convert into high-value products and bioenergy.
Characterization of products
FT-IR spectra
The FTIR spectra of silica and lignin obtained from the pilot-scale are shown in Fig. 2. Overall, both spectra of lignin and silica are similar to each other. A broad peak between 3000 and 3500 cm−1 mainly formed two spectrums that indicated the stretching of H-bonded OH. The peaks at 1510 cm−1 and 1605 cm−1 are attributed to aromatic skeletal vibration (C=C) of lignin (guaiacyl or syringyl). The shape peaks at 1604 cm−1 and 1735 cm−1 are assigned to the C=O stretching of lignin and presences on the silica bands (Esteves et al. 2013). These findings proved an amount of lignin and other organic compounds re-bonding with silica and creating coprecipitation after pretreatment. The interaction between Si and OH groups of water, lignin, and other compounds by hydrogen bonds was also reported by Xia et al. (2018). In contrast with silica bands, lignin bands are insignificantly influenced by silica because of the absence of characteristic peaks of silica at 458–561 cm−1 (Si–O–Si bending), 950–1000 cm−1 (Si–O–Si asymmetric stretching) (Kopani et al. 2017). Therefore, the lignin, recovered in this condition, is purer.
TG analysis
To verify the complicated linkage of silica and other chemicals in black liquor, thermogravimetric analysis (TGA) is used to explain and illustrate silica's thermal stability. Additionally, lignin's thermal properties varied due to the biomass origin difference (Kauldhar and Yadav 2018b). The plots of the thermogravimetric analysis for lignin and silica, shown in Fig. 3a.
The first stage occurred at under 100 °C due to the evaporation of physically adsorbed water. Accordingly, the values of weight loss within 100 °C are 9.3 wt%, 11.9 wt% for lignin and silica, respectively. The second stage was observed around 100–250 °C, which indicated the decomposition of polysaccharides, aliphatic alcohols, and acids (Ramakoti et al. 2019). The mass loss of silica was approximately 2.4 wt%, which confirms the negligible presence of non-lignin compounds co-precipitating with silica, while the weight loss of lignin was around 10 wt%. This noticeable loss implies the coprecipitation of lignin and other organic compounds, which is evidence of the intricate linkages between lignin and another component (Toledano et al. 2013). The increase in the thermal degradation of lignin occurred mainly in temperatures interval of 250–500 °C, similar to the results of Kim et al. (2018). In this stage, the decomposition of lignin happened strongly due to its aromatic structure.
On the other hand, the silica curve is gradually stabilized. The thermal degradation of silica rose to 15 wt% and has a stable trend when increasing temperature. At a temperature over 480 °C, the silica thermogram is steady with a negligible mass loss of less than 5%. The XRD analysis results (Fig. S3) indicated that the obtained lignin and silica have an amorphous structure with a broad peak at 22.6° and 21° of 2θ, respectively.
SEM analysis
The obtained lignin is a dark-brown solid (Fig. 3b), whereas the silica is a white powder (Fig. 3c). The scanning electron microscopy of silica and lignin, as shown in Fig. 4, illustrated the microstructure of silica and lignin. Both lignin and silica appeared with granules of various dimensions. Additionally, the porous structure of silica exhibited a large specific surface area. These grains have an assembly trend to make a more prominent grain. The acquired lignin powder is a slightly smooth surface and no fiber existence, which re-confirmed lignin's purity.
The SEM of silica (a, b) and lignin (c, d) at different magnification (20.0 k—a, 40.0 k—b, 1.0 k—c, 60.0 k—d). The obtained lignin has a slightly smooth surface and no fiber existence, while obtained silica consists of many small particles aggregated together to form SiO2 blocks with porous structure
Reuse of distillation residue as a nitrogen source for SSF
In this study, the waste stream after distillation is used as a nitrogen source for the SSF process to optimize the new biorefinery process for emissions reduction. The reason is that the residue after distillation has a cellulose content of about 25–30% and contains significant protein content (nutritional sources, yeast residues, enzymes) (Supplementary Table S1/Table S1). The residue can be a good source of raw materials for recycling and is used as a substrate for the next fermentation. It can be utilized as an alternative nutrient source for CSL in the fermentation process. Utilizing waste from the production process makes the technology of fermenting straw into alcohol becomes a closed process, reducing production costs, reducing waste in production.
