Abstract
Pearl millet bran is rich source of dietary fiber and several other bioactive compounds and is an unexploited by-product of millet processing industries. The utilization of pearl millet bran for extraction of dietary fiber can be an effective method for its valorization. Hydrothermal extraction of dietary fiber from pearl millet bran is a simple eco-friendly technique in terms of minimal consumption of toxic solvents, increased extraction yield, high purity and considered as an economically viable technique. In the present investigation, extraction and optimization of dietary fiber from pearl millet bran was performed using hydrothermal technique. The highest yield of dietary fiber (74.5%, w/w) was obtained under optimized conditions of water to solid ratio (20:1), temperature (90 °C) and time (15 min). The extracted dietary fiber from pearl millet bran was further assessed for its physico-chemical, functional and structural properties. The studies of functional and physico-chemical properties presented the water holding capacity (6.50 g/g and 3.99 g/g), swelling power (2.0 g/g and 2.05 g/g), oil holding capacity (4.91 g/g and 2.42 g/g), solubility (70%), total phenolic content of 4.24 mg GAE/g and 4.32 mg GAE/g, DPPH reduction of 86.6% and 83.9%, respectively. The results indicated that pearl millet bran can act as rich source of dietary fiber with health enhancing properties and can be utilized as potential food component in preparation of functional food products.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Millets are group of small seeded annual grasses and is growing extensively in the tropical and semi-arid region of the world (Sachdev et al. 2023). Pearl millet (Pennisetum glaucum) is a staple food in Asia, Africa and contributed to 40% of the world millet production. This was followed by foxtail millet (Setaria italica), proso millet (Panicum miliaceum) and finger millet (Eleusine carocana). Pearl millet grains are often referred to as “nutri-cereals’’ and “coarse grain” because of their higher fiber, mineral, protein, antioxidant activity. It constitutes of high functional and nutritional properties and provides a wide range of health benefits (Selladurai et al. 2022). The by-product of millet milling process, millet bran remains underexploited residue, despite the fact that it is rich in polyphenols, dietary fiber, minerals and gluten-free (Barbhai and Hymavathi 2022). Dietary fiber (DF) is defined as that part of a plant in food that is not digested and resistant to enzymatic reaction in the human small intestine, maybe fully or partial fermented in the large intestine, and consists primarily of polysaccharides and a trace amount of protein (Kaur et al. 2021). The DF can be divided into two categories: water-soluble fiber (SDF) and water-insoluble fiber (IDF). SDF and IDF can be found in varying amounts in a variety of food sources, including grains, fruits, vegetables, and legumes (He et al. 2022). DFs can result in beneficial effects, such as lowering the risk of intestinal disorders, diabetes, obesity, hypertension and constipation, regulates blood pressure, thus reducing the risk of heart diseases (Zhu et al. 2018). Dietary fiber is excellent for weight loss as it reduces appetite by adding bulk to the diet. In particular, SDFs (such as glucan, pectin, inulin and oligosaccharides) are readily fermented in the digestive tract as compared to IDFs (such as cellulose, some hemicelluloses and lignin). This has a significant effect on intestinal health of human beings by controlling the composition of gut microbes The consumption of SDF by humans has a positive impact on metabolism and cholesterol, which might be helpful for the treatment of atherosclerosis and other heart diseases (Wei et al. 2022).
The millet bran contains high dietary fiber content, bioactive compounds and micronutrients. However, it is generally discarded as waste or used as a fodder, which doesn’t make use of this potential dietary resource (Mustač et al. 2020). In order to use this unutilized resource, it is therefore of considerable potential to develop a highly efficient method for extracting dietary fiber from pearl millet bran. The hydrothermal (HT) extraction of dietary fiber is an economical and ecofriendly method besides it involves minimal chemicals, no sophisticated equipments and produces high purity dietary fiber. The hydrothermal treatment results in higher extraction yields by modifying the composition of dietary fibers by solubilizing their insoluble fraction and can improve the functionality of dietary fibers, making them more suitable for incorporation in food formulations (Garcia-Amezquita et al. 2020). The present study involves the use of underutilized by-product (bran) of pearl millet which is majorly grown around the world. The hydrothermal method was used as an effective and green solvent for the extraction of dietary fibers from pearl millet bran. The present study involves lower solvent consumption and lesser extraction time which makes the process more economical. Further, the optimization of various extraction variables was carried out to get the maximum yield of dietary fibers from pearl millet bran and characterization (physico-chemical, functional and structural) was also studied.
