Abstract
Genotypic variability was assessed within six Medicago ciliaris genotypes growing symbiotically with Sinorhizobium medicae in order to identify physiological criteria (growth, ion content, and plant health) associated with salt tolerance. Response to salt stress depended on the line and the level of salt. Two lines with lower dry biomass under non-saline conditions (TNC 1.8 from a semi-arid area and TNC 10.8 from a sub-humid area), were more tolerant to NaCl, whereas the most productive lines (TNC 11.5 and TNC 11.9 from a humid bioclime) were more sensitive in terms of growth and nitrogen fixation. Susceptibility of symbiotic nitrogen fixation to saline stress was not associated with a higher accumulation of Na+ in nodules, since the most tolerant lines TNC 1.8 and TNC 10.8 accumulated the highest Na+ amount in nodules. Leaf area and net photosynthate assimilation rate were conserved in line TNC 1.8 and to a lesser extent in line TNC 10.8 potentially owing to a greater ability to protect aerial organs and nodules from Na+ damage and to insure a better supply of leaves with nitrogen. Our results suggest that nodule growth and number and nodule Na+ content should not be used as selection tools for tolerance or susceptibility, since two of the tested lines maintained consistent growth in spite of reduced nodule and high Na+ content. Instead, the most reliable physiological indicators for tolerance appear to be consistent growth (i.e., no growth changes) and reduced leaf Na+ accumulation with increasing concentrations of NaCl.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Soil salinity is considered a major factor affecting crop productivity and agricultural sustainability in arid and semi-arid regions throughout the world (Yamaguchi and Blumwald 2005). Salinity in soils and irrigation water sources restricts yield on almost 40 million hectares, which represent one-third of total irrigated crop land (Norlyn and Epstein 1984). Detrimental effects of salinity on plant growth occurs because of an ionic imbalance, particularly of Ca2+ and K+. The osmotic imbalance resulting from soil salinity causes disturbances in the water balance of the plant, reducing turgor, closing stomata, reducing photosynthesis and inhibiting growth (Munns and Tester 2008). Many plant species respond to NaCl salinity and protect themselves by compartmentalizing excess Na+ and Cl- ions in vacuoles and by accumulating compatible solutes in the cytoplasm, although extreme accumulation of inorganic ions may produce toxic effects. Other plant species protect themselves by excluding ions (Tester and Davenport 2003). A higher tissue K+/Na+ ratio has been proposed as an important selection criterion for salt-tolerance (Munns and Tester 2008).
Traditionally, most legumes have been classified as salt sensitive crop species (Lauchli 1984). Their productivity is particularly constrained by salt when they have to depend exclusively on symbiotic nitrogen fixation (Singleton and Bohlool 1983). The effect of NaCl on legume growth, nodulation and symbiotic nitrogen fixation has been the subject of several recent investigations (Georgiev and Atkins 1993; Saadallah et al. 2001; Ben Salah et al. 2009, 2010, 2011), in which limitations in productivity were linked to poor development of root-nodule bacteria, inefficient sucrose metabolism in root nodules and leaves, and a consequent reduction in nitrogen fixation capacity, leading to slower growth of the host plant. Lauchli (1984) suggested a strong correlation between salt tolerance and Na+ exclusion from legume leaves. This tissue-specific exclusion mechanism, together with accumulation of toxic Na+ ions in roots, was confirmed recently in broad bean (Vicia faba L.), chickpea (Cicer arietinum L.) and the model legume Medicago truncatula L. (Yamamouchi et al. 1997; Tejera et al. 2006; Aydi et al. 2008). However, ion concentrations were not assessed in nodules of plants undergoing symbiotic nitrogen fixation in these studies, and contributions from this organ to host plant tolerance mechanisms are unknown.
Annual medics (Medicago spp.) are winter annual legumes which represent a substantial proportion of the native flora in Tunisia across all bioclimatic areas. They are found in wide-ranging habitats varying in water availability, temperature and geographical location (Abdelkefi et al. 1996; Badri et al. 2008), indicating a strong ability to adapt to local environments. These species are of special interest, since they form symbioses with nitrogen-fixing bacteria and are, therefore, excellent candidates for the low-input improvement of marginal or degraded lands with low fertility. Among these legume species, M. ciliaris (L.) ALL. appeared to be more salt tolerant within a collection that included M. polymorpha, M. truncatula and M. minima (Abdelly et al. 1995), since it maintained its biomass productivity when grown in 100 mM NaCl supplemented with mineral N. If this feature held true for M. ciliaris genotypes growing without supplemented N, they would have strong potential to be used in the reclamation of areas, such as Sebkha (salted depression forming along arid coastlines) edges.