Figure 5a shows the concentration of ethanol consistent with the hydrolyzed fermentation mixture’s fermentation mixture containing 8 wt% of rice straw (dry volume) and 0.1 wt% of CSL, 0.1 wt% of distillation residue, and no additional nutrition. The results show that without using additional nutrition, the final fermentation product only reaches a saturated concentration of 0.7 wt%. In contrast, with additional nutrients being distillation residue or CSL, the achieved saturation concentration after 160 h is 1.6 wt%. Because of the difference in nitrogen content of CSL and distillation residue (Table S1), a mixture containing 8 wt% of dried straw and additional nutrients CSL or distillation residue with an equal in the total amount of nitrogen nutrition was fermented to compare the effects of nutritional nitrogen in these two additional sources. The results shown in Fig. 5b indicated that the amount of distillation residue used increased by up to 0.5 wt% of the whole mixture. The final ethanol concentration did not increase significantly because such nutrients were saturated for the yeast community's growth.
Line graph a depicts the concentration of ethanol according to the fermentation time of the hydrolyzed fermentation mixture containing 8 wt% of rice straw (dry volume) and (1) 0.1 wt% of CSL, (2) 0.1 wt% of distillation residue, and (3) no additional nutrition. Line graph b represents the concentration of ethanol according to the fermentation time of the hydrolyzed fermentation mixture containing 8 wt% of straw and the added amount of nutrients with the same free nitrogen content (1) 0.1 wt% of CSL, (2) 1.5 wt% of the residue, and (3) no additional nutrition. After 160 h, the saturation concentration is 1.6 wt% in both cases of additional nutrients being distillation residue or CSL
Material flow and energy balance of the new sustainable process
The new sustainable process (Fig. S4) was designed by integrating the current bioethanol production system and the new lignin and silica recovery process and reusing the waste stream for cost reduction. In the new system, besides the main product is bioethanol, the high-value co-products were also produced as lignin, silica, and charcoal. The new system’s mass and energy balance were evaluated and synchronized with the whole biorefinery process to demonstrate the new design's sustainability and efficiency compared to the old one.
The material flow of the sustainable process using 203.5 kg dry rice straw was depicted in Fig. S5. The material flow of the process was described by focusing on the changing of rice straw composition, the conversion of rice husk into charcoal, and the recovery of lignin, silica from the waste liquid. In this process, the 203.5 kg rice straw pretreatment was conducted at ambient temperature in 6–8 h and the solid to liquid ratio of 1:5 (kg/kg) by NaOH solution of 1.0 wt%. An amount of lignin and silica was solubilized in an alkaline solution that led to changes in pretreated straw composition with 58.3 wt% of cellulose, 18.6 wt% of hemicellulose, and others (lignin, silica, and other). In the next step, the pretreated rice straw was fermented in SSF, and the fermentation broth then went through two distillation columns to get bioethanol 96 vol%. The decreasing percentage of cellulose indicated the high yield of the SSF process. The sewage after the lignin and silica recovery system has pH 3. The acidic sewage could be reused for acidification as the final liquor after this process contained almost no lignin, silica, cellulose, and hemicellulose. At the same time, the high ash content was attributed to inorganic content. Moreover, according to the high cellulose content, the distillation residue is potential for nanocellulose synthesis (Farinas et al. 2018). Besides upgrading the new process's sustainability and reusing the distillation residue as a nitrogen source for SSF, this residue's nanocellulose recovery is a potential route and needs more investigation for larger-scale applications.
In the new system, the energy flow, as shown in Fig. 6, was gained by various sources, including rice husk, rice straw, kerosene, LPG, and electricity. Human labor was not involved in this energy calculation. Rice husk conversion into charcoal and energy will be reported in the following publication. The reuse waste stream process was also not calculated in this energy balance because of weather issues (e.g., drying distillation residue). Although more than 10,383.8 MJ of energy was required to obtain ethanol, lignin, silica, and charcoal, the electricity consumption only covered 12.5% of this process’s total energy. The carbonization process of rice husk supplied more than 80% of the total energy consumption.
