Abstract
In this study, the effect of 100 mM NaCl on physiological and biochemical responses were investigated in nodules of two Medicago ciliaris lines differing in salt tolerance (TNC 1.8 and TNC 11.9). Results showed that, on the basis of growth and nitrogen fixation, the line TNC 1.8 proved more salt tolerant than TNC 11.9. The salt-induced oxidative stress (membrane lipid peroxidation, leghemoglobin degradation, antioxidant activities reduction) occurred similarly in nodules of both lines. The tolerant line TNC 1.8 showed a better capacity to preserve higher sucrolytic activities and maintained higher nodule malate concentration, although total organic acids decreased in both lines. The higher amount of organic acids in the tolerant line seems to be related to its capacity to maintain higher NH4 nodule concentration in comparison with the sensitive line. Although salt stress reduced concentrations of the majority of amino acid in both lines, the decrease of the most preponderant amino acids glycine, valine, aspartate and glutamate was more accentuated in the sensitive line TNC 11.9. However, alanine concentration increased in the nodules of this sensitive line, suggesting a higher incidence of stress-induced hypoxia. The present study provides further evidence that salt tolerance of nitrogen fixation in the tolerant line is linked to a more effective supply of malate to bacteroids which allows the synthesis of amino acids required to maintain both plant and nodule growth.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Salinization is expected to have overwhelming global effects, resulting in a 30% land loss within the next 25 years and up to 50% by the year 2050 (Wang et al. 2003). This constraint is considered one of the most threatening factors to the natural environment and to limiting agriculture crop production (Chinnusamy et al. 2005; Zadeh and Naeini 2007). Leguminous plants play a critical role in natural ecosystems and agriculture owing to their capacity to fix atmospheric nitrogen.
Symbiotic nitrogen fixation (SNF) process involves a mutualism between legume plants and soil bacteria ‘rhizobia’. This interaction results in the formation of root-nodules, in which the bacteria reduce atmospheric nitrogen to ammonia which is used by the plant for the synthesis of a variety of amino acids, nucleic acids and other nitrogenous compounds (Appels and Haaker 1991). At the same time the bacteria are completely dependent on the host plant for carbon compounds, namely sucrose, and all other nutrients needed for energy production and carbon skeleton for SNF, ammonia assimilation, export of nitrogenous compounds and the building and the maintaining of the machinery of nitrogen fixation which constitutes the nodule (Arrese-Igor et al. 1999; Minchin and Witty 2005). The sucrose supplied to nodules is cleaved by sucrose synthase and alkaline invertase into hexoses which are metabolized through the glycolytic pathway yielding C4-dicarboxylates. Among these C4-diarboxylates, malate has been suggested to be the preferred substrate for bacteroid respiration and to be involved in the regulation of oxygen diffusion (Gálvez et al. 2000).
In addition to having physiological effects, salt stress can induce senescence process in different plant tissues by enhancing production of toxic reactive oxygen species (ROS). NaCl-induced senescence of nodules and decrease in SNF is associated with simultaneous decrease in the levels of antioxidants and the autooxidation of oxygenated leghemoglobin to ferric leghemoglobin yielding O −2 . This radical can dismutate to H2O2, which in turn may attack leghemoglobin, releasing catalytic Fe and producing the highly toxic HO• radical (Puppo and Halliwell 1988). Legume nodules possess a number of enzymatic mechanisms to prevent or limit the toxicity of ROS including superoxide dismutase, catalyse and peroxidase reducing the inherent danger of oxidative damage in the nodules (Jamet et al. 2003).
SNF has been described as being sensitive to salt stress (Jebara et al. 2005; López et al. 2008; Ben Salah et al. 2009). It has been suggested that the salt induced reduction of SNF was concomitant to a decrease in nodule sucrolytic activities, namely that of sucrose synthase (López et al. 2008; Ben Salah et al. 2009) causing a shortage of substrates for bacteroids respiration (Arrese-Igor et al. 1999). Other studies have demonstrated that the salt induced decrease in sucrose synthase activity was associated with an increase in alkaline invertase activity that, however, did not restore SNF (Soussi et al. 1999); thus, the role of sucrose synthase in the response to abiotic stresses was demonstrated (Arrese-Igor et al. 1999). Another major constraint for SNF is the enhanced ROS generation induced by salt stress and the impairment of antioxidant enzyme activities which leads to enhanced lipid peroxidation, protein degradation (namely leghemoglobin) and premature nodule senescence (Bianco and Defez 2009).
