Abstract
The non-conventional sources of dietary fiber such as fruit by-products have recently gained attention due to its well-known functional and physiological properties. Green extraction techniques are of major interest as it helps overcome the drawbacks associated with conventional techniques. Mango peels which are a major by-product of mango fruit are a promising raw material for the recovery of dietary fiber. The present work is focused on the comparison of enzymatic, ultrasound, and ultrasound-assisted enzymatic extraction techniques for dietary fiber concentrate from mango peels considering their structural and certain physicochemical parameters. The highest extraction yield (71%) of total dietary fiber was obtained using ultrasound-assisted enzyme extraction at 25 °C temperature, 40% amplitude, and 1:50 solid-to-liquid ratio after 9 min extraction time. The results specified that mango peels can be effectively utilized for dietary fiber recovery using ultrasound-assisted enzyme extraction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
After the industrial processing of fruit, the discarded by-products if not disposed or treated properly cause serious environmental hazards due to emission of greenhouse gases during decomposition. Moreover, the biomaterials such as oil and pectin as well as sugar present in fruit wastes can stimulate aerobic bacterial action that speeds up the decomposition rate of biodegradable organic matter [1]. The unscrupulous disposal of these by-products in the environment leads to qualitative (nutrition, calorific value, etc.) and quantitative (volume or mass) losses, thus reducing the economic value as well as consumer acceptability of the product [2]. These problems can be circumvented by extraction of bioactive compounds from fruit by-products with functional benefits or its valorization into value-added products by using various green techniques [3].
The mango peels are one of the major by-products of mango fruit and represents about 15–20% of the fresh fruit [4]. Recently, mango peels have gained considerable interest among researchers as it is rich in numerous valuable compounds, such as dietary fiber, polyphenols, and carotenoids possessing functional and antioxidant properties [5, 6]. Dietary fiber is widely used as a functional food ingredient in processed foods as it imparts several health benefits [5]. It constitutes plant-derived or other carbohydrate oligomers and polymers which cannot undergo hydrolysis by endogenous enzymes in the small intestine of humans [7]. Dietary fiber intake causes a significant impact on human body as it may help improve blood cholesterol levels and lipid profiles as well as regulate blood glucose level. Some chronic diseases such as diabetes, obesity, and colon cancer can also be prevented by including dietary fiber in the diet [8,9,10].
Depending on its solubility in water, dietary fiber can be roughly classified into soluble (SDF) and insoluble (IDF) types. Among them, SDF constitutes soluble hemicelluloses, mucilage, pectic substances, and gums, while IDF contains cellulose, lignin, chitosan, and insoluble hemicelluloses [11]. Moreover, they also differ in their physiological response in the body. SDF performs a role in lowering blood glucose, increasing viscosity, and lowering plasma cholesterol, whereas IDF contributes in increasing fecal volume and facilitates defecation [12, 13]. Apart from its health-enhancing effects, the structure-forming properties of dietary fibers are of equal importance. Functional properties like solubility, water/oil holding capacity, gelling potential, and flowability are significantly influenced by soluble dietary fiber fraction [14]. This makes its utilization suitable in food systems as a thickening and gelling agent, stabilizing and encapsulating agent, etc. [8, 9].
The extraction processes of dietary fiber are very expensive and complex which compromises the extraction yield. Therefore, obtaining pure dietary fiber without the presence of impurities, like proteins and lipids, is still a challenging subject [14]. The high anti-radical efficiency and lower content of lipid (2.35%) and protein (4.28%) in mango peels dietary fiber are important parameters in a fiber concentrate [15] and make mango peels suitable for the extraction of dietary fiber and its application in food products. Chemical and enzymatic methods are extensively used for the recovery of dietary fiber from different food sources. The conventional techniques involve the use of high temperature, longer time, and toxic chemicals which can cause degradation of the extracted polysaccharide. Moreover, the processing condition leads to a significant impact on composition and microstructure of dietary fibers effecting the physiochemical and functional properties [16]. In the past years, the ultrasonication technique, due to its eco-friendly nature, has been successfully employed in research and development area in the food industries. It has been utilized in the extraction of polysaccharides, oils, and proteins [17]. Ultrasonic treatment has been reported not only to increase the extraction yield and reaction rate but also reduce the extraction time [18]. Ultrasound-assisted enzymatic extraction is a new and promising extraction method which holds twofold benefits. The involvement of enzymes in ultrasonication treatment may reduce solvent consumption and extraction time as well as improve the extraction yield of the target compound.