The energy balance of the process was calculated based on the law of conservation of energy. The calculation of energy balance was described in Eq. 1:
wherein Erice straw and Erice husk are energy in rice straw and husk, Ekerosene and ELPG are energy input of kerosene and LPG disbursed in the ignition process, Eelectricity is electricity supply for operating the system, Eethanol and Echarcoal are energy in ethanol produced and charcoal discharged, Estream is the energy of steam, Eheatloss+unused is heat loss and unused energy from rice husk carbonization process, Eresidue and unrecovered products are unrecovered energy of product and residues and unused energy from bioethanol production process boundary, Elignin and Esilica are energy in lignin and silica produced, respectively, and Esewage is the energy of liquid residue from black liquor treatment process.
The energy required for the entire process's operation is about 10,383.8 MJ (including the energy of rice husk and straw and another supplier), higher than the traditional process (8661 MJ). The electricity consumption for bioethanol production combined with the black liquor treatment process is 1299 MJ, increased by 554.4 MJ compared to the traditional process (744.6 MJ). In contrast, products and residues' unrecovered energy is expressively reduced nearly 1.5 times compared to the old process (2532.6 MJ). The energy efficiency (EE) of biorefinery was recognized by Eq. 2:
The energy efficiency of 0.529 demonstrated that the new bioethanol production process was upgraded successfully, and utilizing energy for producing high value-added compounds is appropriate. Moreover, the new bioethanol process reduced energy loss from unrecovered and unused material and upgrades this process's value chain in energy efficiency.
Following this process, many products are produced with high applicability, including silica, lignin, charcoal, and bioethanol, while the wastes stream mainly included sodium chloride. Producing more products increase the value of the process and reduces the wastes flux. The simple calculation about economic efficiency was shown in SI to demonstrate this process's possibility toward sustainable production (Table S2). The results indicated that the recovery system could cover the cost for electricity and water use of the entire recovery process and bioethanol process. Moreover, CSL price was also reduced because of the utilization of SSF residue. The calculation does not consider the capital cost to invest a whole process and labor cost for operation.
Overall, based on energy efficiency and small economic calculation, the upgraded biorefinery is promising for mass production to turn biomass into biofuels and bio-chemicals. However, some drawbacks still need to be improved before moving on large-scale production stage.
Perspective and future prospect
Biorefinery has gained attention as a strategic means for sustainable bio-economy in the context of resource depletion (fossil-based) associated with environmental issues (Katakojwala and Mohan 2021). The major challenges of biorefineries are lack of techno-economic feasibility, environmental sustainability, market acceptance, market demands, etc. Production of more high-value products is one of the strategic ways to expand the market and increase the economic feasibility of biorefineries. In this study, the lignin and silica recovery integrated with the bioethanol production system is a candidate for developing the biorefinery approach from lignocellulosic biomass. The novel approach focuses on the compatibility between the recovery process and the pilot-scale biorefinery system, making the process more complex that might be difficult to control. Thus, the biorefinery needs to be comprehensively assessed for their environmental and techno-economic performance by life-cycle assessment (LCA), including life-cycle costs (LCC), life-cycle production (LCP) and life-cycle emissions (LCE). Also, the technologies used for this process are an important factor for consideration. The energy consumption is not efficient (EE = 0.5) because the heat loss and unused energy accounted for more 22% of total energy. This issue demonstrated that the technological configuration of this biorefinery system is less competitive with many other technologies. Our consideration for this issue is that the rest of the energy and heat loss should be reused for producing electricity for operation or sell it back to the grid to maximize the economic value. In fact, the feasibility of this solution was demonstrated by many studies using rice husk as a feedstock for thermo-electricity plants (Quispe et al. 2017). The syngas obtained from rice husk combustion was also applied in dimethyl ether production or bio-oil (Nakyai and Saebea 2019). Simultaneously, the optimization of each process unit should be done to raise productivity. Such R&D activities are promising to create a closing loop process toward circular economy.