As a part of comparative study on the effects of salt stress on growth and nitrogen fixation in wild fodder legume M. ciliaris, we found that nitrogen fixing root-nodules were poorly supplied with sucrose and that the superiority of the tolerant line (TNC 1.8) was associated with its better ability to maintain higher nodule sucrolytic activities namely that of sucrose synthase and alkaline invertase (Ben Salah et al. 2009). We hypothesized therefore that the tolerant line TNC 1.8 would ensure a better supply of bacteroids in organic acids; this would allow an effective supply in nitrogen and thus the maintenance of growth under stress conditions. The objective of the present study was to address the effect of salt stress on antioxidant and sucrolytic activities and the repercussion on the concentration of tricarboxylic acid cycle acids and on amino acids synthesis and their relevance in the tolerance of SNF to salt stress.
2 Materials and methods
2.1 Biological materials and growth conditions
The two lines of M. ciliaris used in this study are originated from local populations from the edge of saline depression of Enfidha (TNC1.8) and non-saline habitat in Mateur (TNC11.9). Since the hard seed coat is impervious to water, seeds were scarified with concentrated H2SO4 for 40 min and were then washed 10 times with sterile distilled water and placed on sterile agar medium at 25°C in the dark. Three-day-seedlings were transferred sterile perlite and were inoculated with Sinorhizobium medicae CI 1.12/E22 strain suspension (about 108 mL−1) (Zribi et al. 2007). After 2 weeks plants were transferred in 250 mL glass bottle wrapped with aluminium foil to maintain darkness in the rooting environment containing Hewitt nutrient solution: KH2PO4 (1.60 mM), MgSO4 (1.50 mM), K2SO4 (1.50 mM), CaSO4 (3.50 mM), H3BO3 (4 μM), MnSO4 (4 μM), ZnSO4 (1 μM), CuSO4 (1 μM), CoCl2 (0.12 μM), (Na)6(Mo)7O24 (0.12 μM). Urea (2 mM N) was added to the nutrient solution only during the first week of irrigation. A month later (after the appearance of functional nodules), plants were divided into 2 lots irrigated with nutrient solution supplemented with 0 mM NaCl (control plants) or 100 mM NaCl (salt-treated plants). The continuously aerated culture medium was renewed weekly. Plants were grown in a greenhouse at 28 ± 5°C / 16 ± 5°C (day/night) temperature, 40 ± 5% / 80 ± 5% (day/night) relative humidity, and 14 h light / 10 h dark regime. The plants were harvested 3 weeks after the start of salt treatment (at late vegetative stage). Plants were uprooted carefully and washed with distilled water.
2.2 Nitrogen-fixation assay and nitrogen determination
Nitrogen fixation was estimated by measuring “in situ” the acetylene reduction activity (ARA) at the end of the vegetative stage. To avoid nodule disturbance, the level of the N-free solution was lowered to 40% of the glass bottle volume one day before the assay. 10% of acetylene was added to the nodulated roots enclosed in the glass bottle. After 20 min of incubation, samples of 1 mL were withdrawn from the flask and ethylene was measured using a gas chromatograph (Cromatix KNK-2000) (Hardy et al. 1968). N was determined as described by Kjeldahl (1983). Nitrogen fixation was estimated as the difference between total N quantities (mg plant−1) before and after salt treatment.
2.3 Ammonium determination
Free ammonium was extracted from frozen material, stored in liquid nitrogen, at 4°C with 0.3 mM H2SO4 and 0.5% (w/v) polyclar AT. The homogenate was centrifuged at 15 000 g for 15 min at 4°C. 100 µL of the supernatant was incubated in presence of 900 µL of phenol-hypochlorite reagent for 30 min at 37°C. Ammonium concentration was estimated measuring the absorbance at 620 nm and calculated from the calibration curve using ammonium sulphate (Weatherburn 1979).
2.4 Nutrients determination
The dried and ground samples (100 mg) were digested in microwave with HNO3: H2O2 (5:3) according to the method of Oliva et al. (2003) and mineral concentration was determined by inductively coupled plasma (ICP) spectrometry.
2.5 Organic and amino acids determination
Organic acids were analysed according to Balibrea et al. (1997). The ethanol extracts (1:3, W:V) were directly filtered through 13 mm diameter and 0.45 mm pore size Millex filters (Millipore Co., Milford, MA, USA) and analyzed by HPLC (Shimadzu Co. Ltd., Kyoto, Japan). Organic acids were determined by using an ion exchange column Interaction ORH-801 (Interaction Chromatography Inc., San Jose´, CA, USA), an UV detector at 210 nm, a mobile phase with H2SO4 0.01 N at a flow rate of 0.6 mL min−1 and at 45.1°C. Quantification was performed by the external standard method by using a data analysis Chrompass program (Jasco Co. Ltd., Tokyo, Japan). Amino acids were analyzed in purified nodule extracts by method described by Godel et al. (1987) using combined orthophthaldehyde (OPA) and fluoreylmthyloxycarbonylchloride (FMOC-CI) precolumn derivatization with OPA in a Shimadzu HPLC system (Shimadzu Corporation, Kyoto, Japan). The injection volume was 10 µL and the samples were eluted by binary gradient during 40 minutes: mobile phase A (sodium acetate 3- hydrate 100 mM, tetrahydrofuran (THF) 0.3% and triethylamine (TEA) 0.018%) and mobile phase B (sodium acetate 3-hidrate 100 mM: acetonitrile: methanol 20:40:40), with a flow 1.2 mL min−1 at 30°C of temperature, and UV detection at 338 nm.