Materials and methods
Materials
The pearl millet (Pennisetum glaucum) was procured from the certified centres at CCSHAU Agricultural Centre (Haryana) and University of Agricultural Sciences Bangalore, the seeds were cleaned to remove the foreign material. Pearl millet bran was obtained through parboiling the pearl millet grains. The whole parboiled-decorticated kernels were milled, polished and sieved through 60 mesh sieve to get bran powder of uniform particle size.
Experimental design
Extraction of dietary fiber
The dietary fiber extraction from pearl millet bran was carried out by hydrothermal method (Elleuch et al. 2008; Cardenas-Toro et al. 2014). The pearl millet bran (1 g) was added into 20 mL distilled water at 100 °C for 5 min. Dietary fiber concentrates were recovered by centrifugation. The residue obtained was subjected to five successful rinsing (100 mL water at 40 °C) followed by centrifugation each time. The recovered residue was dried in an oven (40 °C) to collect the total dietary fiber concentrate (DFC) and yield was estimated using equation below:
Determination of total, insoluble and soluble dietary fiber
The assessment of SDF and IDF content in pearl millet bran was carried out according to the procedure of Wang et al. (2022) with some modifications. The hydrothermally treated mixture was centrifuged (5000 g, 15 min), both supernatant and residue were collected. This was followed by centrifugation, residue was separated out, washed with distilled water, and left to dry to obtain IDF. The filtrate was separated out, mixed with ethanol (95%, four-fold volume) and left for precipitation (2 h). After precipitation, the obtained residue was rinsed with 100% ethanol and left to dry. Total dietary fiber was obtained as the sum of IDF and SDF content (Wang et al. 2022).
Experimental design by response surface methodology
The effect of several extraction parameters including liquid to solid ratio (A) (10:1–30:1), temperature (B) (85–95 °C) and time (C) (10–20 min) on the yield of dietary fiber extraction (%, w/w) was studied using Response Surface Methodology (RSM) with three factors Box–Behnken design (BBD). The data analysis and determination of regression equation coefficients was done using the statistical software Design Expert 13.0.5.0 (Statease Inc., Minneapolis, USA). Model fitting was confirmed by determination of R2 value and each term was tested statistically with F-values of p ≤ 0.05. The analysis of variance (ANOVA) showed the significance of model.
Microstructural characterization
Scanning electron microscopy
Scanning electron microscopy (JSM, 7610 F plus, JEOL, Japan) was used for the structural characterization of SDF and IDF extracted from pearl millet bran.
Fourier transform infrared spectroscopy
The functional groups of dietary fiber from pearl millet bran were observed using Fourier transform infrared spectroscopy (FTIR) spectrophotometer (RX-I Perken Elmer Spectrum, USA). The absorbance range for FTIR was 4000 to 400 cm−1.
X-ray diffraction
The morphology of IDF and SDF samples extracted from pearl millet bran were recorded using XRD (PAN-analytic-X’pert PRO MRD, Almelo, Netherlands). The diffractograms were taken between 5° and 60°.
Physico-chemical characterization
Total phenolic content
The Folin-Ciocalteu calorimetric method was used to determine the total phenolic content (TPC) of dietary fiber samples from pearl millet bran (Ainsworth and Gillespie 2007). Ethanol (50%) was used to prepare the extract (100 µL) of dietary fiber samples and it was mixed with sodium carbonate (750 µL; 7.5% w/v) and Folin-Ciocalteu reagent (750 µL; 10% v/v). The solution was placed at room temperature (30 min) and its absorbance was recorded (765 nm). To calculate TPC, the standard curve of gallic acid was used and the results were expressed as mg gallic acid equivalent per gram of dietary fiber sample (mg GAE/g).
DPPH scavenging activity
To determine antioxidant activity of dietary fiber samples from pearl millet bran, the extract was prepared using distilled water followed by addition of ethanolic DPPH solution (0.1 mM, 1 mL) to extract. It was then placed in dark (30 min) and the absorbance was recorded (517 nm). The DPPH scavenging activity was calculated using the Eq. (1) (Nenadis and Tsimidou 2002)
Particle size
The particle size analyzer (Shimadzu corporation SALD-2300, Japan) was used for the determination of particle size of dietary fiber samples extracted from pearl millet bran.