Understanding the mechanisms which confer salinity tolerance in plants is a key for developing selection and breeding strategies. The main strategy used over the past few years to improve salt tolerance in legumes has been genotype screening and selection (Cordovilla et al. 1995). Thus, the objective of this study was to explore genotypic variation for salt tolerance in tissues of a limited collection of M. ciliaris accessions growing under low input conditions using physiological and nutritional indicators of plant health. This information could then be used to define selection criteria and provide a framework for selecting large numbers of lines from a much broader range of accessions from across the country.
2 Materials and methods
2.1 Biological materials and growth conditions
Six M. ciliaris lines were developed into genetically pure lines from natural populations collected from three widely diverse bioclimatic areas in Tunisia, including a low elevation, inferior semi-arid region by Enfidha (#1; lines TNC 1.3 and TNC 1.8) and two more elevated interior regions, a sub-humid region near Rhayet (#10; lines TNC 10.8 and TNC 10.9), and a humid region near Mateur (#11, TNC 11.5 and TNC 11.9) (Fig. 1; Table 1). During line development, it was assumed that S3 offspring from two generations of spontaneous selfing in a greenhouse should be genetically identical within each line. Consequently, any within-line variation was attributed to environmental (or experimental) variance, while variation between lines was assumed to be due to genetic variability (Badri et al. 2008). Seeds were scarified with concentrated H2SO4 for 40 min, washed 10 times with sterile distilled water, and placed on sterile agar medium at 25°C in the dark. Three-day-seedlings were transferred into pots (20 cm diameter and 30 cm height) filled with sand and were inoculated with a 1 mL suspension (~108/mL) of Sinorhizobium medicae strain CI 1.12/E22 (Zribi et al. 2007). Seeds and the bacterial strain were the property of the Laboratory of Legumes in the Centre of Biotechnologie of Borj Cedria (CBBC). Plants were grown in a greenhouse at CBBC from November to March under semi-controlled conditions: 25 ± 5/15 ± 5°C day/night temperature, day length ranging from 10–12 h, relative humidity 70–90%. Pots were irrigated from the top twice weekly with 150 mL of the following N-free nutrient solution (pH = 5.8) according to Kalia and Drevon (1985): KH2PO4 (0.25 mM), CaCl2 (1.65 mM), MgSO4 (1 mM), K2SO4 (0.7 mM), H3BO3 (4 μM), MnSO4,7H2O (4 μM), ZnSO4,7H2O (1 μM), CuSO4,5H2O (1 μM), CoCl2 (0.12 μM), NaMoO4,2H2O (0.12 μM).
2.2 Salinity treatments
Salinity treatments were arranged in a randomized complete block design (six lines x three NaCl levels x seven replicates). NaCl treatment (75 and 150 mM; electric conductivity is 8.2 and 14.6, respectively) was applied 45 days after sowing after the appearance of functional nodules (spotted by their pink colour). To avoid osmotic shock, salt concentrations were increased daily in a stepwise fashion starting with 50 mM NaCl until the required concentration was reached. Two harvests were made: the first at the beginning of salt treatment and the second at the end of salt treatment (2 months later). Harvested plants were separated into shoots, roots and nodules. Underground organs were washed free of sand and nutrient solution with tap water and then three changes of cold distilled water and blotted dry with filter paper.
2.3 Biomass and photosynthetic measurements
Dry weight of all plant tissues (dry matter, DM) was determined after oven drying at 60°C for 3 days. Total plant DM was calculated as the sum of oven-dried tissue weights. Leaf area was measured using an Area Meter (Li-COR model LI-3000A, Lincoln, NE, USA Lincoln, NE, USA). Photosynthetic activity was measured as the net leaf assimilation rate (NAR, the rate of dry matter production per unit of leaf area per unit of time) calculated as follow: \( [({{\text{W}}_{{2}}} - {{\text{W}}_{{1}}})/({{\text{T}}_{{2}}} - {{\text{T}}_{{1}}})] * [({\text{L}}{{\text{A}}_{{2}}} - {\text{L}}{{\text{A}}_{{1}}})/(ln\,{\text{L}}{{\text{A}}_{{1}}})] \), in mg DM cm−2 d−1 where W represents whole plant DM (mg), LA represents leaf area (cm2), and t represents time (days) subscripted to denote initial1 and final2 harvests.