2.6 Sucrolytic and antioxidant enzymes extraction and assay
After harvest, nodules were immediately frozen in liquid nitrogen and stored at −80°C until analysis. For sucrose synthase and alkaline invertase, samples containing polyvinylpirrolydone (PVP) and Fontainebleau sand were homogenized in 1 mL of extraction buffer containing 50 mM HEPES (pH 7), 10 mM MgCl2, 1 mM EDTA(2H2O), 2.6 mM dithiothreitol (DTT), 10% ethylene glycol and 0.02% Triton X-100 (Pelleschi et al. 1997). After centrifugation at 20,000 g, the supernatant was desalted on G25-Sephadex column pre-equilibrated with 4 mL of the reaction buffer containing 50 mM HEPES pH 7, 2 mM MgCl2, 1 mM Na2EDTA, 2.6 mM dithiothreitol (DTT) and 0.1% bovine serum albumin (BSA). Sucrose synthase and alkaline invertase were assayed by an enzyme-linked assay monitoring NADH formation at 340 nm (Balibrea et al. 2003). Superoxide dismutase (EC 1.15.1.1) and peroxidase (EC 1.11.1.7) were extracted with 10% (w/w) polyvinylpyrrolidone in 50 mM K-phosphate buffer (pH 8) containing 0.1 mM EDTA, 1 mM dithiothreitol and 0.5 mM phenyl-methyl-sulfonyl-fluoride (Scebba et al. 1999). The homogenate was centrifuged at 12 000 g for 30 min. The supernatant protein concentration was determined according to Bradford (1976). Spectrophotometric determination of superoxide dismutase activity was assayed by monitoring the inhibition of photochemical reduction of nitroblue tetrazolium (NBT) at 560 nm (Scebba et al. 1999). Peroxidase activity was assayed by monitoring the formation of tetragaiacol from guaiacol at 470 nm according to Srinivas et al. (1999).
2.7 Oxidative damage
Oxidative damage (estimated by membrane lipid peroxidation) was assessed by measuring the amount of malondialdehyde (MDA) in tissue. Fresh samples were homogenized in 0.1% (w/v) trichloroacetic acid solution. The homogenate was centrifuged at 15,000 g for 15 min. An aliquot of the supernatant was added to 0.5% thiobarbituric acid in 20% trichloroacetic acid. The mixture was incubated at 90°C for 30 min; the reaction was stopped by placing the reaction tubes in an ice water bath. Samples were centrifuged at 10,000 g for 5 min, and the absorbance of the supernatant was read at 532 nm. The value for non-specific absorption at 600 nm was subtracted. The concentration of MDA was calculated from the extinction coefficient 155 mM−1 cm−1.
2.8 Leghemoglobin determination
Nodules were homogenised in Drabkin’s reagent and leghemoglobin was quantified spectrophotometrically at A540 as described by Wilson and Reisenauer (1963). Bovine haemoglobin was used as a standard. Drabkin’s solution was obtained by dissolving 52 mg of potassium cyanide, 198 mg of potassium ferricyanide and 1 mg of sodium bicarbonate in 1,000 ml of distilled water.
2.9 Statistical analysis
Analysis of variance (ANOVA) was used for the statistical analysis of data. Mean separation procedures were carried out using the multiple range tests with Fisher’s least significant difference (LSD) procedure (P < 0.05).
3 Results
3.1 Growth and symbiotic nitrogen fixation
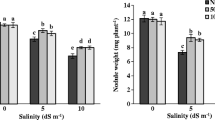
The line from the salty biotope, TNC 1.8, showed the higher production of biomass when compared with the second line TNC 11.9 even in presence of 100 mM NaCl (Fig. 1A). The inhibitory effect of salt treatment on total biomass was detected in both lines but this effect was less pronounced in TNC 1.8. Salinity caused also a decrease in nodule dry matter in both lines; the depressive effect of salt was always more accentuated in TNC 11.9 (Fig. 1B). Specific nitrogen fixation, estimated by acetylene reduction assay was repressed in both lines; TNC 11.9 was more affected by salt than TNC 1.8 (Fig. 1C). This parameter seemed to be more affected by salt than nodule growth (reduction 2.4–1.9 times greater) (Fig. 1C). The amount of fixed nitrogen decreased in both lines (Fig. 1D) and TNC 1.8 showed higher values of N-fixed compared to the other line. Under salt stress, nodule NH4 concentration was significantly more decreased in the sensitive line TNC 11.9 (Fig. 1E).