Functional characterization
Water holding capacity
Estimation of water holding capacity (WHC) of dietary fiber obtained from pearl millet bran was carried out by mixing the sample (0.5 g) with distilled water (20 mL) in a centrifuge tube. It was then placed at room temperature (30 min) followed by centrifugation (3000 g, 20 min) and separation of supernatant (Kurek et al. 2018). The centrifuge tube containing the residue was weighed and WHC was calculated using the Eq. (3).
where M1 is the weight of the sample, M2 is the final weight of the residue.
Oil holding capacity
Estimation of oil holding capacity (OHC) of dietary fiber obtained from pearl millet bran was carried out by mixing the sample (0.5 g) with appropriate amount of oil (10 mL) in a centrifuge tube. The mixture was placed at room temperature (30 min), centrifuged (3000 g, 20 min) and the supernatant was removed (Kurek et al. 2018). The centrifuge tube containing residue was weighed and OHC was estimated using Eq. (4)
where O1 is the sample weight, O2 is the final weight of the residue.
Water solubility
Water solubility (WS) of extracted dietary fiber from pearl millet bran was determined by adding the sample (0.3 g) in distilled water (10 mL) and stirring (100 °C, 30 min). The centrifugation (4000 g, 15 min) was done and supernatant collected was transferred to weighing petri plate and left to dry. The WS was determined using the Eq. (5) (Li et al 2022a, b)
where s1 was the weight of supernatant after drying, s0 was the weight of initial dried sample.
Swelling capacity
To determine the swelling capacity (SC) of dietary fiber from pearl millet bran, the IDF and SDF sample (0.5 g) placed in a test tube, then volume was noted. This was followed by the addition of water (10 mL) and then incubated for 18 h. The final volume achieved by the sample was measured using the Eq. (6) (Li et al. 2022a, b)
where V1 is volume of sample, V2 is sample volume after 18 h, and m is the weight of sample.
Emulsifying activity
To determine the emulsifying activity (EA) of dietary fiber from pearl millet bran, the sample solution (5 mL, 0.5%, w/v) was mixed with oil (5 mL). The mixture was then homogenized and centrifugation (4000 g, 5 min) was done. The emulsion activity was determined using the Eq. (7) (Panwar et al. 2023)
Statistical analysis
The data analysis was done using the statistical software Design Expert 13.0.5.0 (Statease Inc., Minneapolis, USA). The principle component analysis (PCA) was analyzed using SPSS software at a level of significance (p < 0.05).
Results and discussion
The optimum yield of extracted dietary fiber from pearl millet bran was assessed. The physicochemical, functional and structural characteristics of the dietary fibre obtained was evaluated and mentioned below.
Extraction of dietary fiber from pearl millet bran
The total extraction yield of dietary fiber obtained from pearl millet bran isolated using the hydrothermal treatment was found to be 74.5% (w/w). The result obtained was comparable with past studies where it was reported that maximum yield of dietary fiber recovered from millet bran was 73.18 g/100 g (Liu et al. 2012). The effect of various process parameters (temperature 85–95 °C, time 10–20 min, and ratio of liquid to solid 10:1–30:1) on the dietary fiber extraction yield was selected on the basis of primary trials and examined. BBD was used to analyze the interactions between process variables and their effect on the yield of dietary fiber. The three factors BBD design and the responses expressed as IDF and SDF (%, w/w) has been presented in Table 1. The extraction process variables including ratio of liquid to solid (A), temperature (B) and time (C) showed significant effect (p < 0.05) on dietary fiber yield. The quadratic effect of all the process parameters and their interactive effect (AB, AC, and BC) was found significant. F-value was significant with R2 values of 0.996 and 0.997 for IDF and SDF, respectively. The Lack of Fit F-value of 0.439 and 1.55 implies the Lack of Fit is not significant relative to the pure error. There is an 33.29% chance that a “Lack of Fit F-value” this large happens because of noise. The results demonstrated the applicability of the mathematical model for prediction within the range of the selected experimental variables and was found to be appropriate for extraction of IDF and SDF present in pearl millet bran (Table 2).