2.4 Ion content and nitrogen determination
Samples were dried, ground, and analyzed for Na+ and K+ content by extraction in 0.5% HNO3 and flame photometry using a butane-fired ion-calibrated Corning photometer model 410 (United Kingdom) tuned to 589 nm and 767 nm, respectively. Nitrogen was determined as described by Kjeldahl (1883). The effect of NaCl on symbiotic nitrogen fixation was estimated as the difference between total N (mg plant−1) before and after salt treatment in inoculated plants growing without N supplementation
2.5 Statistical analysis
Analysis of variance (ANOVA) and confidence values tests were carried out on the data using Msustat. Means separation procedures were carried out using multiple range tests and Fisher’s least significant difference (LSD) procedure (P < 0.05). Pearson correlation analysis between specific parameters was conducted on individual lines and all six lines combined using SPSS for Windows (version 11.0).
3 Results
3.1 Plant growth and net assimilation
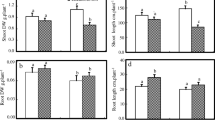
Under control conditions in the absence of applied NaCl, the six Medicago ciliaris lines showed a wide range of dry biomass production (Fig. 2). Lines TNC 11.9 and TNC 11.5 reached the highest dry biomass whereas the line TNC 10.8 showed the lowest. The magnitude of responses to salt also varied with the six lines as well as with the level of applied salt. For example, when plants were irrigated with 75 mM NaCl, lines TNC 1.8, TNC 10.8 and TNC 10.9 maintained biomass production, while the growth of lines TNC 1.3, TNC 11.5 and TNC 11.9 was significantly reduced namely in aerial tissues. Dry biomass was reduced even farther by increasing salinity level to 150 mM for four of the six lines (Fig. 2). In all lines, salinity affected the growth of individual vegetative organs (leaves, stems and roots) similarly to its effect on whole plants.
Regarding leaf area, salt at both levels decreased this parameter in most lines, except for lines TNC 1.3, TNC 1.8 and TNC 10.8, which remained relatively constant (Fig. 3a). Line TNC 11.9 showed the largest decrease (39%) in leaf area than other lines, especially in the presence of 150 mM NaCl. The net phytosynthetic assimilation rate (NAR) also varied according to line and salt level (Fig. 3b). In the presence of 75 mM NaCl, the NAR decreased by 21, 15 and 28% in TNC 1.3, TNC 11.5 and TNC 11.9, respectively. This decrease was even more pronounced with 150 M NaCl in these latter three lines, and also showed up in TNC 10.9 and TNC 10.8. This depressive effect was most pronounced in TNC 1.3 and TNC 11.9 (reduction by 31 and 38%, respectively).
3.2 Nodule growth, nodulation and symbiotic nitrogen fixation
Symbioses established between S. medicae strain CI 1.12/E22 and M. ciliaris showed significant variation between the six lines (Fig. 4). In non-salt treated plants, lines TNC 10.9 and TNC 1.3 showed the highest and the lowest nodule biomass, respectively, whereas nodule biomass for the remaining lines was identical. Nodule number per plant showed also a wide range between lines, with line TNC 10.9 having the highest number. Both of these nodule characteristics were adversely affected by salt treatment (Fig. 4a, b). In TNC 1.8, and to a lesser extent TNC 10.8, nodulation (nodule DM) was less affected by salinity compared with other lines, especially at the highest concentration of salt in which nodulation was only reduced by 52% in TNC 1.8 compared with 69–83% in other lines.
