Abstract
Differential effects of n-3 and n-6 polyunsaturated fatty acids (PUFAs) have been demonstrated on adipose tissue physiology. Facing to the widely recognized beneficial effects of n-3 PUFAs, the n-6 PUFA effects remain controversial. Thus, we further analyzed the linoleic acid (LA) influence on adipocyte functions. To this aim, we treated by LA supplementation at three distinct doses (1, 2.5, or 5 % of energy intake) rats with essential fatty acids deficiency (EFAD). PUFA composition was determined in blood and white adipose tissue (WAT), while lipolytic and lipogenic activities were measured in isolated adipocytes. EFAD rats exhibited reduced WAT mass and increased EFAD biomarkers. WAT mass was completely recovered after supplementation, irrespective of LA dose. However, neither body mass nor EFAD biomarkers returned to control with 1 % LA, while LA abundance doubled in adipocytes from rats supplemented with 5 % LA. Regarding lipolysis, 2.5 % LA normalized the EFAD-induced alterations. A trend to decrease the maximal stimulation of lipolysis was observed with 1 and 5 % LA. Regarding lipogenesis, the lower and higher LA doses increased basal activity and hampered insulin to further stimulate glucose incorporation into lipids whereas 2.5 % LA normalized the basal or insulin-stimulated levels. Our results show that dietary linoleate at 2.5 % restored anatomical, biochemical, and functional disturbances induced by EFAD. At higher dose, LA tended to reduce triacylglycerol breakdown, to increase triacylglycerol assembly, and to provoke insulin resistance. Therefore, LA influence on adipocyte functions does not appear to follow a typical dose–response relationship, adding further complexity to the definition of its dietary requirement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Linoleic acid (C18:2n-6) and alpha-linolenic acid (C18:3n-3) are essential fatty acids (EFAs) that cannot be synthesized in animals while they are necessary for metabolism and must be supplied by an equilibrated diet. They belong to the families of n-3 and n-6 polyunsaturated fatty acids (PUFAs), having more than one double bond located at the third and sixth carbon atom from the methyl group. Other unsaturated fatty acids from the n-7 and n-9 PUFA families can be synthesized in mammals owing to the delta-9 desaturase and are not considered to be dependent on nutritional intake. The absence of EFAs in a diet induces the appearance of specific manifestations (EFA deficiency): growth retardation, dermatitis, alopecia, decreased cold tolerance, and increased susceptibility to infection [28, 45, 49]. EFA deficiency can be detected by biochemical indexes before the appearance of such clinical signs: whatever the blood lipid fraction considered (cells or plasma), there are decreased circulating levels of linoleic and arachidonic acid (C20:4n-6) and increased percentages of eicosatrienoic acid (C20:3n-9). Thus, a ratio of C20:3n-9 to C20:4n-6 (also known as ω9/ω6) higher than 0.2 is a recognized biomarker used to characterize EFA deficiency [30, 45, 49].
However, the complex metabolism of PUFAs, added to the recent evolution of human nutrition (dramatic increase of n-6 PUFA intake resulting from consumption of industrialized foods) [2], has complicated the guidelines for the definition of a regular PUFA intake in our diet [32]. For the consumer, it becomes difficult to maintain the equilibrium between deficiency and excess. Alongside the cases of EFA deficiency in malnutrition, it is the ratio between n-6 and n-3 PUFA intake that has been the matter of numerous nutritional studies. Supplementations with n-3 PUFAs are widely recommended, at least for the mitigation of cardiovascular diseases, and there is clinical evidence that n-3 PUFAs act as possible therapeutic agents in many other diseases: metabolic, neurological, immunological, etc. [7]. Therefore, current nutritional recommendations aim at limiting the commonly called “omega6/omega3 ratio”, by increasing the n-3 PUFA intake (fish oil) and reducing the excessive n-6 PUFA consumption [19]. Reducing the ω6/ω3 ratio (which can reach 20 in certain western diets) is claimed for improving the consumer health and to limit the increased n-6 PUFA intake [1]; however, there is no clear consensus for an optimal value for such ratio and values between 4/1 and 2/1 have been successively proposed [19, 38, 43].
Dose-dependent clinical effects of n-3 PUFA have even been established to depend on the disease to be treated/prevented [7]. Most of the beneficial effects of n-3 PUFAs can be obtained by supplementation with increasing doses of docosahexaenoic acid (C22:6n-3), which is metabolized into products that are thought to be relatively anti-inflammatory (e.g., resolvins) [30]. However, the benefits of increasing n-3 PUFA intake on the improvement of insulin responsiveness and glucose handling [39, 46] can be hampered by enhanced LDL oxidation, at least when increased intake is obtained via fish oil supplementation in man [40].
On the opposite, no substantial evidence of the therapeutic potential of diet supplementation with n-6 PUFA has been reported [44]. Linoleic acid (LA, C18:2n-6), the most important member of n-6 PUFA family, is a precursor of arachidonic acid, promoting prostacyclin production, thus favoring fat cell development [2], and generating pro-inflammatory bioactive lipids involved in cardiometabolic diseases [36]. Moreover, it has been reported that excessive dietary LA would also act as a precursor of various endocannabinoids (e.g., anandamide) and would induce orexigenic hyperactivity and promote obesity via increased food consumption [3]. Anyhow, there is a controversy about the practical utility of the ω6/ω3 ratio in modifying cardiovascular disease risk, and it must be reminded that not all epidemiological studies showed an association of n-6 PUFAs with cardiovascular diseases [47] or with cancer [18, 44], while n-6 PUFA deficiency is undeniably prejudicial [49].