Optimization of process variables for dietary fiber extraction
The interactive effect of different process variables such as temperature, time, and liquid to solid ratio on IDF and SDF yield has been presented in Fig. 1a–f. When the ratio of liquid to solid was increased from 10:1 to 20:1 at temperature up to 90 °C, the extraction yield of IDF was found to increase, however, subsequently its value was found to drop (Fig. 1a). This may be due to the higher concentration difference between the liquid (water) and solid (bran sample), which caused greater diffusion of dietary fiber into the solvent (Wang and Bai 2017). A similar effect of ratio of liquid to solid was observed in earlier studies on corn hull, where increase in ratio of liquid to solid up to 20:1 led to increase in extraction yield of dietary fiber (Wang et al. 2018). An insignificant effect of time and ratio of liquid to solid on IDF yield was observed (p ˃ 0.05). The interactive effect of time and temperature on the IDF yield has been presented in Fig. 1c. The extraction yield of IDF increased, when the treatment time was increase from 10 to 15 min and then found to be decreased. The yield of IDF increased initially when the temperature increased from 85 to 90 °C, but after 90 °C temperature its value eventually decreased. Due to an increase in water solvating capacity at higher temperature, the yield of IDF increased with temperature (Benito et al. 2013).
The interactive effect of temperature and liquid to solid ratio on extracted SDF has been shown in Fig. 1d. The SDF yield increased with temperature from 85 to 90 °C and ratio of liquid to solid 10:1 to 20:1. The increase in solubility of the soluble substances during the hydrothermal treatment at high temperature resulted in the breakdown of millet bran hemicelluloses and release of soluble substances from the sample. The interactive effect of time and ratio of liquid to solid on the SDF yield has been presented in Fig. 1e. The heating time from 10 to 15 min result in increased extracted dietary fiber yield; however, longer duration of hydrothermal treatment led to the thermal disintegration of the soluble dietary fiber (Wang et al. 2018). A similar observation was reported by researchers where it was found that long extraction time resulted in degradation of β-glucan from waxy barley when pressurized hot water was used (Benito et al. 2013). The increase in SDF yield with increasing temperature from 85 to 90 °C may be due to higher solubility and diffusion process (Fig. 1f). When the extraction temperature is too low, it is difficult to dissolve the soluble fibers from the plant cell wall (Kamal et al. 2023).
Verification of the model
The different process variables (extraction time, liquid to solid ratio and temperature) were optimized and resulted in the highest yield of IDF and SDF at 90 °C temperature, liquid to solid ratio of 20:1 and treatment time of 15 min. At this optimized condition, the SDF and IDF yield was predicted to be 8.2%. and 67.8%. The practical experiment gave the yield of IDF and SDF was 67% and 7.5%.
Microstructural characterization
SEM analysis
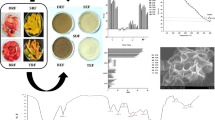
The structural morphology of the dietary fiber samples from pearl millet bran were carried out by scanning electron microscopy indicated in Fig. 2a. SDF-HT sample shown irregular flakes of uneven size and dense surface structure. The IDF-HT revealed a comparatively compact surface and smooth area with fine constituent parts on the surface (Chu et al. 2019). The hydrothermal extraction caused degradation of the cellular structure of dietary fiber samples and resulted in development of clusters and fractures. This may have led to formation of porous structure which corresponds to moderate cholesterol binding capacity and hydration properties of dietary fibers (Wen et al. 2017).
FTIR analysis
The organic functional groups were studied using FTIR spectrum and the molecular structures of IDF and SDF has been represented in Fig. 2b. At about 3272 cm−1 and 3268 cm−1, both samples showed significant signals which were caused by the vibrations of H-bonds linked with the –OH groups of polysaccharides present in the samples. The intensity of absorption peaks at 2800–3000 cm−1 indicated the C–H, –CH2 or –CH groups stretching bands of polysaccharides (Chu et al. 2019). The absorption bands near 1634–1643 cm−1 represented O–H bending vibrations that was characteristic of adsorbed water. The band of absorption peak around 1744 cm−1corresponded to C=O stretching vibrations in the carboxyl groups (–COOH) and suggested that uronic acids existed in the form. Weak intensity peaks near 1200–1435 cm−1 represented the C-O stretching in hemicelluloses, cellulose and C–H bending vibrations (Kaur et al. 2021).