Changes in whole plant dry weight (A), nodule dry weight (B), specific acetylene reduction activity (C), nitrogen fixation (D), NH +4 (E), leghemoglobin (F) and MDA concentration (G) in control ( □) and salt-stressed (■) Medicago ciliaris lines. Mean values followed by the same letter are not significantly different at P < 0.05 (n = 3–7)
3.2 Nodule nutrients concentration
Under control and stressed conditions, nodules of both lines accumulated higher amounts of iron by comparison with the other micronutrients manganese, zinc and copper. Salt treatment decreased to the same extent both lines nodule concentration of potassium. Nodule micronutrient concentrations were unaffected by salt in both lines, except that of manganese which decreased in the tolerant line TNC 1.8, and zinc in the sensitive line TNC 11.9. No differences were observed for sodium accumulation in nodules of either line (Table 1).
3.3 Sucrolytic and antioxidant activities, nodule MDA and leghemoglobin concentration
Salt treatment led to a significant inhibitory effect on sucrose synthase activity in both lines particularly in the sensitive line TNC 11.9 (reduction of 81% against 43% in the tolerant line TNC 1.8). However, alkaline invertase activity was not altered by salt in the tolerant line TNC 1.8 whereas it was repressed by 77% in the sensitive line TNC 11.9. Salt treatment led to a significant inhibitory effect on both antioxidant enzymes activities, superoxide dismutase and peroxydase, at the same extent (Table 2). To estimate Na toxicity-induced oxidative damage, the MDA formation was measured in M. ciliaris nodules (Fig. 1G). Under salt stress, MDA levels increased similarly in both lines. This oxidative damage was associated with decline in nodules leghemoglobin concentration in both lines; this reduction was comparable in both lines (Fig. 1F).
3.4 Nodule metabolite concentration
In absence of 100 mM NaCl and in both lines, succinate and malate were the most abundant organic acids (Table 3) and amounted to 90 and 84% of the total organic acid fraction, whereas oxalate and fumarate were less abundant and represented 3 and 2% of the total organic acid fraction in TNC 1.8 and TNC 11.9 nodules, respectively. Under stress conditions, citrate became the second abundant organic acid following succinate in both lines. Salt stress decreased the total organic acid concentration in both lines (by 40 and 48% in TNC 1.8 and TNC 11.9, respectively). Oxalate was not changed by salt stress in both lines. The concentration of all other organic acids was affected to the same extent in both lines except for the concentration of malate which decreased much higher in the sensitive line TNC 11.9 (reduction of 66 against 30% in the tolerant line TNC 1.8).
Amino acids which were determined in M. ciliaris nodules are listed in Table 3. Under control conditions and in both lines, the most abundant amino acids were alanine, glycine, asparagine, valine, aspartate, glutamate and glutamine. Salt stress induced a decrease in total amino acid concentration in the nodules of both lines to the same extent. This constraint did not affect the pattern of accumulation of amino acid concentration of both lines but it induced a decrease in the concentration of glycine, valine, aspartate, glutamate and other minor amino acids such as arginine, threonine, phenylalanine and methionine. This reduction was more pronounced in the sensitive line TNC 11.9. Asparagine was unmodified by salt stress in both lines; glutamine also remained not modified but this only in the tolerant line TNC 1.8. Alanine concentration increased under salt stress in the sensitive line TNC 11.9; whereas, it remained unchanged in the tolerant line.
4 Discussion
In this study, nodules of M. ciliaris lines subjected to salt stress showed a remarkable inhibition in their nitrogen fixation capacity (measured as the capacity to reduce C2H2). This depressive effect of salt was less pronounced in the line TNC 1.8 originating from the salty biotope. As reported previously (Ashraf and Bashir 2003; Jebara et al. 2005), nodule growth and functioning were more sensitive to salinity than were vegetative organs (Fig. 1A).
As Na negatively affects the acquisition and homeostasis of essential nutrients, salt stress could reduce nodule growth and functioning through its nutritional effect. A previous study showed that in both lines, 100 mM NaCl decreased nodule nutrients concentration particularly that of K, Ca and P (Ben Salah et al. 2009). O’Hara (2001) reported that the symbiotic legume-rhizobium association requires nutrients to a greater extent than both partners individually. The decrease in K and Ca concentration in both lines would lead (i) to a disturbance in the operation of the cortical diffusion barrier and in the control of the ion channel that facilitates voltage dependent transport of monovalent cations including NH4 through the symbiosome membrane, and (ii) to nodules structure alteration, and would then be responsible for the reduction of nitrogenase activity (Tyerman et al. 1995; El-Hamdaoui et al. 2003). The reduction in Mn and Zn concentrations (in the tolerant and sensitive line, respectively) would have some repercussions on the antioxidant response of plants subjected to salt. In fact, these nutrients have been reported to have specific reactions in which the ion brings about optimum conformation of enzyme protein, such superoxide dismutase, and thus its activation and enables electrons transport by valency change (Mengel and Kirkby, 2001). A differential distribution of the toxic ion Na across cell layers (uninfected and infected cells) between the two lines can not be ruled out, as reported by Abd-Alla et al. (2001). Unless Na is sequestrated in vacuoles, this ion would partially induce damage of cellular components, disturbance of enzymatic activities and overproduction of harmful reactive oxygen species (Munns and Tester 2008; Garg and Manchanda 2008).