In this context, our objective was not to re-examine recommendations about the ω6/ω3 ratio but to study the dose dependency of the effects of LA on adipocyte functions. In fat tissue, the adipose conversion of precursor cells is highly enhanced by n-6 PUFAs, which play a key role on gene expression via the prostaglandins they generate [33, 48]. These prostaglandins also activate membrane receptors in mature fat cells [9]. In vitro studies showing that LA reduces insulin influence on adipokine secretion failed to demonstrate a clear-cut dose dependency of the direct effect of the fatty acid on mature adipocytes [37]. In fact, in many cell types, both n-3 and n-6 PUFAs and their metabolites exert pleiotropic actions: they alter membrane fluidity and sensitivity to physicochemical and hormonal stimuli, while they also act on membrane and nuclear receptors. This complexity renders very difficult to evidence a relationship between dose and response, and the situation is even more complicated when optimal values of PUFA dietary intake have to be recommended. So we used the model of LA supplementation at 1, 2.5, or 5 % of energy intake in EFA-deficient rats to focus our attention on the dose dependency of the influence of LA on body weight gain, tissue PUFA composition, and on adipocyte lipolytic and lipogenic activities.
Materials and methods
Animals, diets, and experimental design
The experiment was conducted with a total of 36 male Wistar rats purchased from Janvier (Le Genest Saint Isle, France), with the approval of animal ethics Committee of Institut National de la Santé et de la Recherche Médicale (INSERM Midi-Pyrénées). The rats were individually housed at 21 days of age in cages placed in an air-conditioned room (20 ± 2 °C). All animals had free access to water and were initially fed a standard rodent chow ad libitum for a 5-day adaptation period. At this stage, rats were randomly allocated to one of the two semi-synthetic diets (from UAR laboratories, Villemoisson-Orge, France), the composition of which is given in Table 1. The diets were isocaloric (1,668 kJ/100 g) and contained 22 % of the calories as proteins (casein), 67 % as carbohydrates (dextrose), and 11 % as lipids. The lipids were under the form of a mixture of either peanut plus rapeseed oil for control group (n = 12) or saturated fatty acids, stearate, and palmitate for the EFA-deficient group (n = 24), as previously reported [22]. After 9 weeks, six rats of each group were euthanized for the determination of “supplementation week 0” levels, while the EFA-deficient rats were distributed in three groups of six rats supplemented with different amounts of linoleic acid (LA) at 1, 2.5, and 5 % of calorie intake for 6 weeks. The amounts of added linoleate were chosen in agreement with those reported to be effective in correcting various alterations induced by EFA deficiency [25] or claimed to modify diverse metabolic aspects in rats [12, 41]. The composition of the diets in LA, given by the supplier, was verified by gas chromatography analysis of the lipid fraction, and it was confirmed that the three supplemented diets contained 0.44, 1.1, and 2.2 g of LA/100 g dry chow, corresponding respectively to 1, 2.5, and 5 % of energy, while the EFA-deficient chow contained only 0.02 % of energy under the form of LA (Table 1).
Tissue removal and blood sampling
At the end of experimental period (“supplementation week 6”), rats were killed after overnight fasting. Blood sampling was promptly followed by plasma preparation; then aliquots were stored at −20 °C until analysis. Three different white adipose tissues (WAT) were removed and weighed: inguinal subcutaneous, epididymal, and retroperitoneal. They were pooled and subjected to collagenase digestion at 37 °C in order to obtain freshly isolated adipose cells for immediate explorations of lipolytic and lipogenic activities as in [4]. A portion of the washed fat cell suspension was frozen and stored at −20 °C until biochemical analyses.
Lipolytic activity of rat adipocyte preparations
The chosen index of lipolytic activity was glycerol release. Briefly, adipocytes isolated by collagenase digestion were washed and resuspended in Krebs–Ringer salt solution containing 15 mM sodium bicarbonate, 10 mM HEPES, and 3.5 % of serum bovine albumin (KRBHA) as previously described [34]. For lipolysis measurement, 5.5 mM glucose was present in the medium and the reference lipolytic agents isoprenaline, ACTH, adenosine deaminase (ADA), forskolin, or dibutyryl cAMP (dbcAMP) were tested for 60 min at 37 °C. Then reaction was stopped on ice as described [14]. Antilipolytic responses were measured under the same conditions in the presence of 5 μg/mL ADA and tested antilipolytic agents. A complete antilipolysis was considered as lowering stimulated levels back to basal values (set at 100 %), as previously reported [20]. Glycerol release was determined on aliquots of medium by spectrophotometric measurement at 340 nm, as previously described [4].
Lipogenic activity of rat adipocyte preparations
Lipogenesis was determined by quantifying the D-[3-3H]-glucose incorporation into lipids, according to Moody and co-workers [35]. Fat cells were incubated at 37 °C for 120 min in the same incubation medium as above, containing 0.6 mM of isotopic glucose dilution as unique source of carbohydrates. The same vials (Pony vials from Packard) were used for incubation, for lipid extraction in an organic mixture for liquid scintillation not miscible with aqueous solutions, and for counting of the extracted neo-synthesized lipids, with already reported slight adaptations [11] of the original method of Moody et al. The mean amount of fat cells distributed into the incubation vials represented 73.4, 67.5, 79.5, and 71.3 mg cellular lipids in the control, and in the 1, 2.5, and 5 % LA-supplemented groups, respectively.