XRD analysis
The morphological nature of dietary fiber samples extracted from pearl millet bran was analyzed using X-Ray diffraction. The crystal structure of the IDF and SDF (HT extraction) samples has been presented in Fig. 2c. Amorphous regions in dietary fiber may be characteristic of non-crystalline hemicellulose, cellulose, and lignin whereas, crystalline regions in most part might be due to the presence of cellulose. The SDF-HT sample had weak diffraction peaks which indicated that SDF-HT mainly existed in amorphous form and was difficult to crystallize (Wang et al. 2022). The results showed that the sharp diffraction peaks in IDF-HT observed in the range of 15 to 25 ℃ indicated the presence of typical type I cellulose (Li et al. 2022a, b).
Physico-chemical characterization
TPC of IDF and SDF obtained from pearl millet bran using hydrothermal treatment was found to be 4.24 mg GAE/g and 4.32 mg GAE/g. The lower TPC content may be attributed to the decortication of millet husks and brans. The DPPH activity of IDF and SDF from pearl millet bran obtained was 86.6% and 83.9% (Li et al. 2022a, b). The higher DPPH antioxidant activity exerts positive effects on nutritional profile. The particle size analysis of IDF and SDF obtained from pearl millet bran using hydrothermal extraction indicated values of 181.6 and 161.7 µm, respectively. The particle size directly effects the functional properties of the dietary fibers (Jiang et al. 2022).
Determination of functional properties
WHC is the capability of DF to capture enormous quantity of water and resulted in prevention of water from oozing out (Wang et al. 2022). WHC of IDF and SDF obtained from pearl millet bran using hydrothermal treatment was found to be 6.50 g/g and 3.99 g/g. Similarly, oil holding capacity for IDF and SDF sample obtained was 4.91 g/g and 2.42 g/g, respectively. WHC and OHC are associated with the surface characteristics such as thickness, viscosity, hydrophobic and hydrophilic groups of the dietary fiber components, respectively (Fernández-López et al. 2009). The solubility of pearl millet bran SDF was found to be 70% at 100 °C. Dietary fiber with high WHC makes it suitable in applications as functional food ingredient to alter the viscosity, texture and avoid synaeresis (Elleuch et al. 2011). The SC for IDF and SDF sample from pearl millet bran using hydrothermal treatment obtained was 2.0 g/g and 2.05 g/g. The EA associated with the protein’s ability to absorb water, oil and formation of emulsion (Devisetti et al. 2014). The EA for IDF and SDF sample from pearl millet bran obtained was 24% and 22.5%.
Principle component analysis
The validation of differences among different properties of dietary fiber samples was studied using Principal Component Analysis (Fig. 3). In this case, it was observed that both samples IDF and SDF lie in the same quadrant depicting a strong correlation. Regarding the functional parameters, it was found that emulsifying activity, particle size, water holding capacity and oil holding capacity were a positive correlation with IDF and SDF whereas TPC, DPPH activity, solubility index, and swelling capacity indicate a negative correlation with both samples (Sharma et al. 2022).
Conclusion
Hydrothermal method has been successfully utilized as an efficient ecofriendly technique for the extraction of dietary fiber from pearl millet bran with potential application at the industrial level. The highest yield of total dietary fiber (74.5%, w/w) was found with 20:1 liquid to solid ratio at 90 °C temperature after extraction time of 15 min. SEM images indicated a considerable effect of hydrothermal treatment on dietary fiber. FTIR analysis of IDF and SDF indicated the presence of organic functional groups. XRD analysis of SDF and IDF from pearl millet bran revealed the presence of crystalline form. WHC, OHC and SC of dietary fiber samples from pearl millet bran can help to enhance the texture and rheological properties in food products. The study revealed that pearl millet bran contains considerable amount of dietary fiber and therefore, can be used as a potential food component in preparation of functional food products.
Availability of data and materials
Not applicable.
Code availability
Not applicable.