In nodules of both lines, the decline in leghemoglobin concentration (Fig. 1F), but not in total soluble protein (data not shown) would be indicative of sensitivity of leghemoglobin to degradation compared with other cytosolic proteins. Leghemoglobin degradation could be due to the activation or de-localization of proteases located in the infected cells that display a high affinity for leghemoglobin, especially at the acidic intracellular pH of senescing nodules (Pladys et al. 1991) and would therefore provide catalytic Fe which reacts with H2O2 to produce the toxic HO• radical (Puppo and Halliwell 1988) and participate directly or indirectly (bound to phytoferritin) in lipid peroxidation as occurred in both lines (Fig. 1G). Therefore, it can be suggested that peribacteroid membrane is target of degradative reactions induced by salt stress in the nodules of both lines. The observed oxidative damage have been earlier reported in nodules of soybean, pigeonpea and pea subjected to salt stress (Borucki and Sujkowska 2008; Zilli et al. 2008; Garg and Manchanda 2008). This salt induced oxidative damage could be due to disturbance in the antioxidant defence mechanism namely in the antioxidant enzymes such some superoxide dismutase and peroxidase (Table 2). In both lines, the activity of these enzymes was decreased in presence of salt. This reduction could explain to some extent the depressive effect on the membranes structural integrity and on leghemoglobin concentration. However, the similar response of antioxidant enzymes in both lines suggests that the relative tolerance of TNC 1.8 is not linked to the performance of antioxidant system.
Sucrose synthase represents the key enzyme required for the maintenance of nitrogenase activity (Gordon et al. 1999) and it has been proven to be sensitive to a wide range of stresses such drought, nitrate, salt and defoliation (Ramos et al. 1999; López et al. 2008). In M. ciliaris lines, the salt constraint triggered a drastic inhibition of sucrolytic activities particularly in the sensitive line TNC 11.9 (Table 2). However, although the tolerant line TNC 1.8 maintained constant activity of alkaline invertase, nitrogenase activity was decreased. This implies that the lack of sucrose synthase activity could not be compensated for by alkaline/neutral invertase (Gordon et al. 1999). Because of the decreased sucrose synthase activity, the effectiveness of the nitrogen-fixing capacity of the rhizobial symbiont was decreased and thus altered plant development (Baier et al. 2007).
C4-dicarboxylates are the main products of sucrose breakdown supplied to bacteroids to fuel nitrogen fixation (Lodwig and Poole 2003). Malate and succinate are the preferred substrates to support SNF and are well transported across the peribacteroid membrane via dicarboxylate carrier with high affinity for these two organic acids (Arrese-Igor et al. 1999). Consistent with earlier report (Fougère et al. 1991), salt stress severely decreased the total organic acid concentrations in both lines. The concentration of malate was more reduced in the sensitive line TNC 11.9 whereas changes in concentration of other organic acids was similar in both lines which would highlight the determining involvement of this substrate to fuel SNF. Thus, it can be suggested that the relative tolerance of SNF in TNC 1.8 could be related to its capacity to supply bacteroids effectively in malate. The maintenance of oxalate concentration in both lines (Table 3) could contribute in protecting nodules against the probable salt induced oxidative damage. Indeed, it has been suggested that the complete oxidation of oxalate was of interest in scavenging oxygen and limiting the generation of reactive oxygen species in the vicinity of bacteroids (Trinchant and Rigaud 1996).
The synthesis of organic acids is regulated in response to NH4 (Vance and Gantt 1992). In fact, in non-effective nodules, NH4 is involved in the expression of genes of nodule carbon metabolism enzymes (e. g. sucrose synthase, phosphoenolpyruvate carboxylase (PEPC) and PEPC-Kinase). The higher activities of sucrose synthase and alkaline invertase in M. ciliaris nodules of the tolerant line TNC 1.8 in response to salt stress (Table 2) would explain its better capacity to preserve nitrogen fixation and then to maintain an effective concentration of NH4 (Fig. 1E) and organic acid (Table 3). Since primary assimilation of nitrogen is inseparably linked to carbon metabolism, the differential depressive effect of salt on bacteroid supply in organic acids would lead to a differential amino acid biosynthesis activity in root nodules of both lines.