Changes in nodule dry matter (NDM, A), in nodule number (B) and in total fixed nitrogen (TFN, C) in Medicago ciliaris lines cultivated in the absence or presence of NaCl. TFM was estimated by the difference between total fixed N before and after salt treatment. Mean values of 7 replicates ± confidence interval (P < 0.05)
Nitrogen levels were measured on a whole plant basis and in specific tissues of the six M. ciliaris lines (Table 2). Substantially greater symbiotic nitrogen fixation (mg plant−1) occured in non-saline conditions for line TNC 11.9 (two-fold higher than other lines), followed by TNC 1.3 (Fig. 4c). Under salt treatment, most lines showed decreased nitrogen fixation. In four of the lines, leaf N dropped with increased salt application, whereas in TCN 1.8 it remained constant (and even rose in TNC 10.8) (Table 2). Nodules accumulated two-fold higher N than other tissues, even without salt application. With added salt, the lines displayed variability in nodule N, with several lines showing increased N and others (TNC 11.9) being substantially reduced. Two of the lines (TNC 1.8 and TNC 10.9) remained stable for N over all concentrations.
3.3 Effect of NaCl on ion accumulation
Na+ accumulation was similar in individual aerial and underground tissues for all lines without salt treatment. Accumulation of sodium after salt treatment was markedly higher in underground organs when compared with aerial organs at both concentrations and across all lines. Under saline treatments in the six lines, Na+ accumulated to a similar extent in leaves of all lines treated with 150 mM NaCl, but the lines split into two classes at 75 mM NaCl, in which TNC 1.3, TNC 1.8, and TNC 10.8 accumulated lower amounts than the remaining three lines. Except for line TNC 10.9, which accumulated somewhat lower amounts of Na+ in roots (5-6-fold over non saline conditions), lines generally accumulated 7-8-fold of this toxic ion in roots after sodium supplementation compared to sodium-free growth. Interestingly, nodules of lines TNC 1.8 and TNC 11.9 accumulated more Na+, whereas line TNC 1.3 showed the lowest accumulation in these organs (Table 3).
Under saline conditions, K+ concentration was increased in leaves of three lines (TNC 1.8, TNC 10.8, and TNC 10.9), but remained similar for remaining lines. K+ stayed constant or showed small increases at the moderate salt concentration in stems of most lines, but increased strongly in TCN 10.9 (only at the moderate concentration) and in TCN 11.9 (at the high salt concentration).In roots, K+ was consistently higher under saline conditions when compared with non-saline levels in all lines, but TNC 1.3 and TNC 1.8 accumuated a higher amount of this ion than other lines (Table 4). This contrasted with K+ concentration in nodules of all lines, which generally showed a reduction to the same extent. Lines TNC 1.3 and TNC 1.8 accumulated significantly more potassium in nodules than the other lines regardless of the salt concentration.
3.4 Correlation analysis
In all but one Medicago ciliaris lines, leaf Na+ content was negatively correlated with net photosynthate assimilation rate and this correlation was significant in the line TNC 11.9 (Table 5). Total fixed N (estimated as the difference between total N quantities (mg plant−1) before and after salt treatment) was positively and highly correlated with net photosynthate assimilation rate and leaf reduced nitrogen content for all six lines except the line TNC 10.8 (Table 5). However, total fixed N was poorly correlated to nodule K+ content for all lines except the line TNC 1.8. Again, as with leaf sodium, line TNC 1.8 showed a completely opposite correlation pattern between total fixed N and nodule potassium accumulation.
4 Discussion
A previous study showed tolerance to NaCl in an accession of M. ciliaris growing in the presence of NaCl and N supplementation (Abdelly et al. 1995). In the current study, we evaluated genetic variability for tolerance to NaCl within six M. ciliaris lines growing symbiotically with S. medicae, i.e. in a low-input situation without N supplementation. This was accomplished by comparing the impact of salt on biomass and ion accumulation in aerial and underground tissues; including net photosynthetic assimilation in leaves; nodule number, biomass, and ion accumulation, and total fixed N. The intent was to determine whether genetic variability for low-input (i.e. symbiosis) and salt tolerance existed within a small number of M. ciliaris collections originating from 3 distinct bioclimes. A second objective was to determine which parameters out of a range of physiological and growth traits would be the best tools to use when screening a broader germplasm collection for additional salinity tolerance genotypes.