In the new industrial revolution era (Industry 4.0), the digital transformation will help biorefinery increase its competitiveness (Ungerman et al. 2018). Therein, four components of the Industrial Internet of Things (IIoT), cyber-physical systems (CPS), smart factory, and big data are considered a key for the biorefinery to join in the Industry 4.0 era. The IIoT concept creates the connections between labor, data and machines in biorefinery factories. IIoT required deep knowledge about each process unit to utilize sensors, actuators, and mobile devices to improve operational efficiency and productivity or even detect production risks (Bedhief et al. 2019). CPS or cyber manufacturing, as describes by Rajkumar et al. (2010) is “physical and engineered systems, whose operations are monitored, coordinated, controlled, and integrated by a computing and communicating core”. The CPS system supports labor in controlling and recording data from the vast and intricate biorefineries. Smart factory is defined as “a Factory that context-aware assists people and machines in executing their tasks” (Lucke et al. 2008). “Big data is a term that describes large volumes of high velocity, complex and variable data that require advanced techniques and technologies to enable the capture, storage, distribution, management, and analysis of the information” (Mills et al. 2012). The combination of four components converts normal biorefineries to smart ones that possess high productivity and low environmental impacts. However, the development of biorefinery toward Industry 4.0 brings more technical challenges in analytical competence, sensitive awareness, leadership, and management, that required engineers and researchers, who work directly with the system, have in-depth knowledge and multi-disciplinary skills to study and operate such biorefineries in future.
Conclusion
Addressing the waste stream from biorefinery plants is crucial for the growth of research and development of biorefinery concepts in demand for energy, fuels, and chemicals. Accordingly, the research aims to scale up a recovery system for the biorefinery pilot plant's waste stream and create products from this stream. The system successfully scale-up with an insignificant difference from our previous lab-scale one. The system's compatibility with biorefinery pilot-plant was confirmed via increasing energy efficiency and creating more products than the previous process. The new biorefinery concept promises a potential solution to implement sustainable development goals to provide higher-value creation and is more sustainable than bioenergy production alone. However, some barriers still exist, such as limited technology, unpredictable biomass mass and quality, lignin purification, and less water use. In developing for future biorefineries, it is important to overcome these current limits, minimize impacts to the environment, and apply greener methodologies.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
Data availability statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Change history
24 May 2021
A Correction to this paper has been published: https://doi.org/10.1007/s10098-021-02116-w
References
Abraham A, Mathew AK, Sindhu R et al (2016) Potential of rice straw for bio-refining: an overview. Bioresour Technol 215:29–36
Aresta M, Dibenedetto A, Dumeignil F (2012) Biorefinery: from biomass to chemicals and fuels. Walter de Gruyter, Berlin
Bedhief I, Foschini L, Bellavista P et al (2019) Toward self-adaptive software defined fog networking architecture for IIoT and industry 4.0. In: 2019 IEEE 24th international workshop on computer aided modeling and design of communication links and networks (CAMAD). IEEE, pp 1–5
Bruins ME, Sanders JPM (2012) Small-scale processing of biomass for biorefinery. Biofuels Bioprod Biorefining 6:135–145
Chiew YL, Cheong KY (2011) A review on the synthesis of SiC from plant-based biomasses. Mater Sci Eng B 176:951–964
de Jong E, Higson A, Walsh P, Wellisch M (2012) Bio-based chemicals value added products from biorefineries. IEA Bioenergy, Task42 Biorefinery, 34