Salt stress affected nodule concentration of some amino acids to a greater extent in the sensitive line TNC 11.9 than the tolerant line TNC 1.8 (Table 3). The pronounced increase in alanine observed in the sensitive line TNC 11.9, but not in the tolerant one, is a characteristic response to O2 deficiency reported in many plant tissues (Streeter and Thompson 1972; Reggiani et al. 1988; Fan et al. 1997; Reggiani et al. 2000). Then, it could be suggested that in the sensitive line TNC 11.9 there was an establishment of an anaerobic metabolism in nodules likely following the salt induced decrease in the O2 diffusion to the infected zone (Serraj et al. 1995). In contrast to ethanol, which diffuses out into the media, most of the alanine produced under anaerobic conditions is retained in tissues (Hoffman et al. 1986) and contribute to cytoplasmic homeostasis (Sakano and Tazawa 1984, 1985). The marked depressive effect of salt on glutamate synthesis in the sensitive line TNC 11.9 (probably because of the inhibition of enzymes involved in the glutamine synthetase/glutamate synthase pathway) could explain the higher reduction in the majority of amino acids in this line by comparison with the tolerant line TNC 1.8; glutamate which is assimilated is used by various transaminases to generate all of the other amino acids. Apart from being a central nitrogen metabolite in nodule cells, glutamate, together with glycine, is thought to be involved in the regulation of cytosolic concentration of Ca (Dennison and Spalding 2000; Dubos et al. 2003). Glycine might also function as signalling molecule and favour the growth of nodules (White 1939; Fries 1953; Skinner and Street 1953). This amino acid is also essential for the terminal step of glutathione synthesis (Noctor et al. 1997) and it represents the major component of glycine-rich proteins (it represents up to 60%) found in the cell walls of many higher plants (Ringli et al. 2001). Since, M. ciliaris nodules have indeterminate growth, the maintenance of a relatively higher glycine and glutamate concentration in the tolerant line TNC 1.8 could account for a better growth of nodules and then to better fix nitrogen.
The present data suggest that the tolerance of nitrogen fixation in TNC 1.8 is related to its capacity to maintain sucrolytic activities; this would allow the supply of bacteroids in organic acids, namely malate, and thus a better synthesis of amino acids required for the maintenance of nodule and plant growth. The knowledge of the regulatory mechanisms that simultaneously allow the maintenance of SNF activity and plant growth will help to improve the tolerance of these legumes to saline soils.
References
Abd-Alla MH, El-Enamy AE, Hamada AM, Abdel Wahab AM (2001) Element distribution in faba bean nodules under salinity and its effects on growth, nodulation and nitrogen fixation. Rostlinná Výroba 47:399–404
Appels MA, Haaker H (1991) Glutamate oxaloacetate transaminase in pea root nodules 1 Participation in a malate/aspartate shuttle between plant and bacteroid. Plant Physiol 95:740–747
Arrese-Igor C, Gonzalez EM, Gordon AJ, Minchin FR, Galvez L, Royuela M, Cabrerizo PM, Aparicio-Tejo PM (1999) Sucrose synthase and nodule nitrogen fixation under drought and other environmental stresses. Symbiosis 27:189–212
Ashraf M, Bashir A (2003) Salt induced changes in some organic metabolites and ionic relations in nodules and other parts of two crop plants differing in salt tolerance. Flora 198:486–498
Baier MC, Barsch A, Küster H, Hohnjec N (2007) Antisense repression of the Medicago truncatula nodule-enhanced sucrose synthase leads to a handicapped nitrogen fixation mirrored by specific alterations in the symbiotic transcriptome and metabolome. Plant Physiol 145:1600–1618
Balibrea ME, Cayuela E, Artés F, Pérez-Alfocea F (1997) Salinity effects on some post-harvest quality factors in a commercial tomato hybrid. J Hortic Sci 72:885–892
Balibrea ME, Cuartero J, Bolarín MC, Pérez-Alfocea F (2003) Sucrolytic activities during fruit development of Lycopersicon genotypes differing in tolerance to salinity. Physiol Plant 118:38–46
Ben Salah I, Albacete A, Martínez Andújar C, Haouala R, Labidi N, Zribi F, Martinez V, Pérez-Alfocea F, Abdelly C (2009) Response of nitrogen fixation in relation to nodule carbohydrate metabolism in Medicago ciliaris lines subjected to salt stress. J Plant Physiol 166:477–488
Bianco C, Defez R (2009) Medicago truncatula improves salt tolerance when nodulated by an indole-3-acetic acid-overproducing Sinorhizobium meliloti strain. J Exp Bot 60:3097–3107