Out of the six genotypes evaluated, lines TNC 11.5 and TNC 11.9 originating from the humid bioclime reached the highest photosynthetic assimilation rate, N fixation, and biomass for all vegetative and underground organs under non-saline conditions. Responses to salt with the six genotypes were clearly dependent on the line and the level of applied salt even in such a small collection. Of particular interest are lines TNC 1.8 and TNC 10.8, which were collected from sub-humid and semi-arid sites. These two lines have lower biomass, lower net photosynthetic assimilation rates, and lower fixed N than some of the other lines under low-input non-saline conditions, but they maintain their levels consistently in the presence of 75 mM and 150 mM applied NaCl. Thus, although they show somewhat higher within-line variation than the four other lines, TNC 1.8 and TNC 10.8 may actually have more salt tolerance potential. Genotype variability between the six lines is present in spite of the geographic closeness (~200 km) of the three collection sites. Genotypic variability in plant growth and dry matter accumulation under saline conditions has also been reported for other legumes, such as V. faba, C. arietinum and M. truncatula, when cultivated under symbiotic nitrogen fixation conditions (Cordovilla et al. 1995; Tejera et al. 2006; Aydi et al. 2008).
Na+ accumulated differentially in the tissues of the six lines of M. ciliaris. In M. ciliaris (Ben Salah et al., unpublished data) and M. truncatula (Aydi et al. 2008), Na+ appears to reach a toxic concentration earlier than Cl−; for this reason we only measured Na+ accumulation in this study. Under applied salt, all six lines strongly accumulated Na+. Accumulation was preferential in nodules and roots compared with aerial organs, and leaves grown under saline conditions accumulated the least amount of Na+ of all organs for all the lines. However, line TNC 1.8 and again could be distinguished from the other lines by its low amount of leaf Na+ when tested at 75 mM NaCl. Na+ accumulation into vacuoles may contribute to osmotic adjustment, since it is the least demanding form of physiological or biochemical adjustment (consuming only 3–4 moles of ATP per mole of ion in comparison with 30–50 moles of ATP for the synthesis of organic solutes such as proline or sucrose) (Raven 1985). Low leaf accumulation and higher stem Na+ content could also indicate the export of this toxic ion by phloem in M. ciliaris lines, as shown in Medicago sativa, in which transfer cells play a crucial role at controlling nutrient and ion fluxes (Boughanmi et al. 2003).
A strong, positive correlation should occur between symbiotic nitrogen fixation and photosynthesis, since an actively operating symbiotic apparatus with its high demand for assimilates would promote the export of assimilates from leaves and thus stimulate photosynthetic function (Kirizii et al. 2007). In our study, we observed a strong positive correlation between SNF and photosynthate accumulation. A limited supply of nodules and assimilate would also lead to a decreased supply of leaf nitrogen, which, in turn, could limit chlorophyll replacement through an N-dependent repression mechanism which exists for photosynthesis-related genes (Scheible et al. 2004).
Salt treatment slowed down nodule development (biomass and number) in all six of the M. ciliaris lines, but did not affect nitrogen fixation in TNC 1.8 and TNC 10.8. The reduction of nodule number suggested that salt inhibited the initiation and the development of a second generation of nodules, since salt was applied after nodulation was firmly established in all lines. In several of the lines, salinity treatment also decreased symbiotic nitrogen fixation more than it affected host plant growth. This is consistent with previous studies in M. ciliaris and common bean (Phaseolus vulgaris L.) which reported a particular sensitivity of symbiotic nitrogen fixation to salt (Ashraf and Bashir 2003; Ben Salah et al. 2009, 2010; Jebara et al. 2010). Symbiotic N fixation must be more adaptive in the nodules of TNC 1.8 and TNC 10.8, since neither N fixation nor plant biomass was affected by the drop in nodule number and DM in these two lines.
Nodules of all or several M. ciliaris lines accumulated Na+ to a higher extent compared with aerial organs and roots. This finding is consistent with Na+ accumulation patterns in nodulated soybean, bean, and alfalfa (Serraj et al. 1998). Our results contrast with a study in M. truncatula (Aydi et al. 2008), since they suggest that the susceptibility of symbiotic nitrogen fixation to salt is not associated with higher accumulation of Na+ or K+ in nodules. In our case, nodules of the more saline-tolerant lines TNC 1.8 and TNC 10.8 accumulated high amounts of Na+. In this regard, Singleton and Bohlool (1983) showed that nitrogenase activity and whole plant growth of soybean were only slightly affected by salt in spite of the preferential accumulation of Na+ in nodules and a lower supply of K+. Testing for differential distribution of the toxic ion Na+ across cell layers of bacteria-infected and uninfected cells within these two lines, as conducted by Abd-Alla et al. (2001), as well as comparing these distribution patterns with those of more susceptible M. ciliaris lines, could help to understand the significance of nodule accumulation of Na+ in the future.