The extraction of dietary fiber from food industry by-products including mango peels using conventional methods have been abundantly studied [19,20,21]. However, limited studies have been performed using green techniques for the extraction of dietary fiber from food industry by-products [22]. In view of the above, the present investigation was carried out to evaluate the effect of different green extraction methods (ultrasound-assisted extraction, enzyme-assisted extraction, and ultrasound-assisted enzyme extraction) on the extraction of dietary fiber concentrate from mango peels as well as their structural and physicochemical properties.
2 Materials and methods
2.1 Materials
Mango (Mangifera indica) peels were procured from the local fruit processors, Longowal, Punjab, India. The sample was dried using sun drying method at ambient temperature for 3–4 days, grounded to fine powder, and stored in an air tight vessel at 4 ± 1 °C for further studies. All the chemicals used in the study were of analytical grade.
2.2 Proximate analysis of mango peel powder
Mango peel powder was analyzed for moisture, ash, protein, crude fiber, and fat, using the standard AOAC (1990) methods [23]. Nitrogen content was estimated by Kjeldahl method and was converted to protein by using factor 6.25. Available carbohydrate was estimated by subtracting percentage sum of other proximate constituents from 100, i.e., [100 − (%moisture + % ash + % crude protein + % crude fat + % crude fiber)].
2.3 Extraction of dietary fiber using conventional techniques
The conventional techniques including hot water extraction [24], ethanol extraction [25], and alkaline extraction [26] were utilized for the extraction of dietary fiber concentrate from mango peels.
2.4 Extraction of dietary fiber using green techniques
The green techniques including ultrasound-assisted extraction, enzyme-assisted extraction, and ultrasound-assisted enzyme extraction were utilized for the extraction of dietary fiber concentrate from the mango peels.
2.4.1 Ultrasound-assisted extraction
Extraction of dietary fiber from mango peels was carried out using the ultrasound method [14] with some modifications. The sample (1 g) was weighed in a beaker (400 mL), mixed with phosphate buffer (0.08 M, 50 mL), and pH adjusted to 6.0 ± 1. The mixture was ultrasonicated using ultrasonic probe (Snaptech NexTgen Lab 500) at 25 °C for 9 min with ultrasonic amplitude of 40%. After this, ethanol (twofold volume) was added to the solution, and the suspension was left (60 min) to precipitate. The residues obtained were filtered and washed with ethanol (80%) followed by oven drying (40 °C) to obtain dietary fiber concentrate. The extraction yield was calculated using Eq. (1):
2.4.2 Enzyme-assisted extraction
The extraction of dietary fiber from mango peels was carried out using enzyme-assisted extraction [27]. The sample (1 g) was added to phosphate buffer (50 mL, 0.08 M), and the pH (6.0) was adjusted. Then, α-amylase (50 μL) was added into the mixture, followed by placing it in boiling water bath (98–100 °C, 15 min). Later the mixture was enzymatically digested in sequence using protease (100 μL, pH 7.5) and amyloglucosidase (200 μL, pH 4.5) followed by incubation on water bath (60 °C, 30 min). After incubation, ethanol (twofold volume) was added to the solution, and the suspension was left for 60 min to precipitate. The residue obtained was filtered and washed with ethanol (80%) followed by oven drying (40 °C) to obtain dietary fiber concentrate. The extraction yield was calculated using the above Eq. (1).
2.4.3 Ultrasound-assisted enzyme extraction
Extraction of dietary fiber from mango peels was carried out using ultrasound-assisted enzyme extraction [14] with slight modifications wherein ultrasonication treatment was given prior to enzyme extraction. The sample (1 g) was weighed, mixed with phosphate buffer (0.08 M, 50 mL), and pH was adjusted to 6.0 ± 1. The suspension was sonicated using an ultrasonic probe (Snaptech NexTgen Lab 500) at 25 °C for 9 min with 40% ultrasonic amplitude. The subsequent steps were the same as in the enzymatic extraction procedure. The effect of time (3–12 min), extraction temperature (25–55 °C), amplitude (20–60%), and solid/liquid ratio (1:30–1:60) on yield of dietary fiber concentrate was investigated.