Plasma, erythrocyte, and adipocyte fatty acid composition
Total lipids were extracted from plasma according to the method of Folch et al. [15]. The erythrocytes were washed three times with saline and the lipids extracted as in [10]. The phospholipids (PL) were then separated by thin-layer chromatography as previously described [17]. For adipocyte preparations, Folch’s extraction of total lipids was performed after homogenization. The neutral lipids were separated from the PL according to [27]. Then, triglycerides (TG) were separated and the PL purified by thin-layer chromatography [17].
Gas-chromatography analysis
Plasma and erythrocyte PL fractions were transesterified as well as PL and TG of adipocytes. Fatty acids of each fraction were then analyzed on gas chromatograph according to the method of Hagenfeldt [21], under previously reported conditions [23]. The relative proportion of each FA was expressed as percentage of a total of 13 quantified saturated and mono- or poly-unsaturated fatty acids (FA), which were C16:0, C16:1n-9, C16:1n-7, C18:0, C18:1n-9, C18:1n-7, C18:2n-6, C18:3n-6, C18:3n-3,c20:3n-9, C20:3n-6, C20:4 n-6, and C22:6n-3. For clarity, only a subset of the analyzed FAs is reported in “Results”.
Chemicals
(−) Isoprenaline, forskolin, prostaglandin E1 and E2, insulin, synacthen corresponding to the first 24 amino acids of adrenocorticotropic hormone (ACTH), and other reagents were obtained from Sigma-Aldrich (Saint Quentin Fallavier, France). Brimonidine (UK 14304) was a generous gift from late Dr. H. Paris (Toulouse, France). 3H-glucose and InstaFluorPlus were from PerkinElmer (Boston and Waltham, USA).
Statistical analyses
Results are given as means ± SEM. Statistical significance was assessed by use of Student’s t test. NS denotes non-significantly different from respective control.
Results
Influence of the EFA-deficient diet and of LA supplementation on body mass
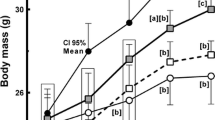
Body weight was significantly reduced in rats fed for 9 weeks with the EFA-deficient diet when compared to age-matched pair-fed controls. However, the EFA-deficient rats rapidly recovered body weight when subjected to LA supplementation (Fig. 1). After 6 weeks of treatment, the mean body weight was no more different in groups receiving the diets at 2.5 or 5 % LA and in the group having free access to control diet. Only the group receiving 1 % LA exhibited a growth retardation at the end of supplementation period, in spite of a delayed—but noticeable—increase in body weight gain, which averaged 48 g during the last 2 weeks of the supplementation protocol, while the corresponding value in control was 35 g.
Influence of linoleic acid supplementation on body weight gain in male rats. Body weight evolution between the age of 13 and 19 weeks in control rats fed a control, semi-synthetic, diet (circles, dotted line), or in previously fed with EFA-deficient diet until the 6-week period of supplementation (open circles, time 0), consisting in having free access to a diet containing 1 (open squares), 2.5 (closed squares), or 5 % (open triangles) of calories as linoleic acid (LA). Each point is the mean ± SEM of at least six males for each group. When not visible, error bar lies within the symbol. Different from respective control (closed circles) at *p < 0.05, **p < 0.01, and ***p < 0.001
Adiposity of control and treated rats
Figure 2 shows that the sum of the dissectible fat stores was dramatically reduced in EFA-deficient rats. These rats exhibited a significant weight reduction in all of the dissected adipose depots, i.e., epididymal, perirenal, mesenteric, and subcutaneous. Rats treated for 6 weeks with diet supplemented in LA showed a complete recovery of this altered parameter since a complete return to control values was reached, irrespective of the LA dose. To further unravel the mechanisms underlying such impressive fattening, which corresponded to more than a doubling of each fat pad mass (whatever its anatomic location, not shown), the lipolytic and lipogenic responses were studied in isolated adipocytes.
Effect of linoleic acid supplementation on fat accumulation in WAT. The total mass of dissected WAT is given before (week 0) and after (week 6) the LA supplementation period for rats fed a control diet (closed circles) or an EFA-deficient diet (open circles) and receiving LA at 1 (open squares), 2.5 (closed squares), or 5 % (open triangles) of calories. Each point is the mean ± SEM of at least three males for each group. Different from respective control at ***p < 0.001
Adipocyte lipolytic activity
First, lipolytic activity of isolated adipocytes was determined in all the six studied groups: control before (cont 0) and after the 6-week supplementation period (cont 6), EFA-deficient group before (defi 0), and after LA supplementation at 1, 2.5, or 5 % (percent of calorie intake). Ex vivo basal glycerol release exhibited only limited variations among the aforementioned groups, with a trend to be reduced after LA supplementation (Fig. 3). Lipolysis was further tested in response to activators and expressed as fold increase over basal.