In conclusion, this study shows that genotypic variation for growth and nitrogen fixation in response to salinity can be found within a limited number of M. ciliaris lines selected from three widely different bioclimatic conditions when grown symbiotically without N supplementation. Our results suggest that nodule growth and number and nodule Na+ should not be used as selection tools for tolerance or susceptibility, since two of the tested lines maintained consistent growth in spite of reduced nodule and high Na+ content. Instead, the most reliable biometric and physiological indicators for tolerance appear to be consistent growth (i.e., no growth changes) and reduced leaf Na+ accumulation with increasing concentrations of NaCl. The limited differences observed between 75 mM or 150 mM NaCl also suggest that screening assays on a much larger spectrum of germplasm would likely be effective using only one salt concentration; hence the cost of the screening per plant could be reduced. The discovery of diversity within such a limited germplasm collection points to an opportunity to develop genetic crosses between tolerant and susceptible M. ciliaris lines and to make mapping populations. Such populations can take advantage of M. truncatula genome resources to develop molecular markers and genetic maps and to use these tools to quickly find salt-tolerant genotypes within a broader range of germplasm from across Tunisia and other countries.
References
Abd-Alla MH, El-Enamy AE, Hamada AM, Abdel Wahab AM (2001) Element distribution in faba bean nodules under salinity and its effects on growth, nodulation and nitrogen fixation. Rostlinná Výroba 47:399–404
Abdelkefi A, Boussaid M, Biborchi A, Haddioui A, Salhi-Hannachi A, Marrakchi M (1996) Genetic diversity inventory and evaluation of spontaneous species belonging to Medicago L. genus in Tunisia. Cah Options Méditerr 18:143–149
Abdelly C, Lachaâl M, Grignon C, Soltani A, Hajji M (1995) Association épisodique de halophytes stricts et de glycophytes dans un écosystème naturel. Agronomie 15:557–68
Ashraf M, Bashir A (2003) Salt stress induced changes in some organic metabolites and ionic relations in nodules and other plant parts of two crop legumes differing in salt tolerance. Flora 198:486–498
Aydi S, Sassi S, Abdelly C (2008) Growth, nitrogen fixation and ion distribution in Medicago truncatula subjected to salt stress. Plant Soil 312:59–67
Badri M, Zitoun A, Soula S, Ilahi H, Huguet T, Aouani ME (2008) Low levels of quantitative and molecular genetic differentiation among natural populations of Medicago ciliaris Kroch. (Fabaceae) of different Tunisian eco-geographical origin. Conserv Genet 9:1509–1520
Ben Salah I, Albacete A, Martínez Andújar C, Haouala R, Labidi N, Zribi F, Martinez V, Pérez-Alfocea F, Abdelly C (2009) Response of nitrogen fixation in relation to nodule carbohydrate metabolism in Medicago ciliaris lines subjected to salt stress. J Plant Physiol 166:477–488
Ben Salah I, Slatni T, Albacete A, Gandour M, Martínez-Andújar C, Houmani H, Ben Hamed K, Martinez V, Pérez-Alfocea F, Abdelly C (2010) Salt tolerance of nitrogen fixation in Medicago ciliaris is related to nodule sucrose metabolism performance rather than antioxidant system. Symbiosis 51:187–195
Ben Salah I, Slatni T, Gruber M, Messedi D, Gandour M, Benzarti M, Haouala R, Zribi K, Ben Hamed K, Perez-Alfocea F, Abdelly C (2011) Relationship between symbiotic nitrogen fixation, sucrose synthesis and anti-oxidant activities in source leaves of two Medicago ciliaris lines cultivated under salt stress. Env Exp Bot 70:66–173
Boughanmi N, Michonneau P, Verdus MC, Piton F, Ferjani E, Bizid E, Fleurat-Lessard P (2003) Structural changes induced by NaCl in companion and transfer cells of Medicago sativa blades. Protoplasma 220:179–187
Cordovilla MP, Ligero F, Lluch C (1995) Influence of host genotypes on growth, symbiotic performance and nitrogen assimilation in faba bean (Vicia faba L.) under salt stress. Plant Soil 172:289–297