Do NH, Pham HH, Le TM et al (2020) The novel method to reduce the silica content in lignin recovered from black liquor originating from rice straw. Sci Rep 10:21263. https://doi.org/10.1038/s41598-020-77867-5
Dowe N, McMillan J (2008) SSF experimental protocols-lignocellulosic biomass hydrolysis and fermentation. NREL chemical analysis and testing laboratory analytical procedures. 2008. NREL/TP-510-42630
Esteves B, Velez Marques A, Domingos I, Pereira H (2013) Chemical changes of heat treated pine and eucalypt wood monitored by FTIR. Maderas Cienc y Tecnol 15:245–258. https://doi.org/10.4067/S0718-221X2013005000020
Farinas CS, Marconcini JM, Mattoso LHC (2018) Enzymatic conversion of sugarcane lignocellulosic biomass as a platform for the production of ethanol, enzymes and nanocellulose. J Renew Mater 6:203–216
García A, Toledano A, Serrano L et al (2009) Characterization of lignins obtained by selective precipitation. Sep Purif Technol 68:193–198. https://doi.org/10.1016/j.seppur.2009.05.001
Hatti-Kaul R (2010) Biorefineries–a path to sustainability? Crop Sci 50:S-152
Jeelani PG, Mulay P, Venkat R, Ramalingam C (2020) Multifaceted application of silica nanoparticles. A review. SILICON 12:1337–1354
Kargbo FR, Xing J, Zhang Y (2010) Property analysis and pretreatment of rice straw for energy use in grain drying: a review. Agric Biol JN Am 1:195–200
Katakojwala R, Mohan SV (2021) A critical view on the environmental sustainability of biorefinery systems. Curr Opin Green Sustain Chem 27:100392. https://doi.org/10.1016/j.cogsc.2020.100392
Kauldhar BS, Yadav SK (2018a) Turning waste to wealth: a direct process for recovery of nano-silica and lignin from paddy straw agro-waste. J Clean Prod 194:158–166
Kauldhar BS, Yadav SK (2018b) Turning waste to wealth: a direct process for recovery of nano-silica and lignin from paddy straw agro-waste. J Clean Prod 194:158–166. https://doi.org/10.1016/j.jclepro.2018.05.136
Kaur A, Kuhad RC (2019) Valorization of rice straw for ethanol production and lignin recovery using combined acid-alkali pre-treatment. BioEnergy Res 12:570–582
Khaleghian H, Molaverdi M, Karimi K (2017) Silica removal from rice straw to improve its hydrolysis and ethanol production. Ind Eng Chem Res 56(35):9793–9798. https://doi.org/10.1021/acs.iecr.7b02830
Khanh LV, Tran UPN, Nguyen QD et al (2015) Self-reuse of distillation residue as a nitrogen source for simultaneous saccharification and fermentation in a bioethanol production process from rice straw. Environ Sci 28:335–342
Kim M, Kim B-C, Nam K, Choi Y (2018) Effect of pretreatment solutions and conditions on decomposition and anaerobic digestion of lignocellulosic biomass in rice straw. Biochem Eng J 140:108–114. https://doi.org/10.1016/j.bej.2018.09.012
Kolfschoten RC, Bruins ME, Sanders JPM (2014) Opportunities for small-scale biorefinery for production of sugar and ethanol in the Netherlands. Biofuels Bioprod Biorefining 8:475–486
Kopani M, Mikula M, Kosnac D et al (2017) Morphology and FT IR spectra of porous silicon. J Electr Eng 68:53–57. https://doi.org/10.1515/jee-2017-0056
Le PK, Le TDT, Nguyen QD et al (2020) Process simulation of the pilot scale bioethanol production from rice straw by Aspen Hysys. In: IOP conference series: materials science and engineering. IOP Publishing, p 12095
Lucke D, Constantinescu C, Westkämper E (2008) Smart factory-a step towards the next generation of manufacturing. In: Manufacturing systems and technologies for the new frontier. Springer, pp 115–118
Mills S, Lucas S, Irakliotis L et al (2012) Demystifying big data: a practical guide to transforming the business of government. TechAmerica Foundation, Washington
Minu K, Jiby KK, Kishore VVN (2012) Isolation and purification of lignin and silica from the black liquor generated during the production of bioethanol from rice straw. Biomass Bioenergy 39:210–217. https://doi.org/10.1016/j.biombioe.2012.01.007