Basal lipolysis in rat adipocytes. Glycerol release is given as μmol/100 mg cellular lipids/60 min for control (closed circles) and EFA-deficient rats (open circles) before and after supplementation with LA at 1 (open squares), 2.5 (closed squares), or 5 % of calories (open triangles). There was no statistical difference between the groups
Lipolytic effects of isoprenaline and non-adrenergic agents
At 1 μM, isoprenaline induced a clear-cut lipolytic activation: basal glycerol release was stimulated by 6–10-fold according to the group, showing that all preparations were responsive to β-adrenergic activation, with the exception of the groups supplemented with 1 or 5 % LA, which tended to respond less than the control (Fig. 4). As expected, the maximal β-adrenergic stimulation remained unchanged in the control group when checked before or after the 6-week supplementation period. This was also the case for synacthen (1 μg/mL, acting via direct activation of Gs-coupled ACTH receptor, also known as melanocortin receptor 2) and for adenosine deaminase (ADA, 5 IU/mL, removing adenosine that inhibits lipolysis via activation of Gi-coupled A1 receptors). There was a trend to observe the highest response in the EFA-deficient group when lipolysis was stimulated by ACTH or ADA. A complete normalization of such enhanced lipolysis was detected mainly in the group supplemented with 2.5 % LA, while those receiving 1 or 5 % LA appeared to be less responsive than control (Fig. 4). This indicated that LA supplementation was not following a monotonous (sigmoidal) dose–response relationship, as far as lipolytic responsiveness was considered, but exhibited a maximum at 2.5 % and decreased at the highest dose tested. The responses to the lipolytic agents forskolin and dbcAMP (directly acting on adenylyl cyclase or on protein kinase A, respectively) also exhibited a tendency to be higher in the EFA-deficient group, which was normalized after 6 weeks of LA supplementation, whatever the dose given (Fig. 5).
Influence of LA supplementation on receptor-mediated stimulation of lipolysis in white adipocytes. The following lipolytic agents were incubated for 1 h with adipocytes: isoprenaline 1 μM (upper section), synacthen 1 μg/mL, an ACTH analogue (central section), or adenosine deaminase 5 IU/mL (lower section). For the indicated groups, the resulting lipolytic responses are given as fold increase over basal glycerol release, set at 1 and visualized by a dotted line. When not visible, error bar lies within the symbol. The tendency to be different from respective control is indicated. Mean ± SEM of three rats
Influence of LA supplementation on non-hormonal stimulation of lipolysis in white adipocytes. Forskolin 10 μM (upper panel) or dibutyryl-cyclic AMP 1 mM (lower panel) were incubated for 1 h with adipocytes of the indicated groups. Lipolysis stimulation was expressed as fold increase over basal. Mean ± SEM of three rats
Antilipolytic responses of EFA-deficient and LA-supplemented adipocytes
To detect putative changes in the antilipolytic responses, two types of antilipolytic agents were tested on sub-maximally stimulated lipolysis and their effects were expressed as percentage of inhibition of stimulated lipolysis. The prostaglandins PGE1 and PGE2 shared a strong antilipolytic effect that, at 10 nM, largely impaired the stimulation induced by ADA in control. This was further increased in EFA-deficient adipocytes since PGE1 and PGE2 totally abolished lipolysis. Such clear-cut lipolysis inhibition was not deeply altered after LA supplementation, and there was no more distinguishable difference in the prostaglandins’ effect between the studied groups (Fig. 6). The α2-adrenergic agonist UK 14304 (brimonidine) was much less antilipolytic than prostaglandins since, at 1 μM, it only partially impaired ADA stimulation, and an even weaker antilipolytic effect was observed in rats supplemented with 1 or 5 % LA (Fig. 6).
Influence of EFA deficiency and LA supplementation on antilipolytic responses. Lipolysis was sub-maximally by ADA 5 IU/mL and adipocytes were incubated with the indicated antilipolytic agents: PGE1 or PGE2 at 10 nM or UK 14304 1 μM. Results as percentage of inhibition, with ADA alone set at 0 % and inhibition down to basal level set at 100 % inhibition. Mean ± SEM of three observations in each of the indicated groups. Different from respective control at *p < 0.05
Adipocyte lipogenic activity
In the context of the impressive fat depot extension occurring in rats recovering from EFA deficiency, it was of interest to study the interplay between LA supplementation level and adipocyte lipogenic activity. Surprisingly, basal lipogenesis exhibited a large inter-individual variability and a tendency to be elevated in the EFA-deficient rats since it was about 2-fold greater than in control even though this difference did not reach statistical significance (Fig. 7). Such apparent in vitro hyper-lipogenic activity disagrees with the limited in vivo fat accumulation of EFA-deficient rats. In fact, the expression of lipogenesis on a per 100 mg cellular lipid basis participated to a somewhat overestimation of adipocyte activity since at the start of supplementation period, there was much numerous smaller fat cells per lipid mass unit in the depleted WAT of EFA-deficient rats than in control. Such artifactual bias was not applicable at the end of supplementation period since the mean fat cell size was similar in treated and control groups (not shown), and since all groups shared the same WAT mass (see Fig. 2). Consequently, basal lipogenic activity was much less variable after the supplementation period: it was equivalent in control and in rats receiving 2.5 % LA, while it remained clearly higher in LA 1 % and LA 5 % groups (Fig. 7). Then, insulin responsiveness was expressed in all the experimental groups as fold increase over basal lipogenesis since such expression is independent of the units used to quantify activity (per 100 mg lipid or per 106 cells), as already documented [11]. In these conditions, insulin increased by approximately 2-fold the spontaneous glucose incorporation into lipids, at least in control before and after the 6-week treatment period (Fig. 8). The trend to exhibit an increased insulin responsiveness found in the EFA-deficient adipocytes totally disappeared in rats receiving 2.5 % LA. On the opposite, rats challenged with 1 and 5 % LA displayed a lower responsiveness to insulin than control (Fig. 8).