2.4.4 Estimation of soluble, insoluble, and total dietary fiber content
The estimation of soluble and insoluble dietary fiber content was carried out using the procedure of Wang et al. [26]. The mixture obtained after each extraction treatment was subjected to centrifugation (5000 g, 15 min) to separate soluble and insoluble dietary fiber content. The residue obtained was separated, rinsed by distilled water, and dried to obtain insoluble dietary fiber. The supernatant was collected, added with fourfold volume of ethanol (95%), and kept for 2 h to precipitate. The collected residue was washed with ethanol (100%) followed by drying to obtain soluble dietary fiber. The total dietary fiber content was determined as the sum of soluble and insoluble dietary fiber content [14].
2.5 Structural properties
2.5.1 Scanning electron microscopy (SEM)
The surface morphology and the microstructure of dietary fiber concentrate obtained using green techniques were studied using scanning electron microscope (JSM, 7610 F plus, JEOL, Japan). The samples were uniformly dispersed on a conductive surface, and coating was done using a thin layer of gold in an argon atmosphere using an iron sputter coater. The images of the samples were taken at different magnifications [28].
2.5.2 X-ray diffraction (XRD)
The XRD patterns of dietary fiber concentrate were obtained using a PAN-analytic-Xpert PRO MRD (Almelo, Netherlands). Diffractograms were taken between 5 and 50° at a rate of 0.21°/s and with a step length of 0.05° [29].
2.6 Physicochemical properties
2.6.1 Water holding capacity
To find the water holding capacity (WHC) of dietary fiber concentrate from mango peels, the sample (0.5 g) was added to distilled water (20 mL) and mixed well. After soaking the sample for 24 h, the slurry was centrifuged (4000 g, 10 min). The supernatant was discarded, and the weight of residue was noted. WHC was calculated as the amount of water retained by the sample using Eq. (2) [30].
where W2 is the final weight of residue and W1 is the initial weight of the sample.
2.6.2 Oil holding capacity
The oil holding capacity (OHC) of dietary fiber concentrate from mango peels was determined by a modified method as given [31]. The sample (0.5 g) and vegetable oil (10 mL) were mixed and centrifuged (4000 g, 10 min) after resting at ambient temperature for 1 h. The supernatant was discarded, and the weight of the residue was noted. The OHC was calculated using Eq. (3):
where O2 is the final weight of residue and O1 is the initial weight of the sample.
3 Statistical analysis
The data obtained for the total, soluble, and insoluble dietary fiber content from mango peels using different green techniques were analyzed by using GraphPad Prism (La Jolla, CA, USA) (version 5.01) software. The results have been expressed as means ± SD. Differences between the means were tested for statistical significance using a two-way ANOVA and followed by Bonferroni post hoc test. The significance level was set at 5% (P < 0.05) for all calculations.
4 Results and discussion
The proximate analysis of the mango peels was carried out, and the yield of dietary fiber concentrate obtained from mango peels along with their structural and physicochemical parameters was evaluated using different extraction techniques which have been discussed below.
4.1 Proximate analysis of mango peel powder
The proximate composition of mango peel powder is shown in Table 1. The results showed that the moisture content was 11.4%, the fat content was 1.55%, the ash content was 3.41%, and the total protein content was 6.4%. The carbohydrate content in mango peel powder was found to be 66.4%. The proximate composition reported was comparable to the earlier reported values of mango peels [32,33,34].
4.2 Effect of conventional and green techniques on extraction yield of dietary fiber concentrate
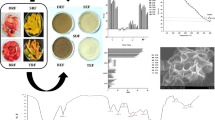
The comparative evaluation of the extraction yield of dietary fiber concentrate obtained using conventional (hot water extraction, ethanol extraction, alkaline extraction) and green techniques (ultrasound-assisted extraction, enzyme-assisted extraction, and ultrasound-assisted enzyme extraction) revealed that the extraction of total dietary fiber using hot water gave the lowest yield (26%) followed by ethanol extraction (44%) and alkaline extraction (59%). Even though extraction of dietary fiber from plant sources using hot water is one of the most commonly utilized methods, long exposure to heating at high temperature may lead to the degradation of the extracted polysaccharide, thereby giving lower yield. Also, the water extractability is lower than that achieved by chemical methods [22]. The extraction yield was found to be more in ethanol extraction but even greater in the case of the alkaline method. This might be because alcohol extraction is particularly suitable for fruits and vegetable matter containing a small amount of starch, intracellular proteins, and polyphenols [35]. Alkaline extraction releases various polysaccharides from the cell wall due to the disruption of hydrogen and covalent bonds. Also, the hydroxyl ions disrupt the hydrogen bonds [22], thus leading to better extraction yield (Fig. 1).