Borucki W, Sujkowska M (2008) The effects of sodium chloride-salinity upon growth, nodulation, and root nodule structure of pea (Pisum sativum L.) plants. Acta Physiol Plant 30:293–301
Bradford M (1976) A rapid and sensitive method for the quantification of microgram quantities of proteins utilising the principal of protein-dye binding. Anal Biochem 72:248–254
Chinnusamy V, Jagendorf A, Zhu JK (2005) Understanding and improving salt tolerance in plants. Crop Sci 45:437–448
Dennison KL, Spalding EP (2000) Glutamate-gated calcium fluxes in Arabidopsis. Plant Physiol 124:1511–1514
Dubos C, Huggins D, Grant GH, Knight MR, Campbell MM (2003) A role for glycine in the gating of plant NMDA-like receptors. Plant J 35:800–810
El-Hamdaoui A, Redondo-Nieto M, Torralba B, Rivella R, Bonilla I, Bolaños L (2003) Influence of boron and calcium on the tolerance to salinity of nitrogen fixing pea plants. Plant Soil 251:93–103
Fan TW-M, Higashi RM, Frenkiel TA, Lane AN (1997) Anaerobic nitrate and ammonium in flood-tolerant rice coleoptiles. J Exp Bot 48:1655–1666
Fougère F, Le Rudulier D, Streeter JG (1991) Effects of salt Stress on amino acid, organic acid, and carbohydrate composition of roots, bacteroids, and cytosol of alfalfa (Medicago sativa L.). Plant Physiol 96:1228–1236
Fries N (1953) Limiting factors in the growth of the pea seedling root. Physiol Plant 6:292–300
Gálvez S, Hirsh AM, Wycoff KL, Hunt S, Layzell DB, Kondorosi A, Crespi M (2000) Oxygen regulation of a nodule-located carbonic anhydrase in alfalfa. Plant Physiol 124:1059–1068
Garg N, Manchanda G (2008) Effect of arbuscular mycorrhizal inoculation on salt-induced nodule senescence in Cajanus cajan (pigeonpea). J Plant Growth Regul 27:115–124
Godel H, Seitz P, Verhoef M (1987) Automated analysis using combined OPA and FMOC-CI precolumn derivatization. LC-LG International 5:44–49
Gordon AJ, Minchin FR, James CL, Komina O (1999) Sucrose synthase in legume nodules is essential for nitrogen fixation. Plant Physiol 120:867–877
Hardy WFR, Holsten R, Jackson E, Burns E (1968) The acetylene ethylene assay for nitrogen fixation: lab and field assay for nitrogen evaluation. Plant Physiol 43:1185–1207
Hoffman NE, Bent AF, Hanson AD (1986) Induction of lactate dehydrogenase isozymes by oxygen deficit in barley root tissue. Plant Physiol 82:658–663
Jamet A, Sigaud S, Van de Sype G, Puppo A, Herouart D (2003) Expression of the bacterial catalase genes during Sinorhizobium meliloti-Medicago sativa symbiosis and their crucial role during the infection process. Mol Plant Microbe Interact 16:217–225
Jebara S, Jebara M, Limam F, Aouani ME (2005) Changes in ascorbate peroxidase, catalase, guaiacol peroxidase and superoxide dismutase activities in common bean (Phaseolus vulgaris) nodules under salt stress. J Plant Physiol 162:929–936
Kjeldahl JZ (1983) New methode zyr Besimming des stickstoffs in organischen Körpen. J Anal Chem 22:366–382
Lodwig E, Poole P (2003) Metabolism of Rhizobium bacteroids. Crit Rev Plant Sci 22:37–78
López M, Herrera-Cervera JA, Iribarne C, Tejera NA, Lluch C (2008) Growth and nitrogen fixation in Lotus japonicus and Medicago truncatula under NaCl stress: nodule carbon metabolism. J Plant Physiol 165:641–650
Mengel K, Kirkby EA (2001) Plant nutrients. In: Dordrecht (ed.). Principles of plant nutrition, pp 1–14
Minchin FR, Witty JF (2005) Respiratory/carbon costs of symbiotic nitrogen fixation in legumes. In: Lambers H, Ribas-Carbo M (ed.) Plant respiration, pp 195–205
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Noctor G, Arisi AC, Jouanin L, Valadier MH, Roux Y, Foyer CH (1997) The role of glycine in determining the rate of glutathione synthesis in poplar. Possible implications for glutathione production during stress. Physiol Plant 100:255–263
O’Hara GW (2001) Nutritional constraints on root nodule bacteria affecting symbiotic nitrogen fixation: a review. Aust J Exp Agr 41:417–433
Oliva SR, Raitio H, Mingorance MD (2003) Comparison of two wet digestion procedures for multielement analysis of plant samples. Communications in Soil Science and Plant Analysis 34:2913–2923
Pelleschi S, Rocher JP, Prioul JL (1997) Effect of water restriction on carbohydrate metabolism and photosynthesis in mature maize leaves. Plant Cell Environ 20:493–503
Pladys D, Dimitrijevic L, Rigaud J (1991) Localization of a protease in protoplast preparations in infected cells of French bean nodules. Plant Physiol 97:1174–1180