Abbreviations
- DF:
-
Dietary fiber
- SDF:
-
Soluble dietary fiber
- IDF:
-
Insoluble dietary fiber
- DFC:
-
Dietary fiber concentrate
- HT:
-
Hydrothermal treatment
- BBD:
-
Box–Behnken design
- RSM:
-
Response surface methodology
- SEM:
-
Scanning electron microscopy
- XRD:
-
X-ray diffraction
- FTIR:
-
Fourier transform infrared spectroscopy
- TPC:
-
Total polyphenolic content
- WHC:
-
Water holding capacity
- OHC:
-
Oil holding capacity
- WS:
-
Water solubility
- SC:
-
Swelling capacity
- EA:
-
Emulsifying activity
- PCA:
-
Principle component analysis
References
Ainsworth E, Gillespie K (2007) Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat Protoc 2:875–877. https://doi.org/10.1038/nprot.2007.102
Barbhai MD, Hymavathi TV (2022) Nutrient, phytonutrient and antioxidant potential of selected underutilized nutri-cereal brans. J Food Meas Charact 16(3):1952–1966
Benito Román Ó, Alonso E, Cocero MJ (2013) Pressurized hot water extraction of β-glucans from waxy barley. J Supercrit Fluids 73:120–125. https://doi.org/10.1016/j.supflu.2012.09.014
Cardenas-Toro FP, Alcazar-Alay SC, Forster-Carneiro T, Meireles MAA (2014) Obtaining oligo-and monosaccharides from agroindustrial and agricultural residues using hydrothermal treatments. Food Public Health 4(3):123–139
Chu J, Zhao H, Lu Z, Lu F, Bie X, Zhang C (2019) Improved physicochemical and functional properties of dietary fiber from millet bran fermented by Bacillus natto. Food Chem 294:79–86
Devisetti R, Yadahally SN, Bhattacharya S (2014) Nutrients and antinutrients in foxtail and proso millet milled fractions: evaluation of their flour functionality. LWT-Food Sci Technol 59(2):889–895. https://doi.org/10.1016/j.lwt.2014.07.003
Elleuch M, Besbse S, Roiseux O, Blecker C, Deroanne C, Drira NE, Attia H (2008) Date flesh: chemical composition and characteristics of the dietary fibre. Food Chem 111(3):676–682. https://doi.org/10.1016/j.foodchem.2008.04.036
Elleuch M, Bedigian D, Roiseux O (2011) Dietary fibre and fibre-rich by-products of food processing: characterisation, technological functionality and commercial applications—a review. Food Chem 124(2):411–421. https://doi.org/10.1016/j.foodchem.2010.06.077
Fernández-López J, Sendra-Nadal E, Navarro C, Sayas E, Viuda-Martos M, Alvarez JAP (2009) Storage stability of a high dietary fibre powder from orange by-products. Int J Food Sci Technol 4:748–756. https://doi.org/10.1111/j.1365-2621.2008.01892.x
Garcia-Amezquita LE, Tejada-Ortigoza V, Torres JA, Welti-Chanes J (2020) Extraction and modification of dietary fiber applying thermal processes. Sci Technol Fibers Food Syst 329–342
He Y, Wang BX, Wen LK, Wang F, Yu H, Chen D, Zhang C (2022) Effects of dietary fiber on human health. Food Sci Hum Wellness 11(1):1–10. https://doi.org/10.1016/j.fshw.2021.07.001
Jiang C, Wang R, Liu X, Wang J, Zheng X, Zuo F (2022) Effect of particle size on physicochemical properties and in vitro hypoglycemic ability of insoluble dietary fiber from corn bran. Front Nutr 9:951821
Kamal MM, Akhtaruzzama M, Sharmin T, Rahman M, Mondal SC (2023) Optimization of extraction parameters for pectin from guava pomace using response surface methodology. J Agric Res 11:100530
Kaur H, Singh B, Singh A (2021) Comparison of dietary fibers obtained from seven Indian cereal grains. J Cereal Sci 102:103331. https://doi.org/10.1016/j.jcs.2021.103331
Kurek MA, Karp S, Wyrwisz J, Niu Y (2018) Physicochemical properties of dietary fibers extracted from gluten-free sources: quinoa (Chenopodium quinoa), amaranth (Amaranthus caudatus) and millet (Panicum miliaceum). Food Hydrocoll 85:321–330. https://doi.org/10.1016/j.foodhyd.2018.07.021
Li M, Chang L, Ren J, Jiang F, Zhao N, Liu Y, Du SK (2022a) Nutritional, physical, functional properties and antioxidant potential of different colors proso millet husks and brans. LWT 171:114092. https://doi.org/10.1016/j.lwt.2022.114092
Li Y, Niu L, Guo Q, Shi L, Deng X, Liu X, Xiao C (2022b) Effects of fermentation with lactic bacteria on the structural characteristics and physicochemical and functional properties of soluble dietary fiber from prosomillet bran. LWT 154:112609. https://doi.org/10.1016/j.lwt.2021.112609