Chemical or thermal extraction methods are highly efficient, but only a portion of the extracted polysaccharides obtained through these processes can be absorbed during digestion in the upper gut. On the other hand, the use of enzymes resulting in extraction conditions similar to those present in the digestive system increases the viscosity and facilitates the greater release of polysaccharides from the mango peel matrix. The yield of dietary fiber concentrate in case of green techniques was found to increase in the following order: enzymatic extraction (55%), ultrasound extraction (60%), and ultrasound-assisted enzymatic extraction (79%). Here, the yield of dietary fiber concentrate in the case of enzymatic extraction was found lesser than obtained through the alkaline method. This may be due to the destruction of the cell wall to a greater extent in case of alkaline solution enabling better penetration and release of fiber contents [36]. Even though thermal and chemical treatments for dietary fiber are efficient to a large extent, they require high temperature, longer extraction time, and toxic solvents. These shortcomings can be overcome using green techniques which involve shorter duration, lower temperature, and nontoxic solvents with better extraction yield. Ultrasonication extraction gave even more yield than enzymatic treatment, and this yield was further enhanced when enzymatic treatment was assisted by ultrasonication. It disrupts the cell structure of fruit wall tissue increasing the accessibility of the solvent to the internal structure, thus releasing the cell components more effectively [22].
4.3 Effect of extraction parameters on extraction yield of dietary fiber concentrate obtained using ultrasound-assisted enzymatic method
The effect of extraction parameters including time, temperature, amplitude, and liquid/solid ratio on extraction yield of dietary fiber concentrate obtained using ultrasound-assisted enzymatic method was investigated. The variation in extraction yield with change in extraction parameters is shown in Fig. 2. The extraction yield was found to decrease with increase in temperature. The yield was found to gradually decrease in initial stage and a significant drop was observed in the later stage which indicates a noteworthy impact of temperature on dietary fiber concentrate yield. This might be due to the sensitivity of the material to temperature. Moreover, it also depends on the polysaccharide to be recovered and matrix characteristics [22]. The findings were similar to that found in case of soybean residue where the highest yield of dietary fiber concentrate was observed at 30 °C [37].
When the extraction time was increased, the yield was also found to increase until 9 min after which a gradual drop in the yield was observed [38]. Ultrasonic amplitude significantly influenced the extraction yield of dietary fiber concentrate. The extraction yield was found to increase with the increase in amplitude up to 50% after which no significant impact was observed. As the ultrasonic waves of larger amplitude progress through the medium, more bubbles are created which are very unstable and collapses over a short time. It may have resulted in increased penetration of solvent into the tissues leading to the disruption of cell wall and release of intracellular product [39]. Similar trend was observed in case of increase in solid/liquid ratio, where the yield of dietary fiber concentrate obtained from mango peels gradually increased from 1:30 to 1:50 after which the yield declined slightly [37].
4.4 SEM analysis
Structural characterization of the fiber samples obtained using ultrasound-assisted extraction, enzyme-assisted extraction, and ultrasound-assisted enzyme extraction techniques was done by means of scanning electron microscopy (SEM) which revealed irregular shaped granules of uneven size and dense surfaces. The compactness of the particles was found more in case of enzyme-assisted extraction method as compared to those in ultrasonicated samples. The ultrasonic treatment disrupted the crosslinks between polysaccharide molecules more effectively and resulted in numerous significantly smaller sized particles [14]. The samples which had undergone ultrasonic treatment showed more disrupted and looser structure which might be due to pore expansion during ultrasonication [40]. The distortedness and number of cracks and holes were even more in samples treated using ultrasound-assisted enzyme technique which indicated the greater efficiency of this method in dietary fiber extraction among all the green techniques (Fig. 3).