Puppo A, Halliwell B (1988) Generation of hydroxyl radicals by soybean nodule leghemoglobin. Planta 173:405–410
Ramos MLG, Gordon AJ, Minchin FR, Sprint JI, Parsons R (1999) Effect of water stress on nodule physiology and biochemistry of a drought tolerant cultivar of common bean (Phaseolus vulgaris L.). Ann Bot 83:57–63
Reggiani R, Cantu CA, Brambilla I, Bertani A (1988) Accumulation and interconversion of amino acids in rice roots under anoxia. Plant Cell Physiol 29:982–987
Reggiani R, Nebuloni M, Mattana M, Brambilla I (2000) Anaerobic accumulation of amino acids in rice roots: role of the glutamine synthetase/glutamate synthase cycle. Amino Acids 18:207–217
Ringli C, Keller B, Ryser U (2001) Glycine-rich proteins as structural components of plant cell walls. Mol Life Sci 58:1430–1441
Sakano K, Tazawa M (1984) Intracellular distribution of free amino acids between the vacuolar and extravacuolar compartments in internodal cells of Chara australis. Plant Cell Physiol 25:1477–1486
Sakano K, Tazawa M (1985) Metabolic conversion of amino acids loaded in the vacuole of Chara australis intemodal cells. Plant Physiol 78:673–677
Scebba F, Sebastiani L, Vitagliano C (1999) Protective enzymes against activated oxygen species in wheat (Triticum aestivum L.) seedlings: responses to cold acclimation. J Plant Physiol 55:762–768
Serraj R, Fleurat-Lessard P, Jaillard B, Drevon JJ (1995) Structural changes in the inner-cortex cells of soybean root nodules are induced by short-term exposure to high salt or oxygen concentrations. Plant Cell Environ 18:455–462
Skinner JC, Street HE (1953) Studies on the growth of excised roots. II. Observations on the growth of excised groundsel roots. New Phytologist 53:44–67
Soussi M, Lluch C, Ocaña A (1999) Comparative study of nitrogen fixation and carbon metabolism in two chick-pea (Cicer arietinum L.) cultivars under salt stress. J Exp Bot 50:1701–1708
Srinivas ND, Rshami KR, Raghavarao KSMS (1999) Extraction and purification of a plant peroxidase by aqueous two-phase extraction coupled with gel filtration. Process Biochem 35:43–48
Streeter JG, Thompson JF (1972) Anaerobic accumulation of g-aminobutyric acid and alanine in radish leaves (Raphanus sativus L.). Plant Physiol 49:572–578
Trinchant JC, Rigaud J (1996) Bacteroid oxalate oxidase and soluble oxalate in nodules of faba beans (Vicia faba L.) submitted to water restricted conditions: possible involvement in nitrogen fixation. J Exp Bot 47:1865–1870
Tyerman SD, Whitehead LF, Day DA (1995) A channel-like transport for NH +4 on the symbiotic interface of N2-fixing plants. Nature 387:629–632
Vance CP, Gantt JS (1992) Control of nitrogen and carbon metabolism in root nodules. Physiol Plant 85:266–274
Wang W, Vinocur B, Altman A (2003) Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta 218:1–14
Weatherburn MW (1979) Phenol-hypochlorite reaction for determination of ammonia. Anal Chem 39:971–974
White PR (1939) Glycine in the nutrition of excised tomato roots. Plant Physiol 14:527–538
Wilson DO, Reisenauer HM (1963) Determination of leghemoglobin in legume nodules. Anal Biochem 6:27–30
Zadeh HM, Naeini MB (2007) Effects of salinity stress on the morphology and yield of two cultivars of canola (Brassica napus L.). J Agr 6:409–414
Zilli CG, Balestrasse KB, Yannarelli GG, Polizio AH, Santa-Cruz DM, Tomaro ML (2008) Heme oxygenase up-regulation under salt stress protects nitrogen metabolism in nodules of soybean plants. Env Exp Bot 64:83–89
Zribi K, Badri Y, Saidi S, van Berkum P, Aouani ME (2007) Medicago ciliaris growing in Tunisian soils is preferentially nodulated by Sinorhizobium medicae. Aust J Soil Res 45:473–477
Acknowledgments
This work was funded by the Tunisian Ministry of Higher Education, Scientific Research and Technology (LR10CBBC02) and by the Fundación Séneca (Communidad Autónoma de Murcia, Spain, project 08712/PI/08). We wish to thank the Laboratory of Legumes (LL) in the Center of Biotechnologies at the technopole of Borj Cedria in Tunis for the generous gift of Sinorhizobium medicae CI 1.12/E22 strain and M. ciliaris seeds. Grateful thanks are due to anonymous reviewers for their constructive comments and for thorough editorial remarks.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ben Salah, I., Slatni, T., Albacete, A. et al. Salt tolerance of nitrogen fixation in Medicago ciliaris is related to nodule sucrose metabolism performance rather than antioxidant system. Symbiosis 51, 187–195 (2010). https://doi.org/10.1007/s13199-010-0073-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13199-010-0073-3