Liu BY, Peng JY, Zeng XM, Zheng HY, Zhong G (2012) Characterization of dietary fiber from millet brans. Appl Mech Mater 140:278–285
Mustač NČ, Novotni D, Habuš M, Drakula S, Nanjara L, Voučko B, Ćurić D (2020) Storage stability, micronisation, and application of nutrient-dense fraction of proso millet bran in gluten-free bread. J Cereal Sci 91:102864. https://doi.org/10.1016/j.jcs.2019.102864
Nenadis N, Tsimidou M (2002) Observations on the estimation of scavenging activity of phenolic compounds using rapid 1,1-diphenyl-2-picrylhydrazyl (DPPH·) tests. J Am Oil Chem Soc 79:1191–1195. https://doi.org/10.1007/s11746-002-0626-z
Panwar D, Panesar PS, Chopra HK (2023) Ultrasound-assisted extraction of pectin from Citrus limetta peels: Optimization, characterization, and its comparison with commercial pectin. Food Bio Sci. 51:102231. https://doi.org/10.1016/j.fbio.2022.102231
Sachdev N, Goomer S, Singh L, Pathak VM, Aggarwal D, Chowhan RK (2023) Current status of millet seed proteins and its applications: a comprehensive review. Appl Food Res. https://doi.org/10.1016/j.afres.2023.100288
Selladurai M, Pulivarthi MK, Raj AS, Iftikhar M, Prasad PV, Siliveru K (2022) Considerations for gluten free foods-pearl and finger millet processing and market demand. Grain Oil Sci Technol. https://doi.org/10.1016/j.gaost.2022.11.003
Sharma R, Sharma S, Singh B (2022) Modulation in the bio-functional & technological characteristics, in vitro digestibility, structural and molecular interactions during bioprocessing of proso millet (Panicum miliaceum L). J Food Compost Anal 107:104372
Wang L, Bai X (2017) The producing technology of resistant starch (RS) from buckwheat using microwave treatment. Sustain Environ 2(3):301–308. https://doi.org/10.22158/se.v2n3p301
Wang L, Liu HM, Xie AJ, Zhu CY, Qin GY (2018) Dietary fiber extraction from defatted corn hull by hot-compressed water. Polish J Food Nutr Sci. https://doi.org/10.1515/pjfns-2017-0015
Wang S, Fang Y, Xu Y, Zhu B, Piao J, Zhu L, Wu J (2022) The effects of different extraction methods on physicochemical, functional and physiological properties of soluble and insoluble dietary fiber from Rubus chingii Hu fruits. J Funct Foods 93:105081. https://doi.org/10.1016/j.jff.2022.105081
Wei C, Ge Y, Liu D, Zhao S, Wei M, Jiliu J, Cao L (2022) Effects of high-temperature, high-pressure, and ultrasonic treatment on the physicochemical properties and structure of soluble dietary fibers of millet bran. Front Nutr 8:820715
Wen Y, Niu M, Zhang B, Zhao S, Xiong S (2017) Structural characteristics and functional properties of rice bran dietary fiber modified by enzymatic and enzyme-micronization treatments. LWT 75:344–351. https://doi.org/10.1016/j.lwt.2016.09.012
Zhu Y, Chu J, Lu Z, Lv F, Bie X, Zhang C, Zhao H (2018) Physicochemical and functional properties of dietary fiber from foxtail millet (Setaria italic) bran. J Cereal Sci 79:456–461
Acknowledgements
The financial support provided by Department of Biotechnology (DBT), Ministry of Science & Technology, Government of India under Grant No. BT/PR36778/PFN/20/1485/2020 is acknowledged. Authors would also acknowledge the infrastructural support provided for carrying out research by Sant Longowal Institute of Engineering and Technology (SLIET), Longowal, India.
Funding
The research project was funded by Department of Biotechnology (DBT), Ministry of Science & Technology, Government of India under Grant No. BT/PR36778/PFN/20/1485/2020.
Author information
Authors and Affiliations
Contributions
RK: Investigation, Methodology, Writing—original draft. PSP: Conceptualization, Writing—review & editing, Supervision, Project administration. BK: Data analysis. CSR: Supervision, Guidance.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest regarding the publication of this paper.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kaur, R., Panesar, P.S., Kaur, B. et al. Hydrothermal extraction of dietary fiber from pearl millet bran: optimization, physico-chemical, structural and functional characterization. J Food Sci Technol 61, 1536–1546 (2024). https://doi.org/10.1007/s13197-023-05921-x
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-023-05921-x