Basal lipogenesis in rat adipocytes. Glucose incorporation into neo-synthesized lipids is given as nmol/100 mg cellular lipids/120 min for control (closed circles) and EFA-deficient rats (open circles) before and after supplementation with LA at 1 (open squares), 2.5 (closed squares), or 5 % of calories (open triangles). Mean ± SEM of three rats. Different from respective control (closed circles) at *p < 0.05
Influence of LA supplementation on stimulation or inhibition of de novo lipogenic activity in white adipocytes. Insulin 2,500 μIU/mL and forskolin 10 μM were incubated for 120 min with adipocytes from control (closed circles), EFA-deficient rats (open circles), or from rats after LA supplementation at 1 (open squares), 2.5 (closed squares), or 5 % (open triangles). Lipogenic responses are given as fold increase over basal glucose incorporation into lipids, set at 1 (dotted line). When not visible, error bar lies within the symbol. Mean ± SEM of three observations. Different from control (closed circles) at *p < 0.05 and **p < 0.01
Lastly, it was examined how forskolin was altering lipogenesis. The diterpene deeply impaired basal lipogenesis in controls, in EFA-deficient and in 2.5 % LA group, while it was unable to hamper the elevated basal lipogenesis of the groups supplemented with 1 or 5 % LA (Fig. 8).
Influence of EFA deficiency and LA supplementation on fatty acid composition in plasma and erythrocyte phospholipids
FA composition of plasma and erythrocyte PL is shown in Tables 2 and 3, respectively. As expected, we observed in EFA-deficient rats an important decrease of C18:2n-6 and C20:4n-6 in both fractions, while the C20:3n-9 percentage increased. As a consequence, the EFA deficiency was characterized by a high ω9/ω6 ratio, especially in plasma PL (Table 2). The FA composition of plasma PL returned to control values within 6 weeks of LA supplementation at 2.5 and 5 %. By contrast, in the group at 1 %, 6 weeks of treatment were not sufficient to recover these biomarkers: the level C18:2n-6 remained low, while C20:3n-9 and ω9/ω6 ratio were elevated. In erythrocytes, all the manifestations of deficiency disappeared after 6 weeks with LA at 5 %. With 2.5 % LA, only the proportion of C20:3n-9 remained elevated (Table 3), while in rats fed with diet at 1 % LA many biomarkers remained indicative of EFA deficiency.
LA supplementation and FA composition in adipocyte phospholipids and triglycerides
In adipocytes (Table 4), FA composition of membrane PL and stored triglycerides displayed the characteristic signs of EFA deficiency: important decrease of linoleic and arachidonic acid (C18:2n-6 and C20:4n-6). Moreover, the increase of C20:3n-9 and ω9/ω6 ratio appeared to be limited in triglycerides, suggesting a buffering action of lipid storage/mobilization relative to changes in FA composition. For most of the FA analyzed, a disappearance of the signs of EFA deficiency was evident after 2.5 % LA supplementation. The higher LA dose allowed a more complete or rapid recovery, but was also accompanied with intriguing rebounds such as increased C18:2n-6 proportion in stored triglycerides (Table 4). Evidently, adipocytes from 1 % LA supplemented group still revealed biochemical signs of EFA deficiency, as for blood fractions.
Discussion
Our results show that, when supplemented with LA at 2.5 %, EFA-deficient rats exhibited a restoration of all observed alterations: anatomical (body and adipose tissue mass), functional (adipocyte lipolytic and lipogenic responses), and biochemical (fatty acid composition). In deficient rats supplemented with either 1 or 5 % LA, the adipose mass was completely regained while adipocytes surprisingly did not completely improve their altered responses and displayed high spontaneous lipogenic activity.
The EFA-deficient state of the rats used for the LA supplementation protocol was clearly evidenced by recognized biomarkers such as decreased n-6 PUFAs and increased C20:3n-9. Consequently, an increased ω9/ω6 ratio, recognized to be a sign of a lack of dietary EFA [22, 30], was found in blood fractions: plasma and erythrocyte phospholipids. This was also the case for the stored triglycerides and the membrane phospholipids of adipocytes. Such clear-cut biochemical changes were accompanied by only a weak trend to elevate basal lipogenesis and to increase the responses to lipolytic activators, at least when adipocyte functions were investigated ex vivo. The moderate tendency to increase insulin lipogenic effect observed after LA restriction cannot be considered as surprising since an increased conversion of glucose to lipids, already observed in rats subjected to EFA deficiency, has been reported to vary according to strain differences [5].