Georgiev GI, Atkins CA (1993) Effects of salinity on N2 fixation, nitrogen metabolism and export and diffusive conductance of cowpea root nodules. Symbiosis 15:239–255
Jebara S, Drevon JJ, Jebara M (2010) Modulation of symbiotic efficiency and nodular antioxidant enzyme activities in two Phaseolus vulgaris genotypes under salinity. Acta Physiol Plant 32:925–932
Kalia VC, Drevon JJ (1985) Variation in nitrogenase activity (C2H2 reduction) during the in-situ incubation of nodules of Glycine max (L.) Merr. C R Acad Sci Franç Microbiol 6:591–596
Kirizii DA, Vorobei NA, Kots SY (2007) Relationships between nitrogen fixation and photosynthesis as the main components of the productivity in alfalfa. Russ J Plant Physiol 54:666–671
Kjeldahl JZ (1883) A new method for the determination of nitrogen in organic bodies. Anal Chem Anal Chem 22:366
Lauchli A (1984) Salt exclusion: an adaptation of legumes for crops and pastures under saline conditions. In: Staples RC, Toenniessen GH (eds) Salinity tolerance in plants. Wiley, New York, pp 171–187
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Norlyn JD, Epstein E (1984) Variability in salt tolerance of four triticale lines at germination and emergence. Crop Sci 24:1090–1992
Raven JA (1985) Regulation of pH and generation of osmolarity in vascular plants: a cost-benefit analysis in relation to efficiency of use of energy, nitrogen and water. New Phytol 101:25–77
Saadallah K, Drevon JJ, Abdelly C (2001) Nodulation et croissance nodulaire chez le haricot (Phaseolus vulgaris) sous contrainte saline. Agronomie 21:627–634
Scheible WR, Morcuende R, Czechowski T, Fritz C, Osuna R, Palacios-Rojas N, Schindelasch D, Thimm O, Udvardi MK, Stitt M (2004) Genome-wide reprogramming of primary and secondary metabolism, protein synthesis, cellular growth processes, and the regulatory infrastructure of Arabidopsis in response to nitrogen. Plant Physiol 136:2483–2499
Serraj R, Vasquez DH, Drevon JJ (1998) Effects of salt stress on nitrogen fixation, oxygen diffusion and ion distribution in soybean, common bean and alfalfa. J Plant Nutr 21:475–488
Singleton PW, Bohlool BB (1983) Effect of salinity on the functional components of the soybean-Rhizobium japonicum symbiosis. Crop Sci 23:815–818
Tejera NA, Soussi M, Lluch C (2006) Physiological and nutritional indicators of tolerance to salinity in chickpea plants growing under symbiotic conditions. Env Exp Bot 58:17–24
Tester M, Davenport R (2003) Na+ tolerance and Na+ transport in higher plants. Ann Bot 91:503–527
Yamaguchi T, Blumwald E (2005) Developing salt-tolerant crop plants: challenges and opportunities. Trends Plant Sci 12:615–620
Yamamouchi M, Tanaka S, Fujiyama H (1997) The cultivarietal differences in salt-tolerance and the effect on the absorption and translocation of K+, Ca2+ and Mg2+ ions in Phaseolus vulgaris L. J Jpn Soc Hortic Sci 65:737–745
Zribi K, Badri Y, Saidi S, van Berkum P, Aouani ME (2007) Medicago ciliaris growing in Tunisian soils is preferentially nodulated by Sinorhizobium medicae. Aust J Soil Res 45:473–477
Acknowledgments
This work was funded by the Tunisian Ministry of Higher Education, Scientific Research and Technology (LR10CBBC02). We wish to thank the Laboratory of Legumes (LL) in the Center of Biotechnologie at the technopole of Borj Cedria in Tunis for the generous gift of Sinorhizobium medicae CI 1.12/E22 strain and M. ciliaris seeds. Grateful thanks are due to Miss Salwa Chalbout for her technical assistance and to anonymous reviewers for their constructive comments and for thorough editorial remarks.
Author information
Authors and Affiliations
Corresponding author
Additional information
Imène Ben Salah and Tarek Slatni have participated equally to this work.
Rights and permissions
About this article
Cite this article
Ben Salah, I., Slatni, T., Gruber, M. et al. Variability in the response of six genotypes of N2-fixing Medicago ciliaris to NaCl. Symbiosis 53, 139–147 (2011). https://doi.org/10.1007/s13199-011-0118-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-011-0118-2