Mochidzuki K, Sato N, Sakoda A, Tuan PD (2006) Development of local biomass-based fuel systems in Mekong Delta area. In: AIChE annual meeting, conference proceedings
Nakyai T, Saebea D (2019) Exergoeconomic comparison of syngas production from biomass, coal, and natural gas for dimethyl ether synthesis in single-step and two-step processes. J Clean Prod 241:118334
Quispe I, Navia R, Kahhat R (2017) Energy potential from rice husk through direct combustion and fast pyrolysis: a review. Waste Manag 59:200–210
Rajkumar R, Lee I, Sha L et al (2010) Cyber-physical systems: the next computing revolution. In: Design Automation Conference, pp 731–736. https://doi.org/10.1145/1837274.1837461
Ramakoti B, Dhanagopal H, Deepa K et al (2019) Solvent fractionation of organosolv lignin to improve lignin homogeneity: structural characterization. Bioresour Technol Rep 7:100293
Ruiz HA, Conrad M, Sun S-N et al (2020) Engineering aspects of hydrothermal pretreatment: from batch to continuous operation, scale-up and pilot reactor under biorefinery concept. Bioresour Technol 299:122685
Saini JK, Saini R, Tewari L (2015) Lignocellulosic agriculture wastes as biomass feedstocks for second-generation bioethanol production: concepts and recent developments. 3 Biotech 5:337–353
Serna-Loaiza S, García-Velásquez CA, Cardona CA (2019) Strategy for the selection of the minimum processing scale for the economic feasibility of biorefineries. Biofuels Bioprod Biorefining 13:107–119
Sluiter A, Hames B, Ruiz R et al (2008) Determination of structural carbohydrates and lignin in biomass. Lab Anal Proced 1617:1–16
Sun Z, Fridrich B, de Santi A et al (2018) Bright side of lignin depolymerization: toward new platform chemicals. Chem Rev 118:614–678
Toledano A, Serrano L, Labidi J et al (2013) Heterogeneously catalysed mild hydrogenolytic depolymerisation of lignin under microwave irradiation with hydrogen-donating solvents. ChemCatChem 5:977–985
Tran UPN, Phan DD, Tran ATT et al (2015) Sodium bicarbonate pretreatment on rice straw for bioethanol production and utilizing the waste water of this process to absorb CO2 in biogas. Sci Technol Dev J 18:96–107
Tran UPN, Van Vu KL, Nguyen QD et al (2013) Energy balance of small-scale biorefinery system. Environ Sci 26:489–496
Ungerman O, Dedkova J, Gurinova K (2018) The impact of marketing innovation on the competitiveness of enterprises in the context of industry 4.0. J Compet 10:132
Vu LVK, Tran PNU, Phan DT et al (2013) Agricultural residues as alternative supplemental nutrients for Bioethanol fermentation process from rice straw. J Sci Technol Vietnam B 5:32–36
Xia Q, Liu Y, Meng J et al (2018) Multiple hydrogen bond coordination in three-constituent deep eutectic solvents enhances lignin fractionation from biomass. Green Chem 20(12):2711–2721. https://doi.org/10.1039/C8GC00900G
Zhang C (2020) Lignocellulosic ethanol: technology and economics. In: Alcohol fuels-current Technol Futur Prospect 1–21. https://doi.org/10.5772/intechopen.86701
Acknowledgements
This research is funded by Vietnam National University under Grant Number B2019-20-11. We acknowledge the support of time and facilities from Ho Chi Minh City University of Technology (HCMUT), VNU-HCM for this study.
Author information
Authors and Affiliations
Contributions
Conceptualization, formal analysis, investigation and writing: TML, Writing and investigation: UPNT; Writing—review and editing, investigation: YHPD; investigation and writing: QDN; supervision, methodology, and conceptualization: VTT; writing, methodology, conceptualization: PTM; Funding acquisition, supervision, methodology, and conceptualization PKL.
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: Affiliation information of all the authors were updated.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Le, T.M., Tran, U.P.N., Duong, Y.H.P. et al. Sustainable bioethanol and value-added chemicals production from paddy residues at pilot scale. Clean Techn Environ Policy 24, 185–197 (2022). https://doi.org/10.1007/s10098-021-02097-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-021-02097-w