The biomarker alterations such as increased ω9/ω6 ratio were recovered after 6-week supplementation with LA in a typical dose-dependent manner. Our study show that even the FA composition of lipids stored in fat cells was sensitive to dietary n-6 PUFAs. This confirmed that ingested LA can accumulate in fat tissues, as already demonstrated by kinetic studies using stable isotope tracers [31]. The restoration of the FA composition found in diverse lipid fractions also demonstrated that the percentages of LA in the supplemented diets were adequately chosen. Among the three different levels of LA, the lower set at 1 % of energy can be considered as insufficient, and that at 2.5 % close to the recommended levels. Notably, the 5 % level provoked an LA accumulation in fat cell droplets that exceeded normal values. Indeed, recommendations for daily LA human intake vary from 2 to 3 % of total energy for adequate intake to assure good growth, development, and biological functions [44]. An upper limit has been fixed to 4–6 % of energy, considering the risk of inflammation or obesity linked with significant LA consumption as non-negligible [44]. Accordingly, the supplementation at 2.5 % of LA was sufficient in our model to allow body weight, biomarkers, and most of the adipocyte responses to return to control values. By contrast, 6 weeks of supplementation at 1 % LA were not sufficient for a total recovery of body mass and of most of biochemical markers, except the C20:4n-6 blood levels. Nevertheless, the lower LA dose surprisingly allowed a complete recovery of fat depot mass (subcutaneous, epididymal, and retroperitoneal). The elevated basal lipogenesis, together with the impaired antilipolysis (at least that of α2-adrenergic nature) and the blunted lipolytic capacity (lowered maximal responses to isoprenaline or ADA) found in adipocytes from the 1 % LA group, indicated that WAT was still recovering from the EFA deficiency-induced disturbances by exaggerating mechanisms that favor lipid storage. Undoubtedly, upon LA administration, WAT was the site of an intense anabolism leading to fat store repletion. The supply of only 1 % LA was probably moderating such adipose store repletion, though leading to a complete recovery in 6 weeks, while one can suppose that a shorter period was necessary with 2.5 or 5 % supplementation. More intriguingly, the higher LA dose of 5 % induced a state of adipocyte responsiveness that was resembling that found in rats supplemented with 1 % LA. With the lower and the higher doses, there was a tendency to reduce maximal lipolysis and to increase lipogenesis up to a state that was resistant to further stimulation by insulin. The insulin resistance found at the higher LA dose deals with that previously analyzed in 3T3 adipocytes challenged with 300 μM LA [16]. Indeed, the higher dose of dietary LA (5 % of energy) resulted in an impressive accumulation of LA in fat cell triglycerides that was twice that found in control. This is likely a sign of LA overload. In this case, the deleterious effect of excessive n-6 PUFAs on insulin resistance originally proposed by Simopoulos on the basis of clinical observations [42] was confirmed by our model. Nevertheless, high intake of LA has been implicated in the impressive WAT development through several mechanisms that compensated for this apparent decreased insulin responsiveness. It is accepted that high LA levels raise arachidonate abundance in adipose tissue, which, in turn, increases prostacyclin production and stimulates signaling pathways implicated in adipogenesis [33, 36]. Finally, the exaggerated lipid deposition and the increased lipogenesis we found in WAT of rats treated with n-6 PUFA was perfectly mirroring the opposite effects of n-3 PUFAs, which activate lipid oxidation and reduce lipogenesis in liver [8].
The inability of forskolin to reduce the elevated basal lipogenesis in the 1 and 5 % LA groups remains unclear but underlines how elevated was the spontaneous triglyceride synthesis under these conditions. Forskolin was used to inhibit lipogenesis not as a direct adenylyl cyclase activator but rather as a glucose uptake blocker since it binds to cell-surface glucose transporters and impairs their function [24]. Such blockade was blunting the glucose incorporation into lipids in the control, EFA-deficient, and 2.5 % LA-supplemented groups, but appeared inoperative in rats exhibiting an exaggerated spontaneous lipogenesis (1 and 5 % LA). Probably the elevated activity of the intracellular enzymes involved in triglyceride assembly, as already reported for animals fed LA-enriched diets [29], has rendered less limiting the transport step in the pathway of glucose incorporation into lipids. The exact nature of the acceleration of the glucose incorporation into neo-synthesized cellular lipids in the fat cells after 1 and 5 % LA supply remains to be determined. In spite of the absence of any mechanistic demonstration, our observations are in total accordance with the concept that not only dietary fat amount but also dietary fat composition influence adipose tissue metabolism via perturbations of membrane functions and cellular metabolic processes [13].
Regarding lipolysis, EFA-deficient rats displayed an increased sensitivity to activation by various lipolytic agents, irrespective of their mechanism of action. This apparently contrasts with the loss of sensitivity reported for epinephrine-stimulated lipase in similarly deficient rats [26], but likely fits with the need to mobilize most of the PUFAs stored in the adipocytes to limit the impact of EFA deficiency. As with other troubles, these increased lipolytic responses were recovered with LA supplementation. The α2-adrenergic antilipolytic component, known to be larger in obese than in lean rats [6], was almost unaltered by EFA deficiency, but limited in the groups supplemented with the low and the high LA doses, indicating either an incomplete recovery from deficiency or a dysregulation by excess of n-6 PUFAs.
Since LA has been reported to be a precursor of prostaglandins [25], it was of special interest to test the effects of a few prostaglandins on mature adipocyte lipolysis. We observed that PGE1 and PGE2 induced antilipolytic effects that were slightly more important in EFA-deficient adipocytes, an alteration that was corrected by LA supplementation, independently of the PUFA dose.
Lastly, our observations were unable to demonstrate a typical dose dependency of the effects of LA on several adipocyte functions. They agree with a study in which linoleate decreased basal adiponectin secretion and insulin-stimulated leptin secretion when tested in vitro with rat adipocytes for 96 h [37]. This study brought evidence that the LA was acting directly on adipocytes, but raised a concern about the dose dependency of the n-6 PUFA since the effects obtained at 1 and 200 μM were almost similar. Thus, adipocyte functions (related to lipid handling or to adipokine secretion) do not seem to follow a typical, sigmoidal, dose–response curve in the presence of LA. Based on the three LA supplementation levels we tested, it appeared that the maximal effect was not induced by the highest tested dose. Although limited to a reduced number of observations, and in spite of possible changes in the proportion of dietary fatty acids other than LA among the different experimental semi-synthetic diets we compared in this study, our findings support that a dietary intake of LA at 5 % of energy supply is not an optimal dose for adipocyte functions. Taken together, our results bring evidence that dietary LA exquisitely influences lipolytic and lipogenic activities and that incomplete or excessive intake of this n-6 PUFA may rapidly promote deleterious effects on adipose tissue.