4.5 XRD analysis
The influence of different extraction treatments on the crystal structure of the polymers in the fiber concentrate of mango peels were analyzed by X-ray diffraction. Different XRD spectra corresponding to the extraction techniques (ultrasound-assisted extraction, enzyme-assisted extraction, and ultrasound-assisted enzyme extraction) have been given in Fig. 4. The results showed several low- or high-intensity peaks at 2ϴ° = 21.9; 22.1, 25.9, 31.6, and 45.4°. The high-intensity peaks indicated the presence of cellulose molecules, whereas the low-intensity peaks were linked to the presence of hemicellulose and lignin. It has been reported that dietary fiber comprises 70% orderly crystalline and 30% amorphous regions. The crystalline cellulose is characterized by sharp XRD peaks with an extended crystalline, whereas amorphous region is composed of non-crystalline cellulose, hemicellulose, and lignin [29]. It should be noted that the degree of crystallinity could be affected by the treatment method used for extraction of dietary fiber concentrate from mango peel [25]. In this case, ultrasonicated samples were found to possess more crystallinity as compared to individual enzyme-treated sample. This might be due to the ultrasonication treatment causing the hydrolysis of hemicellulose and removal of amorphous portion of cellulose resulting in increased crystallinity [41].
4.6 Total, soluble, and insoluble dietary fiber content, water holding capacity, and oil holding capacity of dietary fiber concentrate obtained using different green techniques
Total dietary fiber content of the fiber concentrate obtained from mango peels using ultrasound-assisted extraction, enzyme-assisted extraction, and ultrasound-assisted enzyme extraction is presented in Table 2. The results indicated that the highest total dietary fiber content was obtained using ultrasound-assisted enzyme extraction (71 g/100 g) followed by ultrasound-assisted extraction (68 g/100 g) and enzyme-assisted extraction (66 g/100 g). There was not much difference observed between the total dietary fiber content obtained using ultrasound-assisted and enzymatic method. However, their combined effect led to an enhancement in the total dietary fiber content. The result was in coherence with previous studies where the maximum content of the total dietary fiber obtained from mango peels was found to be 70–72.5% [5, 19].
The soluble dietary fiber content was found maximum using enzymatic extraction (27.2 g/100 g) followed by ultrasound-assisted enzyme method (26.9 g/100 g) and ultrasound method (26.4 g/100 g). This indicated that enzymatic hydrolysis had a significant impact on the content and composition of fiber samples [28]. On the other hand, ultrasound treatment caused cell wall damage and negatively influenced soluble dietary fiber content [30]. The results were in accordance with a previous study on quinoa, amaranth, and millet, where enzymatic treatment gave higher content of soluble dietary fiber as compared to ultrasound- and ultrasound-assisted enzymatic extraction [42]. In the case of insoluble dietary fiber content, least value was obtained with enzymatic extraction (38.8 g/100 g). This might be due to the amount of protein remaining in the sample due to the formation of protein–polysaccharide complex. A comparatively higher content of insoluble dietary fiber content was obtained using ultrasound method (41.1 g/100 g) and ultrasound-assisted enzymatic extraction method (44.6 g/100 g). The results correlated with the previous findings on flaxseed gum where least content of insoluble dietary fiber was obtained using enzymatic method in comparison to ultrasound-assisted enzymatic extraction [14].
The water holding capacity is an important functional property of food hydrocolloids. The WHC of a food material expresses its ability to retain water after processes like compression and centrifugation. The WHC of dietary fiber concentrate obtained using different extraction methods revealed the highest WHC in case of enzymatic method (4.5 g/g) followed by ultrasound-assisted enzyme extraction (4.3 g/g) and ultrasound-assisted extraction (4.0 g/g). The greater value of WHC in case of enzymatic method might be due to the breakage of intermolecular hydrogen bonds of cellulose and hemicelluloses present in fiber concentrates. This increased the surface exposure of hydrated hydroxyl and carboxyl groups, thus increasing the capillary action of the fibers that, in turn, led to an increase in WHC [43].
Oil holding capacity is another important technological property which depends on the chemical and physical structure of plant polysaccharides. The OHC of fiber concentrate plays a significant role in the prevention of fat loss during food processing and in the reduction of serum cholesterol levels [44]. The values obtained were similar to that reported in earlier studies [19]. The lower content of OHC observed in the fiber concentrate of mango peels indicates that the food supplemented with this fruit by-product will not have high oil retention capacity.