References
Ailhaud G, Guesnet P, Cunnane SC (2008) An emerging risk factor for obesity: does disequilibrium of polyunsaturated fatty acid metabolism contribute to excessive adipose tissue development? Br J Nutr 100:461–470
Ailhaud G, Massiera F, Weill P, Legrand P, Alessandri JM, Guesnet P (2006) Temporal changes in dietary fats: role of n-6 polyunsaturated fatty acids in excessive adipose tissue development and relationship to obesity. Prog Lipid Res 45:203–236
Alvheim AR, Malde MK, Osei-Hyiaman D, Hong Lin Y, Pawlosky RJ, Madsen L, Kristiansen K, Frøyland L, Hibbeln JR (2012) Dietary linoleic acid elevates endogenous 2-AG and anandamide and induces obesity. Obesity (Silver Spring) 20:1984–1994
Atgié C, Sauvant P, Ambid L, Carpéné C (2009) Possible mechanisms of weight loss of Siberian hamsters (Phodopus sungorus sungorus) exposed to short photoperiod. J Physiol Biochem 65:377–386
Berdanier CD, Baltzell JK (1986) Comparative studies of the responses of two strains of rats to an essential fatty acid deficient diet. Comp Biochem Physiol A Comp Physiol 85:725–727
Carpéné C, Rebourcet MC, Guichard C, Lafontan M, Lavau M (1990) Increased alpha 2-adrenergic binding sites and antilipolytic effect in adipocytes from genetically obese rats. J Lipid Res 31:811–819
Cicero AFG, Reggi A, Parini A, Borghi C (2012) Application of polyunsaturated fatty acids in internal medicine: beyond the established cardiovascular effects. Arch Med Sci 8:784–793
Clarke SD (2001) Nonalcoholic steatosis and steatohepatitis. I. Molecular mechanism for polyunsaturated fatty acid regulation of gene transcription. Am J Physiol Gastrointest Liver Physiol 281:G865–G869
Cohen-Luria R, Sigler L, Rimon G (1989) Biphasic effect of sodium fluoride and guanyl nucleotides on binding to prostaglandin E2 receptors in rat epididymal adipocyte membranes. Cell Signal 1:561–568
Dodge JT, Phillips GB (1967) Composition of phospholipids and of phospholipids fatty acids and aldehydes in human red cells. J Lipid Res 8:667–675
Duffaut C, Bour S, Prévot D, Marti L, Testar X, Zorzano A, Carpéné C (2006) Prolonged treatment with the β3-adrenergic agonist CL 316243 induces adipose tissue remodeling in rat but not in guinea pig: 2) modulation of glucose uptake and monoamine oxidase activity. J Physiol Biochem 62:101–112
Eder E, Wacker M, Lutz U, Nair J, Fang X, Bartsch H, Beland FA, Schlatter J, Lutz WK (2006) Oxidative stress related DNA adducts in the liver of female rats fed with sunflower-, rapeseed-, olive- or coconut oil supplemented diets. Chem Biol Interact 159:81–89
Fernández-Quintela A, Churruca I, Portillo MP (2007) The role of dietary fat in adipose tissue metabolism. Public Health Nutr 10:1126–1131
Ferrand C, Redonnet A, Prévot D, Carpéné C, Atgié C (2006) Prolonged treatment with the β3-adrenergic agonist CL 316243 induces adipose tissue remodeling in rat but not in guinea pig: 1) fat store depletion and desensitization of β-adrenergic responses. J Physiol Biochem 62:89–100
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Gao Z, Zhang X, Zuberi A, Hwang D, Quon MJ, Lefevre M, Ye J (2004) Inhibition of insulin sensitivity by free fatty acids requires activation of multiple serine kinases in 3T3-L1 adipocytes. Mol Endocrinol 18:2024–2034
Garcia J, Ghisolfi J, Lapalu-Traon C, Periquet B, Olives JP, Boyer MJ, Thouvenot JP (1986) Screening for essential fatty acid deficiency in the infant. Ann Biol Clin 44:380–383
Gerber M (2009) Background review paper on total fat, fatty acid intake and cancers. Ann Nutr Metab 55:140–161
Gómez Candela C, Bermejo López LM, Loria Kohen V (2011) Importance of a balanced omega 6/omega 3 ratio for the maintenance of health: nutritional recommendations. Nutr Hosp 26:323–329
Gomez-Zorita S, Tréguer K, Mercader J, Carpéné C (2013) Resveratrol directly affects in vitro lipolysis and glucose transport in human fat cells. J Physiol Biochem 69:585–593
Hagenfeldt L (1966) A gas-chromatographic method for the determination of individual free fatty acids in plasma. Clin Chim Acta 13:266–268
Harant I, Carpéné C, Garcia J, Thouvenot JP, Ghisolfi J (1990) Correction by dietary linoleic acid of rat adipocyte metabolic disorders in essential fatty acid deficiency. Food Addit Contam 7:S134–S137
Harant I, Ghisolfi J, Couvaras O, Garcia J, Vaysse P, Thouvenot JP (1990) Fatty acid composition of adipocyte membrane phospholipids and stored triglycerides in infants receiving total parenteral nutrition. J Parenter Enteral Nutr 14:42–46
Hellwig B, Brown FM, Schürmann A, Shanahan MF, Joost HG (1992) Localization of the binding domain of the inhibitory ligand forskolin in the glucose transporter GLUT-4 by photolabeling, proteolytic cleavage and a site-specific antiserum. Biochim Biophys Acta 1111:178–184
Huang YS, Mitchell J, Jenkins K, Manku MS, Horrobin DF (1984) Effect of dietary depletion and repletion of linoleic acid on renal fatty acid composition and urinary prostaglandin excretion. Prostaglandins Leukot Med 15:223–228