5 Conclusions
Green techniques (ultrasound-assisted extraction, enzyme-assisted extraction, and ultrasound-assisted enzyme extraction) had been successfully utilized in the present study for the extraction of total dietary fiber from mango peels. Among these techniques, ultrasound-assisted enzyme extraction gave the highest yield of total dietary fiber (71%) at 25 °C temperature, 40% ultrasonic amplitude, and 1:50 solid-to-liquid ratio after 9 min of extraction time. The study indicated that mango peels possess considerably high content of dietary fiber, which can serve as a potential functional food ingredient in applications of food processing, and the ultrasound-assisted enzyme extraction method is an effective green technique for the extraction of dietary fiber. However, further studies can be performed to scale up the process for increasing the extraction efficiency of dietary fiber from mango peels, thus expanding areas of their application.
References
Oloye MT, Jabar JM, Adetuyi AO et al (2021) Extraction and characterization of pectin from fruit peels of Irvingia gabonensis and pulp of Cola millenii and Theobroma cacao as precursor for industrial applications. Biomass Conv Bioref. https://doi.org/10.1007/s13399-021-01366-4
Saini A, Panesar PS, Bera MB (2020) Valuation of Citrus reticulata (kinnow) peels for the extraction of lutein using ultrasonication technique. Biomass Conv Bioref. https://doi.org/10.1007/s13399-020-00605-4
Saini A, Panesar PS, Bera MB (2019) Valorization of fruits and vegetables waste through green extraction of bioactive compounds and their nanoemulsions-based delivery system. Bioresour Bioprocess 6(26). https://doi.org/10.1186/s40643-019-0261-9
Serna-Cock L, García-Gonzales E, Torres-Leon C (2016) Agro-industrial potential of the mango peels based on its nutritional and functional properties. Food Reviews International 32(4):364–376. https://doi.org/10.1080/87559129.2015.1094815
Ajila CM, Rao UJS (2013) Mango peels dietary fibre: composition and associated bound phenolics. J Funct Foods 5(1):444–450. https://doi.org/10.1016/j.jff.2012.11.017
Sogi D, Siddiq M, Greiby I, Dolan K (2013) Total phenolics, antioxidant activity, and functional properties of ‘Tommy Atkins’ mango peels and kernel as affected by drying methods. Food Chem 141(3):2649–2655. https://doi.org/10.1016/j.foodchem.2013.05.053
(2010) Joint FAO/WHO Food Standards Programme CODEX Alimentarius Commission Codex Alimentarius (CODEX) Guidelines on nutrition labelling CAC/GL 2–1985 as last amended 2010. Rome (Italy): FAO
Li YO, Komarek AR (2017) Dietary fibre basics: health, nutrition, analysis, and applications. Food Qual Saf 1(1):47–59. https://doi.org/10.1093/fqs/fyx007
Ozyurt VH, Ötles S (2016) Effect of food processing on the physicochemical properties of dietary fibre. Acta Sci Pol Technol Aliment 15(3):233–245 https://doi.org/10.17306/J.AFS.2016.3.23
Yangilar F (2013) The application of dietary fibre in food industry: structural features, effects on health and definition, obtaining and analysis of dietary fibre: a review. J Food Nutr Res 1(3):13–23 https://doi.org/10.12691/jfnr-1-3-1
Cheng L, Zhang X, Hong Y, Li Z, Li C, Gu Z (2017) Characterisation of physicochemical and functional properties of soluble dietary fibre from potato pulp obtained by enzyme-assisted extraction. Int J Biol Macromol 101:1004–1011
Beretta MV, Bernaud FR, Nascimento C, Steemburgo T, Rodrigues TC (2018) Higher fiber intake is associated with lower blood pressure levels in patients with type 1 diabetes. Arch of Endocrinol Metabol 62(1):47–54
Dreher ML (2018) Overview of the health benefits of adequate fiber intake. Nutr Health 19–40. https://doi.org/10.1007/978-3-319-50557-2_2
Moczkowska M, Karp S, Niu Y, Kurek M (2019) Enzymatic, enzymatic-ultrasonic and alkaline extraction of soluble dietary fibre from flaxseed – a physicochemical approach. Food Hydrocoll 90:105–112. https://doi.org/10.1016/j.foodhyd.2018.12.018
Masibo M, He Q (2009) Mango bioactive compounds and related nutraceutical properties—a review. Food Rev Int 25(4):346–370. https://doi.org/10.1080/87559120903153524
Peerajit P, Chiewchan N, Devahastin S (2012) Effects of pretreatment methods on health-related functional properties of high dietary fibre powder from lime residues. Food Chem 132(4):1891–1898
Jia X, Chen M, Wan JB, Su H, He C (2015) Review on the extraction, characterization and application of soybean polysaccharide. RSC Adv 5(90):73525–73534. https://doi.org/10.1039/c5ra12421b
Bagherian H, Ashtiani F, Fouladitajar A, Mohtashamy M (2011) Comparisons between conventional, microwave- and ultrasound-assisted methods for extraction of pectin from grapefruit. Chem Eng Process Process Intensif 50(11–12):1237–1243. https://doi.org/10.1016/j.cep.2011.08.002
Oliveira A, Paula D, Oliviera E, Saraiva S, Stringheta P, Ramos A (2018) Optimization of pectin extraction from Ubá mango peel through surface response methodology. Int J Biol Macromol 113:395–402. https://doi.org/10.1016/j.ijbiomac.2018.02.154
Sommano SR, Ounamornmas P, Nisoa M, Sriwattana S, Page P et al (2018) Characterisation and physiochemical properties of mango peel pectin extracted by conventional and phase control microwave-assisted extractions. Int Food Res J 25(6):2657–2665
Dhingra D, Michael M, Rajput H, Patil R (2012) Dietary fibre in foods: a review. J Food Sci Technol 49(3):255–266. https://doi.org/10.1007/s13197-011-0365-5
Tejada-Ortigoza V, Garcia-Amezquita LE, Serna-Saldívar SO et al (2018) High hydrostatic pressure and mild heat treatments for the modification of orange peels dietary fiber: effects on hygroscopic properties and functionality. Food Bioprocess Technol 11:110–121. https://doi.org/10.1007/s11947-017-1998-9
AOAC (1990) Official methods of analysis. Association of Official Analytical Chemists, Washington, DC
Elleuch M, Besbse S, Roiseux O, Blecker C, Deroanne C, Drira N, Attia H (2008) Date flesh: chemical composition and characteristics of the dietary fibre. Food Chem 111(3):676–682. https://doi.org/10.1016/j.foodchem.2008.04.036
Rouhou M, Abdelmoumen S,Thomas S, Attia H, Ghorbel D (2018) Use of green chemistry methods in the extraction of dietary fibers from cactus rackets (Opuntia ficus indica): structural and microstructural studies. International Journal of Biological Macromolecules 116(901–910). https://doi.org/10.1016/j.ijbiomac.2018.05.090
Wang K, Li M, Wang Y, Liu Z, Ni Y (2021) Effects of extraction methods on the structural characteristics and functional properties of dietary fiber extracted from kiwifruit (Actinidia deliciosa). Food Hydrocolloids 110. https://doi.org/10.1016/j.foodhyd.2020.106162
Prosky L, Asp NG, Schweizer T, Devries J, Furda I (1988) Determination of insoluble, soluble, and total dietary fiber in foods and food products: interlaboratory study. J Assoc Off Analytic Chem 71(5):1017–1023. https://doi.org/10.1093/jaoac/71.5.1017
Jiang Y, Yin H, Zheng Y, Wang D, Liu Z, Deng Y, Zhao Y (2020) Structure, physicochemical and bioactive properties of dietary fibers from Akebia trifoliata (Thunb.) Koidz. seeds using ultrasonication/shear emulsifying/microwave-assisted enzymatic extraction. Food Res Int 136. https://doi.org/10.1016/j.foodres.2020.109348
Ma M, Mu T (2016) Effects of extraction methods and particle size distribution on the structural, physicochemical, and functional properties of dietary fiber from deoiled cumin. Food Chem 194:237–246. https://doi.org/10.1016/j.foodchem.2015.07.095
Zhang W, Zeng G, Pan Y, Chen W, Huang W, Chen H et al (2017) Properties of soluble dietary fiber-polysaccharide from papaya peels obtained through alkaline or ultrasound-assisted alkaline extraction. Carbohydr Polym 172:102–112. https://doi.org/10.1016/j.carbpol.2017.05.030