Iritani N, Ikeda Y, Fukuda H (1984) Physiological impairment in linoleic acid deficiency of rats and the effect of n-3 polyunsaturated fatty acids. J Nutr Sci Vitaminol (Tokyo) 30:179–185
Juaneda P, Rocquelin G (1985) Rapid and convenient separation of phospholipids and non-phosphorus lipids from rat heart using silica cartridges. Lipids 20:40–41
Kirby A, Woodward A, Jackson S, Wang Y, Crawford MA (2010) The association of fatty acid deficiency symptoms (FADS) with actual essential fatty acid status in cheek cells. Prostaglandins Leukot Essent Fatty Acids 83:1–8
Kouba M, Mourot J (1998) Effect of a high linoleic acid diet on delta 9-desaturase activity, lipogenesis and lipid composition of pig subcutaneous adipose tissue. Reprod Nutr Dev 38:31–37
Le HD, Fallon EM, Kalish BT, de Meijer VE, Meisel JA, Gura KM, Nose V, Pan AH, Bistrian BR, Puder M (2013) The effect of varying ratios of docosahexaenoic acid and arachidonic acid in the prevention and reversal of biochemical essential fatty acid deficiency in a murine model. Metabolism 62:499–508
Lin YH, Salem N (2007) Whole body distribution of deuterated linoleic and alpha-linolenic acids and their metabolites in the rat. J Lipid Res 48:2709–2724
Makrides M, Smithers LG, Gibson RA (2010) Role of long-chain polyunsaturated fatty acids in neurodevelopment and growth. Nestlé Nutr Workshop Ser Pediatr Program 65:123–136
Massiera F, Saint-Marc P, Seydoux J, Murata T, Kobayashi T, Narumiya S, Guesnet P, Amri EZ, Negrel R, Ailhaud G (2003) Arachidonic acid and prostacyclin signaling promote adipose tissue development: a human health concern? J Lipid Res 44:271–279
Mercader J, Wanecq E, Chen J, Carpéné C (2011) Isopropylnorsynephrine is a stronger lipolytic agent in human adipocytes than synephrine and other amines present in Citrus aurantium. J Physiol Biochem 67:443–452
Moody AJ, Stan MA, Stan M, Gliemann J (1974) A simple free fat cell bioassay for insulin. Horm Metab Res 6:12–16
Muhlhausler BS, Ailhaud GP (2013) Omega-6 polyunsaturated fatty acids and the early origins of obesity. Curr Opin Endocrinol Diabetes Obes 20:56–61
Pérez-Matute P, Martinez JA, Marti A, Moreno-Aliaga MJ (2007) Linoleic acid decreases leptin and adiponectin secretion from primary rat adipocytes in the presence of insulin. Lipids 42:913–920
Pickova J (2009) Importance of knowledge on lipid composition of foods to support development towards consumption of higher levels of n-3 fatty acids via freshwater fish. Physiol Res 58(Suppl 1):S39–S45
Pinel A, Morio-Liondore B, Capel F (2014) Omega 3 fatty acids modulate metabolism of insulin-sensitive tissues: implication for the prevention of type 2 diabetes. J Physiol Biochem. doi:10.1007/s13105-013-0303-2
Prince MJ, Deeg MA (1997) Do n-3 fatty acids improve glucose tolerance and lipemia in diabetics? Curr Opin Lipidol 8:7–11
Schlemmer CK, Coetzer H, Claassen N, Kruger MC (1999) Oestrogen and essential fatty acid supplementation corrects bone loss due to ovariectomy in the female Sprague Dawley rat. Prostaglandins Leukot Essent Fatty Acids 61:381–390
Simopoulos AP (1994) Is insulin resistance influenced by dietary linoleic acid and trans fatty acids? Free Radic Biol Med 17:367–372
Simopoulos AP (2002) The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother 56:365–379
Simopoulos AP (2008) The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med (Maywood) 233:674–688
Soderhjelm L, Weise HF, Holman RT (1970) The role of polyunsaturated fatty acid in human nutrition and metabolism. Prog Chem Fats Lipids 9:555–585
Taouis M, Dagou C, Ster C, Durand G, Pinault M, Delarue J (2002) N-3 polyunsaturated fatty acids prevent the defect of insulin receptor signaling in muscle. Am J Physiol Endocrinol Metab 282:E664–E671
Willett WC (2007) The role of dietary n-6 fatty acids in the prevention of cardiovascular disease. J Cardiovasc Med (Hagerstown) 8:S42–S45
Wolf G (1996) Adipocyte differentiation is regulated by a prostaglandin liganded to the nuclear peroxisome proliferator-activated receptor. Nutr Rev 54:290–292
Yamanaka WK, Clemans GW, Hutchinson ML (1980) Essential fatty acids deficiency in humans. Prog Lipid Res 19:187–215
Acknowledgement
The authors express gratitude to Brigitte Periquet (Univ. Paul Sabatier, Toulouse, France) for her knowledge on adipocyte biology and dietary fatty acids, and to the staff of Biochemical analyses of Rangueil Hospital (Toulouse, France) for facilitating access to their devices.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Harant-Farrugia, I., Garcia, J., Iglesias-Osma, MC. et al. Is there an optimal dose for dietary linoleic acid? Lessons from essential fatty acid deficiency supplementation and adipocyte functions in rats. J Physiol Biochem 70, 615–627 (2014). https://doi.org/10.1007/s13105-014-0315-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-014-0315-6