Huang X, Dou JY, Li D, Wang LJ (2018) Effects of superfine grinding on properties of sugar beet pulp powders. LWT- Food Sci Technol 87(2018):203–209. https://doi.org/10.1016/j.lwt.2017.08.067
Romelle F, P A, Manohar R (2016) Chemical composition of some selected fruit peels. Eur J Food Sci Technol 4(4):12–21
Ajila C, Leelavathi K, Rao P, Ummiti (2008) Improvement of dietary fiber content and antioxidant properties in soft dough biscuits with the incorporation of mango peels powder. J Cereal Sci 48:319–326. https://doi.org/10.1016/j.jcs.2007.10.001
Baddi J, Vijayalakshmi D, Durgannavar A, Chandru R (2015) Mango peels: a potential source of natural bioactive phyto-nutrients in functional food. Asian J Dairy Food Res 34(1):75–77
Legentil A, Guichard I, Piffaut B, Haluk JP (1995) Characterization of strawberry pectin extracted by chemical means. LWT- Food Sci Technol 28(6):569–576. https://doi.org/10.1016/0023-6438(95)90003-9
Wikiera A, Mika M, Starzynska-Janiszewska A, Stodolak B (2015) Application of celluclast 1.5L in apple pectin extraction. Carbohydr Polym 134:251–257
Sun J, Zhang Z, Xiao F, Wei Q, Jing Z (2018) Ultrasound-assisted alkali extraction of insoluble dietary fiber from soybean residues. IOP Conference Series: Material Science and Engineering 392. https://doi.org/10.1088/1757-899X/392/5/052005
Guandalini B, Rodrigues N, Marczak L (2018) Sequential extraction of phenolics and pectin from mango peels assisted by ultrasound. Food Res Int 119:455–461. https://doi.org/10.1016/j.foodres.2018.12.011
Zhang Z, Wang L, Jiao S, Chen X, Mao Z (2008) Ultrasound-assisted extraction of oil from flaxseed. Sep Purif Technol 62(1):192–198. https://doi.org/10.1016/j.seppur.2008.01.014
Zheng Y, Li Y (2018) Physicochemical and functional properties of coconut (Cocos nucifera L) cake dietary fibres: effects of cellulase hydrolysis, acid treatment and particle size distribution. Food Chem 257:135–142. https://doi.org/10.1016/j.foodchem.2018.03.012
Begum YA, Deka SC (2019) Effect of processing on structural, thermal, and physicochemical properties of dietary fiber of culinary banana bracts. J Food Process Preserv 43(12). https://doi.org/10.1111/jfpp.14256
Kurek MA, Karp S, Wyrwisz J, Niu Y (2018) Physicochemical properties of dietary fibers extracted from gluten-free sources: quinoa (Chenopodium quinoa), amaranth (Amaranthus caudatus) and millet (Panicum miliaceum). Food Hydrocoll 85:321–330. https://doi.org/10.1016/j.foodhyd.2018.07.021
Daou C, Zhang H (2014) Functional and physiological properties of total, soluble, and insoluble dietary fibres derived from defatted rice bran. J Food Sci Technol 51(12):3878–3885. https://doi.org/10.1007/s13197-013-0925-y
Navarro-González I, García-Valverde V, García-Alonso J, Periago MJ (2011) Chemical profile, functional and antioxidant properties of tomato peels fiber. Food Res Int 44(5):1528–1535. https://doi.org/10.1016/j.foodres.2011.04.005
Acknowledgements
The authors would like to acknowledge the financial support provided by ASEAN India Science and Technology Development Fund (AISTDF) and by Science and Engineering Research Board, Department of Science and Technology (SERB-DST), under Grant No. CRD/2019/000141. Further, the infrastructural support provided by Sant Longowal Institute of Engineering and Technology (SLIET), for carrying out the research, is also acknowledged.
Funding
The research project was funded by ASEAN India Science and Technology Development Fund (AISTDF) and Science and Engineering Research Board, Department of Science and Technology (SERB-DST), under Grant No. CRD/2019/000141.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kaur, B., Panesar, P.S. & Thakur, A. Extraction and evaluation of structural and physicochemical properties of dietary fiber concentrate from mango peels by using green approach. Biomass Conv. Bioref. (2021). https://doi.org/10.1007/s13399-021-01740-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-021-01740-2